Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
- Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2024;39(1):1-11. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1922
- 1,887 View
- 74 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
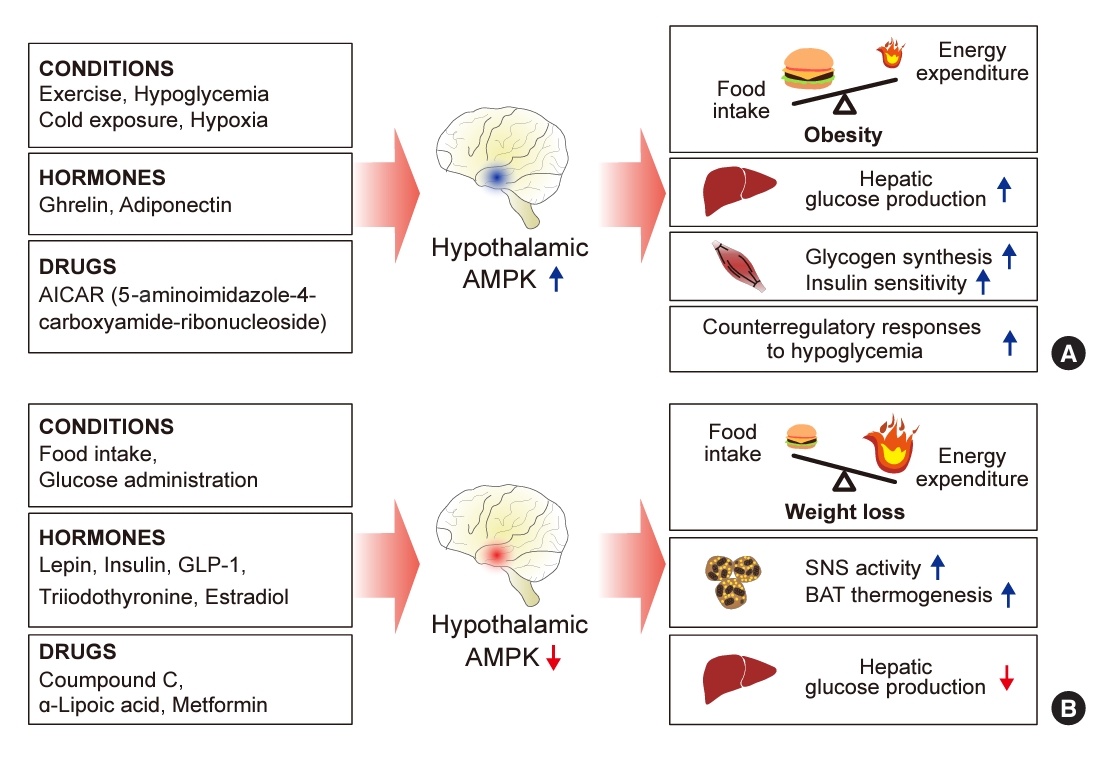
ePub - 5´-Adenosine monophosphate (AMP)-activated protein kinase (AMPK), a cellular energy sensor, is an essential enzyme that helps cells maintain stable energy levels during metabolic stress. The hypothalamus is pivotal in regulating energy balance within the body. Certain neurons in the hypothalamus are sensitive to fluctuations in food availability and energy stores, triggering adaptive responses to preserve systemic energy equilibrium. AMPK, expressed in these hypothalamic neurons, is instrumental in these regulatory processes. Hypothalamic AMPK activity is modulated by key metabolic hormones. Anorexigenic hormones, including leptin, insulin, and glucagon-like peptide 1, suppress hypothalamic AMPK activity, whereas the hunger hormone ghrelin activates it. These hormonal influences on hypothalamic AMPK activity are central to their roles in controlling food consumption and energy expenditure. Additionally, hypothalamic AMPK activity responds to variations in glucose concentrations. It becomes active during hypoglycemia but is deactivated when glucose is introduced directly into the hypothalamus. These shifts in AMPK activity within hypothalamic neurons are critical for maintaining glucose balance. Considering the vital function of hypothalamic AMPK in the regulation of overall energy and glucose balance, developing chemical agents that target the hypothalamus to modulate AMPK activity presents a promising therapeutic approach for metabolic conditions such as obesity and type 2 diabetes mellitus.

- Obesity and Metabolism
- Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress
- Min Jeong Choi, Saet-Byel Jung, Joon Young Chang, Minho Shong
- Endocrinol Metab. 2021;36(1):1-11. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.956

- 5,488 View
- 228 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Paracrine interactions are imperative for the maintenance of adipose tissue intercellular homeostasis, and intracellular organelle dysfunction results in local and systemic alterations in metabolic homeostasis. It is currently accepted that mitochondrial proteotoxic stress activates the mitochondrial unfolded protein response (UPRmt) in vitro and in vivo. The induction of mitochondrial chaperones and proteases during the UPRmt is a key cell-autonomous mechanism of mitochondrial quality control. The UPRmt also affects systemic metabolism through the secretion of cell non-autonomous peptides and cytokines (hereafter, metabokines). Mitochondrial function in adipose tissue plays a pivotal role in whole-body metabolism and human diseases. Despite continuing interest in the role of the UPRmt and quality control pathways of mitochondria in energy metabolism, studies on the roles of the UPRmt and metabokines in white adipose tissue are relatively sparse. Here, we describe the role of the UPRmt in adipose tissue, including adipocytes and resident macrophages, and the interactive roles of cell non-autonomous metabokines, particularly growth differentiation factor 15, in local adipose cellular homeostasis and systemic energy metabolism.
