Search
- Page Path
- HOME > Search
- Calcium & Bone Metabolism
- Updates on Paget’s Disease of Bone
- Yong Jun Choi, Young Bae Sohn, Yoon-Sok Chung
- Endocrinol Metab. 2022;37(5):732-743. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1575
- 3,779 View
- 317 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Paget’s disease of the bone is a prevalent bone disease characterized by disorganized bone remodeling; however, it is comparatively uncommon in East Asian countries, including China, Japan, and Korea. The exact cause still remains unknown. In genetically susceptible individuals, environmental triggers such as paramyxoviral infections are likely to cause the disease. Increased osteoclast activity results in increased bone resorption, which attracts osteoblasts and generates new bone matrix. Fast bone resorption and formation lead to the development of disorganized bone tissue. Increasing serum alkaline phosphatase or unique radiographic lesions may serve as the diagnostic indicators. Common symptoms include bone pain, bowing of the long bones, an enlarged skull, and hearing loss. The diagnosis is frequently confirmed by radiographic and nuclear scintigraphy of the bone. Further, bisphosphonates such as zoledronic acid and pamidronate are effective for its treatment. Moreover, biochemical monitoring is superior to the symptoms as a recurrence indicator. This article discusses the updates of Paget’s disease of bone with a clinical case.
-
Citations
Citations to this article as recorded by- Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats
Radina Vasileva, Tsvetan Chaprazov, Aneliya Milanova
Journal of Functional Biomaterials.2024; 15(4): 106. CrossRef - Newly Diagnosed Monostotic Paget’s Disease of Bone during Living Kidney Donor Candidate Evaluation
Diana Jędrzejuk, Paweł Poznański, Paweł Szewczyk, Oktawia Mazanowska, Marek Bolanowski, Magdalena Krajewska, Dorota Kamińska
Biomedicines.2023; 11(2): 401. CrossRef - Paget's disease of bone in the patient presented with a bowed leg
Mehrzad Hajialiloo, Sepideh Tahsini Tekantapeh
Clinical Case Reports.2023;[Epub] CrossRef
- Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats

- Thyroid
- Thyroid Function across the Lifespan: Do Age-Related Changes Matter?
- John P. Walsh
- Endocrinol Metab. 2022;37(2):208-219. Published online April 14, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1463
- 6,013 View
- 338 Download
- 12 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
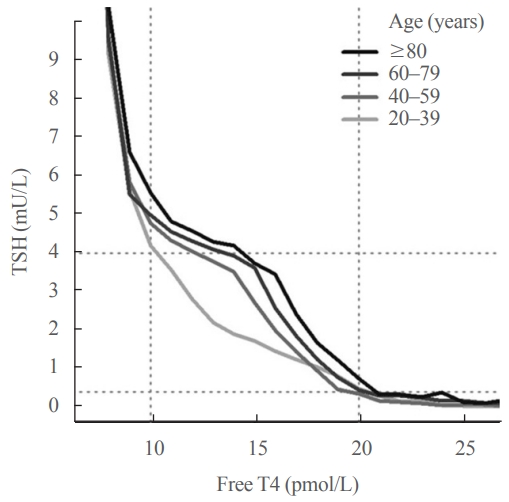
ePub - Circulating concentrations of thyrotropin (TSH) and thyroxine (T4) are tightly regulated. Each individual has setpoints for TSH and free T4 which are genetically determined, and subject to environmental and epigenetic influence. Pituitary-thyroid axis setpoints are probably established in utero, with maturation of thyroid function continuing until late gestation. From neonatal life (characterized by a surge of TSH and T4 secretion) through childhood and adolescence (when free triiodothyronine levels are higher than in adults), thyroid function tests display complex, dynamic patterns which are sexually dimorphic. In later life, TSH increases with age in healthy older adults without an accompanying fall in free T4, indicating alteration in TSH setpoint. In view of this, and evidence that mild subclinical hypothyroidism in older people has no health impact, a strong case can be made for implementation of age-related TSH reference ranges in adults, as is routine in children.
