Search
- Page Path
- HOME > Search
Review Article
- Calcium & bone metabolism
- Skeletal Senescence with Aging and Type 2 Diabetes
- Joshua Nicholas Farr
- Endocrinol Metab. 2023;38(3):295-301. Published online June 14, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1727
- 2,783 View
- 127 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
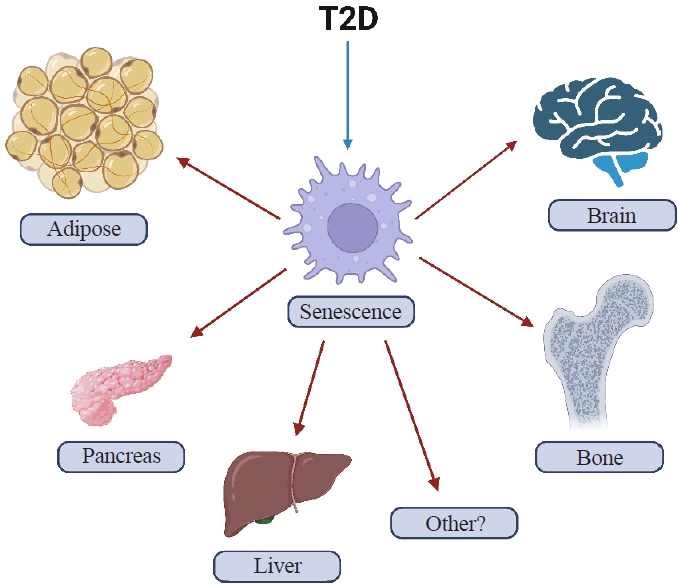
ePub - Osteoporosis and type 2 diabetes (T2D) are common diseases that often coexist. While both of these diseases are associated with poor bone quality and increased fracture risk, their pathogenesis of increased fracture risk differs and is multifactorial. Mounting evidence now indicates that key fundamental mechanisms that are central to both aging and energy metabolism exist. Importantly, these mechanisms represent potentially modifiable therapeutic targets for interventions that could prevent or alleviate multiple complications of osteoporosis and T2D, including poor bone quality. One such mechanism that has gained increasing momentum is senescence, which is a cell fate that contributes to multiple chronic diseases. Accumulating evidence has established that numerous boneresident cell types become susceptible to cellular senescence with old age. Recent work also demonstrates that T2D causes the premature accumulation of senescent osteocytes during young adulthood, at least in mice, although it remains to be seen which other bone-resident cell types become senescent with T2D. Given that therapeutically removing senescent cells can alleviate age-related bone loss and T2D-induced metabolic dysfunction, it will be important in future studies to rigorously test whether interventions that eliminate senescent cells can also alleviate skeletal dysfunction in context of T2D, as it does with aging.
-
Citations
Citations to this article as recorded by- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Cellular Neuroscience.2024;[Epub] CrossRef - The synergistic effect of diabetes mellitus and osteoporosis on the all-cause mortality: a cohort study of an American population
Weihua Li, Siyu Xie, Shengdong Zhong, Liting Lan
Frontiers in Endocrinology.2024;[Epub] CrossRef - Identification of systemic biomarkers and potential drug targets for age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Aging Neuroscience.2024;[Epub] CrossRef
- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration

Original Article
- Diabetes, Obesity and Metabolism
- Inhibition of miR-146a-5p and miR-8114 in Insulin-Secreting Cells Contributes to the Protection of Melatonin against Stearic Acid-Induced Cellular Senescence by Targeting Mafa
- Shenghan Su, Qingrui Zhao, Lingfeng Dan, Yuqing Lin, Xuebei Li, Yunjin Zhang, Chunxiao Yang, Yimeng Dong, Xiaohan Li, Romano Regazzi, Changhao Sun, Xia Chu, Huimin Lu
- Endocrinol Metab. 2022;37(6):901-917. Published online December 7, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1565

- 2,558 View
- 220 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Chronic exposure to elevated levels of saturated fatty acids results in pancreatic β-cell senescence. However, targets and effective agents for preventing stearic acid-induced β-cell senescence are still lacking. Although melatonin administration can protect β-cells against lipotoxicity through anti-senescence processes, the precise underlying mechanisms still need to be explored. Therefore, we investigated the anti-senescence effect of melatonin on stearic acid-treated mouse β-cells and elucidated the possible role of microRNAs in this process.
Methods
β-Cell senescence was identified by measuring the expression of senescence-related genes and senescence-associated β-galactosidase staining. Gain- and loss-of-function approaches were used to investigate the involvement of microRNAs in stearic acid-evoked β-cell senescence and dysfunction. Bioinformatics analyses and luciferase reporter activity assays were applied to predict the direct targets of microRNAs.
Results
Long-term exposure to a high concentration of stearic acid-induced senescence and upregulated miR-146a-5p and miR- 8114 expression in both mouse islets and β-TC6 cell lines. Melatonin effectively suppressed this process and reduced the levels of these two miRNAs. A remarkable reversibility of stearic acid-induced β-cell senescence and dysfunction was observed after silencing miR-146a-5p and miR-8114. Moreover, V-maf musculoaponeurotic fibrosarcoma oncogene homolog A (Mafa) was verified as a direct target of miR-146a-5p and miR-8114. Melatonin also significantly ameliorated senescence and dysfunction in miR-146a-5pand miR-8114-transfected β-cells.
Conclusion
These data demonstrate that melatonin protects against stearic acid-induced β-cell senescence by inhibiting miR-146a- 5p and miR-8114 and upregulating Mafa expression. This not only provides novel targets for preventing stearic acid-induced β-cell dysfunction, but also points to melatonin as a promising drug to combat type 2 diabetes progression. -
Citations
Citations to this article as recorded by- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice
Yuxuan Chen, Rou Peng, Yi Qian, Yizhou Lu, Liyao Chen, Meiling Yu, Minjiao Jiang, Wei Wu, Shengfeng Lu
Gene.2024; 897: 148090. CrossRef - MiR-126 and miR-146a as Melatonin-Responsive Biomarkers for Neonatal Brain Ischemia
Maria Cristina Albertini, Tania Vanzolini, Serafina Perrone, Michael D. Weiss, Giuseppe Buonocore, Valentina Dell’Orto, Walter Balduini, Silvia Carloni
Journal of Molecular Neuroscience.2023; 73(9-10): 763. CrossRef
- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice


 KES
KES

 First
First Prev
Prev