-
Citations
Citations to this article as recorded by- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior
Carla Igual Gil, Bethany M Coull, Wenke Jonas, Rachel N Lippert, Susanne Klaus, Mario Ost
Life Science Alliance.2022; 5(11): e202201495. CrossRef - Stress-induced FGF21 and GDF15 in obesity and obesity resistance
Susanne Keipert, Mario Ost
Trends in Endocrinology & Metabolism.2021; 32(11): 904. CrossRef
- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior

- Diabetes-Related Cardiac Dysfunction
- Lamario J. Williams, Brenna G. Nye, Adam R. Wende
- Endocrinol Metab. 2017;32(2):171-179. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.171
- 12,218 View
- 45 Download
- 37 Web of Science
- 36 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The proposal that diabetes plays a role in the development of heart failure is supported by the increased risk associated with this disease, even after correcting for all other known risk factors. However, the precise mechanisms contributing to the condition referred to as diabetic cardiomyopathy have remained elusive, as does defining the disease itself. Decades of study have defined numerous potential factors that each contribute to disease susceptibility, progression, and severity. Many recent detailed reviews have been published on mechanisms involving insulin resistance, dysregulation of microRNAs, and increased reactive oxygen species, as well as causes including both modifiable and non-modifiable risk factors. As such, the focus of the current review is to highlight aspects of each of these topics and to provide specific examples of recent advances in each area.
-
Citations
Citations to this article as recorded by-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats
Afolabi C. Akinmoladun, Morenikejimi Bello, Emmanuel Oluwafemi Ibukun
Archives of Physiology and Biochemistry.2023; 129(6): 1219. CrossRef - An Overview of Cardiotonic Medicinal Plants from the Perspective of Iranian Traditional Medicine
Akram Alembagheri, Homa Hajimehdipoor, Rasool Choopani, Somayeh Esmaeili
Jundishapur Journal of Natural Pharmaceutical Products.2023;[Epub] CrossRef - Nanoformulations for the Delivery of Dietary Anthocyanins for the Prevention and Treatment of Diabetes Mellitus and Its Complications
Ana R. Nunes, Elisabete C. Costa, Gilberto Alves, Luís R. Silva
Pharmaceuticals.2023; 16(5): 736. CrossRef - Cyp2e1 knockdown attenuates high glucose-induced apoptosis and oxidative stress of cardiomyocytes by activating PI3K/Akt signaling
Jianying Wang, Han Yang, Chao Wang, Cuie Kan
Acta Diabetologica.2023; 60(9): 1219. CrossRef - Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
Yu-Qi Li, Lei Xin, Yu-Chi Zhao, Shang-Qi Li, Ya-Nuo Li
World Journal of Hepatology.2023; 15(6): 786. CrossRef - Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine
Ahmed S. Mandour, Ahmed Farag, Mahmoud A. Y. Helal, Gamal El-Masry, Salim Al-Rejaie, Ken Takahashi, Tomohiko Yoshida, Lina Hamabe, Ryou Tanaka
Animals.2023; 13(15): 2452. CrossRef - Diet‐induced prediabetes: Effects on the activity of the renin–angiotensin–aldosterone system in selected organs
Bongeka Cassandra Mkhize, Palesa Mosili, Phikelelani Sethu Ngubane, Ntethelelo Hopewell Sibiya, Andile Khathi
Journal of Diabetes Investigation.2022; 13(5): 768. CrossRef - Knowledge domain and emerging trends in diabetic cardiomyopathy: A scientometric review based on CiteSpace analysis
Shiyi Tao, Deshuang Yang, Lanxin Zhang, Lintong Yu, Zihan Wang, Lingling Li, Jin Zhang, Ruiqi Yao, Li Huang, Mingjing Shao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Sodium–Glucose Co-transporter-2 Inhibitors
Joshua J Neumiller, Fredrick J Lienhard, Radica Z Alicic, Katherine R Tuttle
European Endocrinology.2022; 18(2): 106. CrossRef - Protective effects of medicinal plant against diabetes induced cardiac disorder: A review
Sadegh Shabab, Zahra Gholamnezhad, Maryam Mahmoudabady
Journal of Ethnopharmacology.2021; 265: 113328. CrossRef - Toward a broader view of mechanisms of drug cardiotoxicity
Polina Mamoshina, Blanca Rodriguez, Alfonso Bueno-Orovio