-
Citations
Citations to this article as recorded by- The ageing thyroid: implications for longevity and patient care
Diana van Heemst
Nature Reviews Endocrinology.2024; 20(1): 5. CrossRef - Incidence and Determinants of Spontaneous Normalization of Subclinical Hypothyroidism in Older Adults
Evie van der Spoel, Nicolien A van Vliet, Rosalinde K E Poortvliet, Robert S Du Puy, Wendy P J den Elzen, Terence J Quinn, David J Stott, Naveed Sattar, Patricia M Kearney, Manuel R Blum, Heba Alwan, Nicolas Rodondi, Tinh-Hai Collet, Rudi G J Westendorp,
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1167. CrossRef - Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
Rosalie B. T. M. Sterenborg, Inga Steinbrenner, Yong Li, Melissa N. Bujnis, Tatsuhiko Naito, Eirini Marouli, Tessel E. Galesloot, Oladapo Babajide, Laura Andreasen, Arne Astrup, Bjørn Olav Åsvold, Stefania Bandinelli, Marian Beekman, John P. Beilby, Jette
Nature Communications.2024;[Epub] CrossRef - Evaluation of multiple organophosphate insecticide exposure in relation to altered thyroid hormones in NHANES 2007‐2008 adult population
Massira Ousseni Diawara, Songtao Li, Mingzhi Zhang, Francis Manyori Bigambo, Xu Yang, Xu Wang, Tianyu Dong, Di Wu, Chenghao Yan, Yankai Xia
Ecotoxicology and Environmental Safety.2024; 273: 116139. CrossRef - Thyroid-function reference ranges in the diagnosis of thyroid dysfunction in adults
Salman Razvi
Nature Reviews Endocrinology.2024; 20(5): 253. CrossRef - Association between exposure to chemical mixtures and epigenetic ageing biomarkers: Modifying effects of thyroid hormones and physical activity
Wanying Shi, Jianlong Fang, Huimin Ren, Peijie Sun, Juan Liu, Fuchang Deng, Shuyi Zhang, Qiong Wang, Jiaonan Wang, Shilu Tong, Song Tang, Xiaoming Shi
Journal of Hazardous Materials.2024; 469: 134009. CrossRef - DNA Methylation in Autoimmune Thyroid Disease
Nicole Lafontaine, Scott G Wilson, John P Walsh
The Journal of Clinical Endocrinology & Metabolism.2023; 108(3): 604. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - Serum Lipidomic Analysis Reveals Biomarkers and Metabolic Pathways of Thyroid Dysfunction
Hua Dong, Wenjie Zhou, Xingxu Yan, Huan Zhao, Honggang Zhao, Yan Jiao, Guijiang Sun, Yubo Li, Zuncheng Zhang
ACS Omega.2023; 8(11): 10355. CrossRef - Developmental and environmental modulation of fecal thyroid hormone levels in wild Assamese macaques (Macaca assamensis)
Verena Behringer, Michael Heistermann, Suchinda Malaivijitnond, Oliver Schülke, Julia Ostner
American Journal of Primatology.2023;[Epub] CrossRef - Prevalence of Functional Alterations and the Effects of Thyroid
Autoimmunity on the Levels of TSH in an Urban Population of Colombia:
A Population-Based Study
Hernando Vargas-Uricoechea, Valentina Agredo-Delgado, Hernando David Vargas-Sierra, María V. Pinzón-Fernández
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(6): 857. CrossRef - Genetic determinants of thyroid function in children
Tessa A Mulder, Purdey J Campbell, Peter N Taylor, Robin P Peeters, Scott G Wilson, Marco Medici, Colin Dayan, Vincent V W Jaddoe, John P Walsh, Nicholas G Martin, Henning Tiemeier, Tim I M Korevaar
European Journal of Endocrinology.2023; 189(2): 164. CrossRef - Relationship between Thyroid CT Density, Volume, and Future TSH Elevation: A 5-Year Follow-Up Study
Tomohiro Kikuchi, Shouhei Hanaoka, Takahiro Nakao, Yukihiro Nomura, Takeharu Yoshikawa, Md Ashraful Alam, Harushi Mori, Naoto Hayashi
Life.2023; 13(12): 2303. CrossRef - Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status
Katleen Van Uytfanghe, Joel Ehrenkranz, David Halsall, Kelly Hoff, Tze Ping Loh, Carole A. Spencer, Josef Köhrle
Thyroid®.2023; 33(9): 1013. CrossRef - Blood hormones and suicidal behaviour: A systematic review and meta-analysis
Xue-Lei Fu, Xia Li, Jia-Mei Ji, Hua Wu, Hong-Lin Chen