Cell Reports Medicine.2021; 2(3): 100216. CrossRef - Cardioprotective Effect of Glycyrrhizin on Myocardial Remodeling in Diabetic Rats
Vikram Thakur, Narah Alcoreza, Monica Delgado, Binata Joddar, Munmun Chattopadhyay
Biomolecules.2021; 11(4): 569. CrossRef - Cardioprotective Action of Glycyrrhizin on Diabetic Rats with Myocardial Remodeling
Fuxu Chen, Jie Song, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Management of inflammation in cardiovascular diseases
Sumanta Kumar Goswami, Prabhat Ranjan, Roshan Kumar Dutta, Suresh Kumar Verma
Pharmacological Research.2021; 173: 105912. CrossRef - Diabetic Cardiomyopathy: Clinical and Metabolic Approach
Dragan B. Djordjevic, Goran Koracevic, Aleksandar D. Djordjevic, Dragan B. Lovic
Current Vascular Pharmacology.2021; 19(5): 487. CrossRef - Effect of Acute Chemotherapy on Glucose Levels in Rats
Ahmad H. Alhowail, Gena S. Alfawzan, Maha A. Aldubayan, Lolwah S. Alsalam
International Journal of Pharmacology.2020; 16(3): 276. CrossRef - Transplantation of adipose tissue lacking PAI-1 improves glucose tolerance and attenuates cardiac metabolic abnormalities in high-fat diet-induced obesity
Sijing Liu, Yi Li, Xin Fan, Kai Li, Chunrong Xu, Liping Zhang, Mao Luo, Liqun Wang, Rong Li, Jianbo Wu
Adipocyte.2020; 9(1): 170. CrossRef - Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers
Jeffrey I. Mechanick, Michael E. Farkouh, Jonathan D. Newman, W. Timothy Garvey
Journal of the American College of Cardiology.2020; 75(5): 525. CrossRef - Associated Targets of the Antioxidant Cardioprotection of Ganoderma lucidum in Diabetic Cardiomyopathy by Using Open Targets Platform: A Systematic Review
Fahmi Shaher, Hongbin Qiu, Shuqiu Wang, Yu Hu, Weiqun Wang, Yu Zhang, Yao Wei, Hisham AL-ward, Mahfoudh A. M. Abdulghani, Sattam Khulaif Alenezi, Salem Baldi, Shaobo Zhou
BioMed Research International.2020; 2020: 1. CrossRef - Human trophoblast-derived exosomes attenuate doxorubicin-induced cardiac injury by regulating miR-200b and downstream Zeb1
Jie Ni, Yihai Liu, Lina Kang, Lian Wang, Zhonglin Han, Kun Wang, Biao Xu, Rong Gu
Journal of Nanobiotechnology.2020;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Glucagon-like Peptide-1 Receptor Agonists
Emily J Cox, Radica Z Alicic, Joshua J Neumiller, Katherine R Tuttle
US Endocrinology.2020; 16(2): 80. CrossRef - Hyperbaric Oxygen Therapy Dampens Inflammatory Cytokine Production and Does Not Worsen the Cardiac Function and Oxidative State of Diabetic Rats
Rita Benkő, Zsuzsanna Miklós, Viktor Antal Ágoston, Katrine Ihonvien, Csaba Répás, Roland Csépányi-Kömi, Margit Kerék, Nóra Judit Béres, Eszter Mária Horváth
Antioxidants.2019; 8(12): 607. CrossRef - Heart Failure in Type 2 Diabetes Mellitus
Helena C. Kenny, E. Dale Abel
Circulation Research.2019; 124(1): 121. CrossRef - SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice
Damilola D. Adingupu, Sven O. Göpel, Julia Grönros, Margareta Behrendt, Matus Sotak, Tasso Miliotis, Ulrika Dahlqvist, Li-Ming Gan, Ann-Cathrine Jönsson-Rylander
Cardiovascular Diabetology.2019;[Epub] CrossRef - Depressive symptoms in asymptomatic stage B heart failure with Type II diabetic mellitus
Paul J. Mills, Pam R. Taub, Ottar Lunde, Meredith A. Pung, Kathleen Wilson, Christopher Pruitt, Thomas Rutledge, Alan Maisel, Barry H. Greenberg
Clinical Cardiology.2019; 42(6): 637. CrossRef - Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate
Amir M. Al Hroob, Mohammad H. Abukhalil, Omnia E. Hussein, Ayman M. Mahmoud
Biomedicine & Pharmacotherapy.2019; 109: 2155. CrossRef - The Janus face of HMGB1 in heart disease: a necessary update
Angela Raucci, Stefania Di Maggio, Francesco Scavello, Alessandro D’Ambrosio, Marco E. Bianchi, Maurizio C. Capogrossi
Cellular and Molecular Life Sciences.2019; 76(2): 211. CrossRef - Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model
Sabri Sudirman, Ching-Shu Lai, Yi-Ling Yan, Hung-I Yeh, Zwe-Ling Kong
Scientific Reports.2019;[Epub] CrossRef - Microarray profiling analysis identifies the mechanism of miR‐200b‐3p/mRNA‐CD36 affecting diabetic cardiomyopathy via peroxisome proliferator activated receptor‐γ signaling pathway
Liqiong Xu, Wei Chen, Min Ma, Anfang Chen, Chengyue Tang, Chengwei Zhang, Lin Cai
Journal of Cellular Biochemistry.2019; 120(4): 5193. CrossRef - Plasma Low-Density Lipoprotein Cholesterol Correlates With Heart Function in Individuals With Type 2 Diabetes Mellitus: A Cross-Sectional Study
Po-Chung Cheng, Shang-Ren Hsu, Jung-Chi Li, Ching-Pei Chen, Szu-Chi Chien, Shih-Te Tu, Yun-Chung Cheng, Yu-Hsiu Liu, Jeng-Fu Kuo
Frontiers in Endocrinology.2019;[Epub] CrossRef - Impact of diabetes mellitus on the contractile properties of the left and right atrial myofilaments†
Constanze Bening, Khaled Alhussini, Elena-Aura Mazalu, Jonathan Yaqub, Khaled Hamouda, Dejan Radakovic, Christoph Schimmer, Grzegorz Hirnle, Rainer Leyh
European Journal of Cardio-Thoracic Surgery.2018; 54(5): 826. CrossRef - LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling
Lu Gao, Yuan Liu, Sen Guo, Lili Xiao, Leiming Wu, Zheng Wang, Cui Liang, Rui Yao, Yanzhou Zhang
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2018; 1864(10): 3322. CrossRef - Gene expression profiles of rat MMECs with different glucose levels and fgl2 gene silencing
Zhenzhong Zheng, Fan Zhang, Dengpeng Gao, Yujing Wu, Hao Wu
Diabetes/Metabolism Research and Reviews.2018;[Epub] CrossRef - Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes
Kwong-Man Ng, Yee-Man Lau, Vidhu Dhandhania, Zhu-Jun Cai, Yee-Ki Lee, Wing-Hon Lai, Hung-Fat Tse, Chung-Wah Siu
Scientific Reports.2018;[Epub] CrossRef - Apelin‑13 ameliorates metabolic and cardiovascular disorders in a rat model of type 2 diabetes with a high‑fat diet
Meng Li, Huijuan Fang, Jian Hu
Molecular Medicine Reports.2018;[Epub] CrossRef - Adriamycin-induced cardiomyopathy can serve as a model for diabetic cardiomyopathy – a hypothesis
Kaviyarasi Renu, V.G. Abilash, P.B. Tirupathi Pichiah, Thabassum Akthar Syeda, Sankarganesh Arunachalam
Asian Pacific Journal of Tropical Biomedicine.2017; 7(11): 1041. CrossRef
-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats

- Obesity and Metabolism
- Brain Regulation of Energy Metabolism
- Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2016;31(4):519-524. Published online December 20, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.4.519
- 9,868 View
- 182 Download
- 53 Web of Science
- 53 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader In healthy individuals, energy intake is in balance with energy expenditure, which helps to maintain a normal body weight. The brain's inability to control energy homeostasis underlies the pathology of hyperphagia and obesity. The brain detects body energy excess and deficit by sensing the levels of circulating metabolic hormones and nutrients and by receiving metabolic information from the periphery via the autonomic nervous system. A specialized neuronal network coordinates energy intake behavior and the metabolic processes affecting energy expenditure. Here, we briefly review neuronal mechanisms by which our body maintains energy balance.
-
Citations
Citations to this article as recorded by- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
Endocrinology and Metabolism.2024; 39(1): 1. CrossRef - Central inhibition of stearoyl-CoA desaturase has minimal effects on the peripheral metabolic symptoms of the 3xTg Alzheimer’s disease mouse model
Laura K. Hamilton, Paule E. H. M’Bra, Sophia Mailloux, Manon Galoppin, Anne Aumont, Karl J. L. Fernandes
Scientific Reports.2024;[Epub] CrossRef - Adipokines from white adipose tissue in regulation of whole body energy homeostasis
Bijayashree Sahu, Naresh C. Bal
Biochimie.2023; 204: 92. CrossRef - Growth hormone receptor (GHR) in AgRP neurons regulates thermogenesis in a sex-specific manner
Lukas Stilgenbauer, Juliana Bezerra Medeiros de Lima, Lucas Kniess Debarba, Manal Khan, Lisa Koshko, John J. Kopchick, Andrzej Bartke, Augusto Schneider, Marianna Sadagurski
GeroScience.2023; 45(3): 1745. CrossRef - Living high - training low model applied to C57BL/6J mice: Effects on physiological parameters related to aerobic fitness and acid-base balance
Pedro Paulo Menezes Scariot, Marcelo Papoti, Emanuel Elias Camolese Polisel, Juan Bordon Orsi, Paul R. Van Ginkel, Tomas A. Prolla, Fúlvia Barros Manchado-Gobatto, Claudio Alexandre Gobatto