Neuroscience & Biobehavioral Reviews.2022; 139: 104725. CrossRef
- The ageing thyroid: implications for longevity and patient care

- Diabetes
- Pathophysiology of Type 2 Diabetes in Koreans
- Soo Heon Kwak, Kyong Soo Park
- Endocrinol Metab. 2018;33(1):9-16. Published online March 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.9
- 5,425 View
- 111 Download
- 12 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The pathophysiology of type 2 diabetes is characterized by variable degrees of insulin resistance and impaired insulin secretion. Both genetic and environmental factors serve as etiologic factors. Recent genetic studies have identified at least 83 variants associated with diabetes. A significant number of these loci are thought to be involved in insulin secretion, either through β-cell development or β-cell dysfunction. Environmental factors have changed rapidly during the past half century, and the increased prevalence of obesity and diabetes can be attributed to these changes. Environmental factors may affect epigenetic changes and alter susceptibility to diabetes. A recent epidemiologic study revealed that Korean patients with type 2 diabetes already had impaired insulin secretion and insulin resistance 10 years before the onset of diabetes. Those who developed diabetes showed impaired β-cell compensation with an abrupt decrease in insulin secretion during the last 2 years before diabetes developed. The retrograde trajectory of the disposition index differed according to the baseline subgroups of insulin secretion and insulin sensitivity. We hope that obtaining a more detailed understanding of the perturbations in the major pathophysiologic process of diabetes on the individual level will eventually lead to the implementation of precision medicine and improved patient outcomes.
-
Citations
Citations to this article as recorded by- Stress-Reducing Psychological Interventions as Adjuvant Therapies for
Diabetic Chronic Wounds
Isadora Pombeiro, João Moura, M. Graça Pereira, Eugénia Carvalho
Current Diabetes Reviews.2022;[Epub] CrossRef - Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
Diabetes & Metabolism Journal.2021; 45(2): 260. CrossRef - Dose-Dependent Effect of Smoking on Risk of Diabetes Remains after Smoking Cessation: A Nationwide Population-Based Cohort Study in Korea
Se Eun Park, Mi Hae Seo, Jung-Hwan Cho, Hyemi Kwon, Yang-Hyun Kim, Kyung-Do Han, Jin-Hyung Jung, Yong-Gyu Park, Eun-Jung Rhee, Won-Young Lee
Diabetes & Metabolism Journal.2021; 45(4): 539. CrossRef - DNA Methylation Changes Associated With Type 2 Diabetes and Diabetic Kidney Disease in an East Asian Population
Hakyung Kim, Jae Hyun Bae, Kyong Soo Park, Joohon Sung, Soo Heon Kwak
The Journal of Clinical Endocrinology & Metabolism.2021; 106(10): e3837. CrossRef - Associations among Obesity Degree, Glycemic Status, and Risk of Heart Failure in 9,720,220 Korean Adults
Eun-Jung Rhee, Hyemi Kwon, Se Eun Park, Kyung-Do Han, Yong-Gyu Park, Yang-Hyun Kim, Won-Young Lee
Diabetes & Metabolism Journal.2020; 44(4): 592. CrossRef - Smoking as a Target for Prevention of Diabetes
Ye Seul Yang, Tae Seo Sohn
Diabetes & Metabolism Journal.2020; 44(3): 402. CrossRef - Clinical characteristics of diabetic ketoacidosis in users and non-users of SGLT2 inhibitors
J.Y. Jeon, S.-K. Kim, K.-S. Kim, S.O. Song, J.-S. Yun, B.-Y. Kim, C.-H. Kim, S.O. Park, S. Hong, D.H. Seo, J.A. Seo, J.H. Noh, D.J. Kim
Diabetes & Metabolism.2019; 45(5): 453. CrossRef - Identifying Pathogenic Variants of Monogenic Diabetes Using Targeted Panel Sequencing in an East Asian Population
Seung Shin Park, Se Song Jang, Chang Ho Ahn, Jung Hee Kim, Hye Seung Jung, Young Min Cho, Young Ah Lee, Choong Ho Shin, Jong Hee Chae, Jae Hyun Kim, Sung Hee Choi, Hak C Jang, Jee Cheol Bae, Jong Cheol Won, Sung-Hoon Kim, Jong-Il Kim, Soo Heon Kwak, Kyong
The Journal of Clinical Endocrinology & Metabolism.2019; 104(9): 4188. CrossRef - Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease
Daniela Stols-Gonçalves, Luca Schiliró Tristão, Peter Henneman, Max Nieuwdorp
Current Diabetes Reports.2019;[Epub] CrossRef - The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES)
Bongyoung Kim, Hyun Young Choi, Wonhee Kim, Chiwon Ahn, Juncheol Lee, Jae Guk Kim, Jihoon Kim, Hyungoo Shin, Jae Myung Yu, Shinje Moon, Taulant Muka
PLOS ONE.2018; 13(11): e0206994. CrossRef
- Stress-Reducing Psychological Interventions as Adjuvant Therapies for
Diabetic Chronic Wounds

- Update on Familial Hypercholesterolemia: Diagnosis, Cardiovascular Risk, and Novel Therapeutics
- Sang-Hak Lee
- Endocrinol Metab. 2017;32(1):36-40. Published online January 19, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.36
- 4,034 View
- 57 Download
- 11 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader In recent studies, the reported prevalence of heterozygous familial hypercholesterolemia (FH) has been higher than in previous reports. Although cascade genetic screening is a good option for efficient identification of affected patients, diagnosis using only clinical criteria is more common in real clinical practice. Cardiovascular risk is much higher in FH patients due to longstanding low density lipoprotein cholesterol (LDL-C) burden and is also influenced by other risk factors. Although guidelines emphasize aggressive LDL-C reduction, the majority of patients cannot reach the LDL-C goal by conventional pharmacotherapy. Novel therapeutics such as proprotein convertase subtilisin/kexin type 9 inhibitors have shown strong lipid lowering efficacy and are expected to improve treatment results in FH patients.
-
Citations
Citations to this article as recorded by- Effectiveness and Safety of a Fixed-Dose Combination of Valsartan and Rosuvastatin (Rovatitan® Tablet) in Patients with Concomitant Hypertension and Hyperlipidemia: An Observational Study
Kwang Je Lee, Jae-Kean Ryu, Yun-Hyeong Cho, Won Yong Shin, Jeong Su Kim, Young Won Yoon, Ji Yong Jang, Won Ho Kim, Jong Wook Beom, Seok-Min Kang
Drug Design, Development and Therapy.2023; Volume 17: 1047. CrossRef - Role of PCSK9 Inhibitors in Patients with Familial Hypercholesterolemia
Brian Tomlinson, Nivritti Gajanan Patil, Manson Fok, Christopher Wai Kei Lam
Endocrinology and Metabolism.2021; 36(2): 279. CrossRef - Identification and Functional Characterization of a Low-Density Lipoprotein Receptor Gene Pathogenic Variant in Familial Hypercholesterolemia
Hong-Yan Shu, Wei Zhang, Cong-Cong Zheng, Man-Yun Gao, Yong-Cun Li, Yan-Gang Wang
Frontiers in Genetics.2021;[Epub] CrossRef - Gut Microbiota and Complications of Type-2 Diabetes
Camelia Oana Iatcu, Aimee Steen, Mihai Covasa
Nutrients.2021; 14(1): 166. CrossRef - LDLR Gene Mutation p.Asp360His and Familial Hypercholesterolemia in a Mexican Community
Teresita De Jesús Hernández Flores, Juan Ramón González García, Yoaly Josefina Sánchez López, Norma Alejandra Vázquez Cárdenas, Ana Gabriela Colima Fausto, Sergio Yair Rodríguez Preciado, María Teresa Magaña Torres
Archives of Medical Research.2020; 51(2): 153. CrossRef - A Rare Double Heterozygous Mutation in Low-Density Lipoprotein Receptor and Apolipoprotein B-100 Genes in a Severely Affected Familial Hypercholesterolaemia Patient
Lilla Juhász, István Balogh, László Madar, Beáta Kovács, Mariann Harangi
Cureus.2020;[Epub] CrossRef - Efficacy and Safety of a Fixed-Dose Combination of Candesartan and Rosuvastatin on Blood Pressure and Cholesterol in Patients With Hypertension and Hypercholesterolemia: A Multicenter, Randomized, Double-Blind, Parallel Phase III Clinical Study