Life Sciences.2023; 317: 121443. CrossRef - Whole Transcriptome Analysis of Hypothalamus in Mice during Short-Term Starvation
Eun-Young Oh, Byong Seo Park, Hye Rim Yang, Ho Gyun Lee, Thai Hien Tu, Sunggu Yang, Mi-Ryung Han, Jae Geun Kim
International Journal of Molecular Sciences.2023; 24(4): 3204. CrossRef - Hormonal Gut–Brain Signaling for the Treatment of Obesity
Eun Roh, Kyung Mook Choi
International Journal of Molecular Sciences.2023; 24(4): 3384. CrossRef - Neuronal Blockade of Thyroid Hormone Signaling Increases Sensitivity to Diet-Induced Obesity in Adult Male Mice
Eva Rial-Pensado, Laurence Canaple, Romain Guyot, Christoffer Clemmensen, Joëlle Wiersema, Shijia Wu, Sabine Richard, Anita Boelen, Timo D Müller, Miguel López, Frédéric Flamant, Karine Gauthier
Endocrinology.2023;[Epub] CrossRef - Genetic Contributors to Obesity
Ramya Sivasubramanian, Sonali Malhotra
Gastroenterology Clinics of North America.2023; 52(2): 323. CrossRef - Neurocomputational mechanisms of food and physical activity decision-making in male adolescents
Seung-Lark Lim, Amanda S. Bruce, Robin P. Shook
Scientific Reports.2023;[Epub] CrossRef - Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound
Yaoheng Yang, Jinyun Yuan, Rachael L. Field, Dezhuang Ye, Zhongtao Hu, Kevin Xu, Lu Xu, Yan Gong, Yimei Yue, Alexxai V. Kravitz, Michael R. Bruchas, Jianmin Cui, Jonathan R. Brestoff, Hong Chen
Nature Metabolism.2023; 5(5): 789. CrossRef - Changes in hypothalamic mu-opioid receptor expression following acute olanzapine treatment in female rats: Implications for feeding behavior
Maiken Krogsbaek, Nick Yao Larsen, Anne M. Landau, Connie Sanchez, Jens Randel Nyengaard
Journal of Chemical Neuroanatomy.2023; 132: 102324. CrossRef - Insulin Resistance and Glucose Metabolism during Infection
Borros Arneth
Endocrines.2023; 4(4): 685. CrossRef - The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis
Nikki Le, Sarah Sayers, Veronica Mata-Pacheco, Edward J. Wagner
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Link Between Energy-Related Sensations and Metabolism: Implications for Treating Fatigue
Marco Filippi, Rainer Krähenmann, Patrick Fissler
Frontiers in Psychology.2022;[Epub] CrossRef - Unaltered Tonic Inhibition in the Arcuate Nucleus of Diet-induced Obese Mice
Moonsun Sa, Jung Moo Lee, Mingu Gordon Park, Jiwoon Lim, Jong Min Joseph Kim, Wuhyun Koh, Bo-Eun Yoon, C. Justin Lee
Experimental Neurobiology.2022; 31(3): 147. CrossRef - Hypothalamus–Muscle Parallel Induction of Metabolic Pathways Following Physical Exercise
Almog Katz, Meital Gonen, Yael Shahar, Asael Roichman, Batia Lerrer, Haim Yosef Cohen
Frontiers in Neuroscience.2022;[Epub] CrossRef - Monocarboxylate transporters (MCTs) in skeletal muscle and hypothalamus of less or more physically active mice exposed to aerobic training
P.P.M. Scariot, F.B. Manchado-Gobatto, W.R. Beck, M. Papoti, P.R. Van Ginkel, C.A. Gobatto
Life Sciences.2022; 307: 120872. CrossRef - Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction
Ana S. Correia, Sara C. Pereira, Tiago Morais, Ana D. Martins, Mariana P. Monteiro, Marco G. Alves, Pedro F. Oliveira
Biomedicines.2022; 10(10): 2609. CrossRef - A pilot study of contrast-enhanced electrical impedance tomography for real-time imaging of cerebral perfusion
Yuyan Zhang, Jian’an Ye, Yang Jiao, Weirui Zhang, Tao Zhang, Xiang Tian, Xuetao Shi, Feng Fu, Liang Wang, Canhua Xu
Frontiers in Neuroscience.2022;[Epub] CrossRef - Repercussions of maternal exposure to high-fat diet on offspring feeding behavior and body composition: a systematic review
Wenicios Ferreira Chaves, Isabeli Lins Pinheiro, Jacqueline Maria da Silva, Raul Manhães-de-Castro, Raquel da Silva Aragão
Journal of Developmental Origins of Health and Disease.2021; 12(2): 220. CrossRef - Obesity-associated Pathways of Anthocyanins
Elif YILDIZ, Metin GULDAS, Pinar ELLERGEZEN, Asli Gul ACAR, Ozan GURBUZ
Food Science and Technology.2021; 41( suppl 1): 1. CrossRef - Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism
Ming-Liang Lee, Hirokazu Matsunaga, Yuki Sugiura, Takahiro Hayasaka, Izumi Yamamoto, Taiga Ishimoto, Daigo Imoto, Makoto Suematsu, Norifumi Iijima, Kazuhiro Kimura, Sabrina Diano, Chitoku Toda
Nature Communications.2021;[Epub] CrossRef - Sleep and Cardiovascular Risk
Lyudmila Korostovtseva, Mikhail Bochkarev, Yurii Sviryaev
Sleep Medicine Clinics.2021; 16(3): 485. CrossRef - Evaluation and Management of Early Onset Genetic Obesity in Childhood
Sonali Malhotra, Ramya Sivasubramanian, Gitanjali Srivastava
Journal of Pediatric Genetics.2021; 10(03): 194. CrossRef - Gene expression atlas of energy balance brain regions
Maria Caterina De Rosa, Hannah J. Glover, George Stratigopoulos, Charles A. LeDuc, Qi Su, Yufeng Shen, Mark W. Sleeman, Wendy K. Chung, Rudolph L. Leibel, Judith Y. Altarejos, Claudia A. Doege
JCI Insight.2021;[Epub] CrossRef - New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases
Magdalena Czerwińska, Katarzyna Czarzasta, Agnieszka Cudnoch-Jędrzejewska
Frontiers in Physiology.2021;[Epub] CrossRef - A putative role for lncRNAs in epigenetic regulation of memory
Ashleigh B. Irwin, Rudhab Bahabry, Farah D. Lubin
Neurochemistry International.2021; 150: 105184. CrossRef - Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis
Kirill V. Zhukov, Alexandre A. Vetcher, Bagrat A. Gasparuan, Alexander Y. Shishonin
Polymers.2021; 13(23): 4248. CrossRef - Placental NEGR1 DNA methylation is associated with BMI and neurodevelopment in preschool-age children
E Breton, V Gagné-Ouellet, K Thibeault, R Guérin, Rj Van Lieshout, P Perron, Mf Hivert, L Bouchard
Epigenetics.2020; 15(3): 323. CrossRef - The dorsomedial hypothalamus and nucleus of the solitary tract as key regulators in a rat model of chronic obesity
Chen Zhang, Pernille Barkholt, Jens Christian Nielsen, Ditte Dencker Thorbek, Kristoffer Rigbolt, Niels Vrang, David Paul Drucker Woldbye, Jacob Jelsing
Brain Research.2020; 1727: 146538. CrossRef - Hypothalamic NAD+-Sirtuin Axis: Function and Regulation
Eun Roh, Min-Seon Kim
Biomolecules.2020; 10(3): 396. CrossRef - The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain
Jaewoo Hong, Yurim Kim, Sudhirkumar Yanpallewar, P. Charles Lin
International Journal of Molecular Sciences.2020; 21(4): 1341. CrossRef - Sirtuin (SIRT)-1: At the crossroads of puberty and metabolism
Carlos F. Aylwin, Alejandro Lomniczi
Current Opinion in Endocrine and Metabolic Research.2020; 14: 65. CrossRef - Metabolomics Reveals the Alteration of Metabolic Pathway by Alpha-Melanocyte-Stimulating Hormone in B16F10 Melanoma Cells
Seung-Ho Seo, Jae Kwon Jo, Eun-Ju Kim, Seong-Eun Park, Seo Yeon Shin, Kyung Mok Park, Hong-Seok Son
Molecules.2020; 25(15): 3384. CrossRef - Noninvasive real-time detection of cerebral blood perfusion in hemorrhagic shock rabbits based on whole-brain magnetic induction phase shift: an experimental study
Wencai Pan, Wei Zhuang, Yinbao Chong, Mingxin Qin, Yang Li, Jingjing Xiao, Qing Wang, Shihui Zhang, Shuanglin Zhao, Peng Zhao
Physiological Measurement.2020; 41(9): 095004. CrossRef - Neurochemical regulators of food behavior for pharmacological treatment of obesity: current status and future prospects
Gayane Sargis Vardanyan, Hasmik Samvel Harutyunyan, Michail Iosif Aghajanov, Ruben Sargis Vardanyan
Future Medicinal Chemistry.2020; 12(20): 1865. CrossRef - The Co-occurrence of Pediatric Obesity and ADHD: an Understanding of Shared Pathophysiology and Implications for Collaborative Management
Valerie M. O’Hara, Jennifer L. Curran, Nancy T. Browne
Current Obesity Reports.2020; 9(4): 451. CrossRef - Constitutive Androstane Receptor: A Peripheral and a Neurovascular Stress or Environmental Sensor
Fabiana Oliviero, Céline Lukowicz, Badreddine Boussadia, Isabel Forner-Piquer, Jean-Marc Pascussi, Nicola Marchi, Laila Mselli-Lakhal
Cells.2020; 9(11): 2426. CrossRef - Automated diffusion-based parcellation of the hypothalamus reveals subunit-specific associations with obesity
Melanie Spindler, Jale Özyurt, Christiane M. Thiel
Scientific Reports.2020;[Epub] CrossRef - SIRT1 in Astrocytes Regulates Glucose Metabolism and Reproductive Function
Irene Choi, Emily Rickert, Marina Fernandez, Nicholas J G Webster