Kyoung Im Cho, Bo Hyun Kim, Yong Hyun Park, Jeong-Cheon Ahn, Sang Hyun Kim, Wook Jin Chung, Weon Kim, Il Suk Sohn, Jin Ho Shin, Yong Jin Kim, Kiyuk Chang, Cheol Woong Yu, Soe Hee Ahn, Seok Yeon Kim, Jae Kean Ryu, Jong Young Lee, Bum Kee Hong, Taek Jong Ho
Clinical Therapeutics.2019; 41(8): 1508. CrossRef - Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia
Mary P. McGowan, Seyed Hamed Hosseini Dehkordi, Patrick M. Moriarty, P. Barton Duell
Journal of the American Heart Association.2019;[Epub] CrossRef - Autosomal recessive hypercholesterolemia: Case report
Zaneta Petrulioniene, Urte Gargalskaite, Violeta Mikstiene, Rimvydas Norvilas, Egle Skiauteryte, Algirdas Utkus
Journal of Clinical Lipidology.2019; 13(6): 887. CrossRef -
Q192R polymorphism in the
PON1
gene and familial hypercholesterolemia in a Saudi population
Khalid Khalaf Alharbi, May Salem Alnbaheen, Fawiziah Khalaf Alharbi, Rana M. Hasanato, Imran Ali Khan
Annals of Saudi Medicine.2017; 37(6): 425. CrossRef
- Effectiveness and Safety of a Fixed-Dose Combination of Valsartan and Rosuvastatin (Rovatitan® Tablet) in Patients with Concomitant Hypertension and Hyperlipidemia: An Observational Study

- Obesity and Metabolism
- Genetic Studies on Diabetic Microvascular Complications: Focusing on Genome-Wide Association Studies
- Soo Heon Kwak, Kyong Soo Park
- Endocrinol Metab. 2015;30(2):147-158. Published online June 30, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.2.147
- 4,258 View
- 39 Download
- 15 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetes is a common metabolic disorder with a worldwide prevalence of 8.3% and is the leading cause of visual loss, end-stage renal disease and amputation. Recently, genome-wide association studies (GWASs) have identified genetic risk factors for diabetic microvascular complications of retinopathy, nephropathy, and neuropathy. We summarized the recent findings of GWASs on diabetic microvascular complications and highlighted the challenges and our opinion on future directives. Five GWASs were conducted on diabetic retinopathy, nine on nephropathy, and one on neuropathic pain. The majority of recent GWASs were underpowered and heterogeneous in terms of study design, inclusion criteria and phenotype definition. Therefore, few reached the genome-wide significance threshold and the findings were inconsistent across the studies. Recent GWASs provided novel information on genetic risk factors and the possible pathophysiology of diabetic microvascular complications. However, further collaborative efforts to standardize phenotype definition and increase sample size are necessary for successful genetic studies on diabetic microvascular complications.
-
Citations
Citations to this article as recorded by- Genetics of diabetes
Shiwali Goyal, Jyoti Rani, Mohd Akbar Bhat, Vanita Vanita
World Journal of Diabetes.2023; 14(6): 656. CrossRef - Plasma thrombin-activatable fibrinolysis inhibitor and the 1040C/T polymorphism are risk factors for diabetic kidney disease in Chinese patients with type 2 diabetes
Qinghua Huang, Dujin Feng, Lianlian Pan, Huan Wang, Yan Wu, Bin Zhong, Jianguang Gong, Huijun Lin, Xianming Fei
PeerJ.2023; 11: e16352. CrossRef - The G Allele of the rs12050217 Polymorphism in the BDKRB1 Gene Is Associated with Protection for Diabetic Retinopathy
Leticia A. Brondani, Daisy Crispim, Julia Pisco, Jorge A. Guimarães, Markus Berger
Current Eye Research.2019; 44(9): 994. CrossRef - Genome‐wide association study identifies new susceptibility loci for diabetic nephropathy in Korean patients with type 2 diabetes mellitus
Kyung H. Jeong, Jin S. Kim, Jeong‐Taek Woo, Sang Y. Rhee, Yu H. Lee, Yang G. Kim, Ju‐Young Moon, Su K. Kim, Sun W. Kang, Sang H. Lee, Yeong H. Kim
Clinical Genetics.2019; 96(1): 35. CrossRef - Diabetic polyneuropathy, deep white matter lesions, and carotid atherosclerosis: is there any association?