Endocrinology.2019; 160(6): 1547. CrossRef - Hypothalamic mechanisms associated with corticotropin-releasing factor-induced anorexia in chicks
Jinxin Wang, Justin Matias, Elizabeth R. Gilbert, Tetsuya Tachibana, Mark A. Cline
Neuropeptides.2019; 74: 95. CrossRef - HMG-CoA synthase 2 drives brain metabolic reprogramming in cocaine exposure
Xue Shao, Yunxuan Tang, Hailei Long, Hui Gu, Jie Zhang, Pengchi Deng, Yinglan Zhao, Xiaobo Cen
Neuropharmacology.2019; 148: 377. CrossRef - The Effect of Feeding Behavior on Hypothalamus in Obese Type 2 Diabetic Rats with Glucagon-like Peptide-1 Receptor Agonist Intervention
Ke Lu, Xiaoyan Chen, Jianhua Yan, Xinchun Li, Chen Huang, Qi Wan, Xuelian Deng, Qiao Zou
Obesity Facts.2018; 11(3): 181. CrossRef - The Long-Term Impact of High Levels of Alpha-Melanocyte-Stimulating Hormone in Energy Balance Among Obese Adolescents
Ana Claudia Pelissari Kravchychyn, Raquel Munhoz da Silveira Campos, Flávia Campos Corgosinho, Deborah Cristina Landi Masquio, Sofia Emanuelle de Castro Ferreira Vicente, Yasmin Alaby Martins Ferreira, Patrícia Leão Silva, Aline de Piano Ganen, Lila Missa
Annals of Nutrition and Metabolism.2018; 72(4): 279. CrossRef - Psychopharmacological advances in eating disorders
Hubertus Himmerich, Janet Treasure
Expert Review of Clinical Pharmacology.2018; 11(1): 95. CrossRef - Food engineering into the XXI century
José Miguel Aguilera
AIChE Journal.2018; 64(1): 2. CrossRef - The Role of Glucagon-Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP-1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain
Choon Bae, Juhyun Song
International Journal of Molecular Sciences.2017; 18(11): 2493. CrossRef - “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression
Juan M. Lima-Ojeda, Rainer Rupprecht, Thomas C. Baghai
Frontiers in Psychiatry.2017;[Epub] CrossRef - Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity
Katharina Timper, Jens C. Brüning
Disease Models & Mechanisms.2017; 10(6): 679. CrossRef - Brain glucose metabolism: Role of Wnt signaling in the metabolic impairment in Alzheimer’s disease
Pedro Cisternas, Nibaldo C. Inestrosa
Neuroscience & Biobehavioral Reviews.2017; 80: 316. CrossRef - Astrocyte-Specific Deletion of Peroxisome-Proliferator Activated Receptor-γ Impairs Glucose Metabolism and Estrous Cycling in Female Mice
Marina O Fernandez, Katherine Hsueh, Hyun Tae Park, Consuelo Sauceda, Vicky Hwang, Deepak Kumar, Sun Kim, Emily Rickert, Sumana Mahata, Nicholas J G Webster
Journal of the Endocrine Society.2017; 1(11): 1332. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator

- Thyroid
- Mitochondrial Energy Metabolism and Thyroid Cancers
- Junguee Lee, Joon Young Chang, Yea Eun Kang, Shinae Yi, Min Hee Lee, Kyong Hye Joung, Kun Soon Kim, Minho Shong
- Endocrinol Metab. 2015;30(2):117-123. Published online June 30, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.2.117
- 4,317 View
- 50 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Primary thyroid cancers including papillary, follicular, poorly differentiated, and anaplastic carcinomas show substantial differences in biological and clinical behaviors. Even in the same pathological type, there is wide variability in the clinical course of disease progression. The molecular carcinogenesis of thyroid cancer has advanced tremendously in the last decade. However, specific inhibition of oncogenic pathways did not provide a significant survival benefit in advanced progressive thyroid cancer that is resistant to radioactive iodine therapy. Accumulating evidence clearly shows that cellular energy metabolism, which is controlled by oncogenes and other tumor-related factors, is a critical factor determining the clinical phenotypes of cancer. However, the role and nature of energy metabolism in thyroid cancer remain unclear. In this article, we discuss the role of cellular energy metabolism, particularly mitochondrial energy metabolism, in thyroid cancer. Determining the molecular nature of metabolic remodeling in thyroid cancer may provide new biomarkers and therapeutic targets that may be useful in the management of refractory thyroid cancers.
-
Citations
Citations to this article as recorded by- Exploring the clinical utility of DPP-IV and SGLT2 inhibitors in papillary thyroid cancer: a literature review
Angelika Buczyńska, Maria Kościuszko, Adam Jacek Krętowski, Anna Popławska-Kita
Frontiers in Pharmacology.2024;[Epub] CrossRef - Liquid Biopsy as a Method for Minimally Invasive Diagnosis of Thyroid Cancer
Tagir I. Rakhmatullin, Mark Jain, Larisa M. Samokhodskaya, Vladimir A. Zhivotov
Journal of Clinical Practice.2023; 14(3): 69. CrossRef - Development of Metabolic Synthetic Lethality and Its Implications for Thyroid Cancer
Sang-Hyeon Ju, Seong Eun Lee, Yea Eun Kang, Minho Shong
Endocrinology and Metabolism.2022; 37(1): 53. CrossRef - Monensin Inhibits Anaplastic Thyroid Cancer via Disrupting Mitochondrial Respiration
and AMPK/mTOR Signaling
Yanli Li, Qianshu Sun, Sisi Chen, Xiongjie Yu, Hongxia Jing
Anti-Cancer Agents in Medicinal Chemistry.2022; 22(14): 2539. CrossRef - Growth Differentiation Factor 15 is a Cancer Cell-Induced Mitokine That Primes Thyroid Cancer Cells for Invasiveness
Yea Eun Kang, Jin Man Kim, Mi Ae Lim, Seong Eun Lee, Shinae Yi, Jung Tae Kim, Chan Oh, Lihua Liu, Yanli Jin, Seung-Nam Jung, Ho-Ryun Won, Jae Won Chang, Jeong Ho Lee, Hyun Jung Kim, Hyun Yong Koh, Sangmi Jun, Sun Wook Cho, Minho Shong, Bon Seok Koo
Thyroid.2021; 31(5): 772. CrossRef - Clinical Significance of the D-Loop Gene Mutation in Mitochondrial DNA in Laryngeal Cancer
Lei Wang, He-Xiang Cheng, Yan-Hui Zhou, Min Ma
OncoTargets and Therapy.2021; Volume 14: 3461. CrossRef - Transcriptomic and Genetic Associations between Alzheimer’s Disease, Parkinson’s Disease, and Cancer
Jaume Forés-Martos, Cesar Boullosa, David Rodrigo-Domínguez, Jon Sánchez-Valle, Beatriz Suay-García, Joan Climent, Antonio Falcó, Alfonso Valencia, Joan Anton Puig-Butillé, Susana Puig, Rafael Tabarés-Seisdedos
Cancers.2021; 13(12): 2990. CrossRef - KLF5 influences cell biological function and chemotherapy sensitivity through the JNK signaling pathway in anaplastic thyroid carcinoma
Zheng Wang, Xinguang Qiu, Hao Zhang, Weihan Li
Journal of Biochemical and Molecular Toxicology.2020;[Epub] CrossRef - Metabolic reprogramming related to whole-chromosome instability in models for Hürthle cell carcinoma
Ruben D. Addie, Sarantos Kostidis, Willem E. Corver, Jan Oosting, Sepideh Aminzadeh-Gohari, René G. Feichtinger, Barbara Kofler, Mehtap Derya Aydemirli, Martin Giera, Hans Morreau
Scientific Reports.2020;[Epub] CrossRef - Inhibition of mitochondrial respiration by tigecycline selectively targets thyroid carcinoma and increases chemosensitivity
Yuehua Wang, Fei Xie, Dejie Chen, Ling Wang
Clinical and Experimental Pharmacology and Physiology.2019; 46(10): 890. CrossRef - Investigating Therapeutic Effects of Retinoic Acid on Thyroid Cancer via Protein-Protein Interaction Network Analysis
Majid Rezaei-Tavirani, Mostafa Rezaei-Tavirani, Mona Zamanian Azodi
International Journal of Cancer Management.2019;[Epub] CrossRef - CASE REPORT: An Extensively Necrotic Hürthle-Cell Carcinoma Mimicked a Thyroid Abscess
Sanders H. Lin, Shih-Ming Huang, Su-Lin Peng
Clinical Thyroidology.2018; 30(11): 529. CrossRef - Atovaquone enhances doxorubicin’s efficacy via inhibiting mitochondrial respiration and STAT3 in aggressive thyroid cancer
Zhuo Lv, Xintong Yan, Liying Lu, Chun Su, Yin He
Journal of Bioenergetics and Biomembranes.2018; 50(4): 263. CrossRef - Identification of novel biomarker and therapeutic target candidates for diagnosis and treatment of follicular carcinoma
Xianyin Lai, Christopher B. Umbricht, Kurt Fisher, Justin Bishop, Qiuying Shi, Shaoxiong Chen
Journal of Proteomics.2017; 166: 59. CrossRef - Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer
Marika H Tesselaar, Johannes W Smit, James Nagarajah, Romana T Netea-Maier, Theo S Plantinga
Journal of Molecular Endocrinology.2017; 59(4): R141. CrossRef - Integrated microRNA, gene expression and transcription factors signature in papillary thyroid cancer with lymph node metastasis
Nurul-Syakima Ab Mutalib, Sri Noraima Othman, Azliana Mohamad Yusof, Shahrun Niza Abdullah Suhaimi, Rohaizak Muhammad, Rahman Jamal
PeerJ.2016; 4: e2119. CrossRef
- Exploring the clinical utility of DPP-IV and SGLT2 inhibitors in papillary thyroid cancer: a literature review


 KES
KES

 First
First Prev
Prev