Sevgi Ferik, Hayat Güven, Mehlika Panpallı Ateş, Işık Conkbayır, Selçuk Çomoğlu, Bülent Güven
Neurological Sciences.2018; 39(1): 103. CrossRef - Altered expression of WFS1 and NOTCH2 genes associated with diabetic nephropathy in T2DM patients
Sahar A. Sharaf, Nagwa A. Kantoush, Dina F. Ayoub, Alshaymaa A. Ibrahim, Amaal A. Abdelaal, Rokaya Abdel Aziz, Mahmoud M. ElHefnawi, Amira N. Ahmed
Diabetes Research and Clinical Practice.2018; 140: 304. CrossRef - Precision medicine in diabetes prevention, classification and management
Fangying Xie, Juliana CN Chan, Ronald CW Ma
Journal of Diabetes Investigation.2018; 9(5): 998. CrossRef - Clinical worthlessness of genetic prediction of common forms of diabetes mellitus and related chronic complications
R. Buzzetti, S. Prudente, M. Copetti, M. Dauriz, S. Zampetti, M. Garofolo, G. Penno, V. Trischitta
Nutrition, Metabolism and Cardiovascular Diseases.2017; 27(2): 99. CrossRef - Diabetic macular oedema: clinical risk factors and emerging genetic influences
Ebony Liu, Jamie E Craig, Kathryn Burdon
Clinical and Experimental Optometry.2017; 100(6): 569. CrossRef - Normoalbuminuric diabetic kidney disease
Chao Chen, Chang Wang, Chun Hu, Yachun Han, Li Zhao, Xuejing Zhu, Li Xiao, Lin Sun
Frontiers of Medicine.2017; 11(3): 310. CrossRef - Biomarkers of Diabetic Retinopathy
Daniel Shu Wei Ting, Kara-Anne Tan, Val Phua, Gavin Siew Wei Tan, Chee Wai Wong, Tien Yin Wong
Current Diabetes Reports.2016;[Epub] CrossRef - Association Between Heme Oxygenase-1 Promoter Polymorphisms and the Development of Albuminuria in Type 2 Diabetes
Eun Young Lee, Yong-ho Lee, Soo Hyun Kim, Kyu Sik Jung, Obin Kwon, Beom Seok Kim, Chung Mo Nam, Chun Sik Park, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee
Medicine.2015; 94(43): e1825. CrossRef
- Genetics of diabetes

- Reproduction and Metabolism: Insights from Polycystic Ovary Syndrome.
- Prathima Jasti, Andrea Dunaif
- Endocrinol Metab. 2012;27(3):180-190. Published online September 19, 2012
- DOI: https://doi.org/10.3803/EnM.2012.27.3.180
- 2,048 View
- 29 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Until the 1980s, polycystic ovary syndrome (PCOS) was considered to be a poorly defined reproductive disorder. During that decade, it was recognized that PCOS was associated with profound insulin resistance and a substantially increased risk for type 2 diabetes mellitus in young women. Accordingly, the mechanisms linking the reproductive and metabolic features of the syndrome became the subject of intense investigation. Insulin is now recognized as a reproductive as well as a metabolic hormone and insulin signaling in the central nervous system participates in normal reproductive function. These insights have been directly translated into a novel therapy for PCOS with insulin sensitizing drugs. Androgens also have reversible metabolic actions to decrease insulin sensitivity and increase visceral fat. Prenatal androgen administration to non-human primates, sheep and rodents produces reproductive and metabolic features of PCOS suggesting that the disorder also has developmental origins. PCOS is highly heritable and male as well as female relatives have reproductive and metabolic phenotypes. A number of confirmed genetic susceptibility loci have now been mapped for PCOS and genes in well-known as well as novel biologic pathways have been implicated in disease pathogenesis.
-
Citations
Citations to this article as recorded by- The Role of Foxo3 in Leydig Cells
Young Suk Choi, Joo Eun Song, Byung Soo Kong, Jae Won Hong, Silvia Novelli, Eun Jig Lee
Yonsei Medical Journal.2015; 56(6): 1590. CrossRef - FoxO1 Is a Negative Regulator of FSHβ Gene Expression in Basal and GnRH-Stimulated Conditions in Female
Young-Suk Choi, Hyeon Jeong Lee, Cheol Ryong Ku, Yoon Hee Cho, Mi Ran Seo, Yoo Jeoung Lee, Eun Jig Lee
Endocrinology.2014; 155(6): 2277. CrossRef
- The Role of Foxo3 in Leydig Cells


 KES
KES

 First
First Prev
Prev



