Search
- Page Path
- HOME > Search
- Endocrine Research
- Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
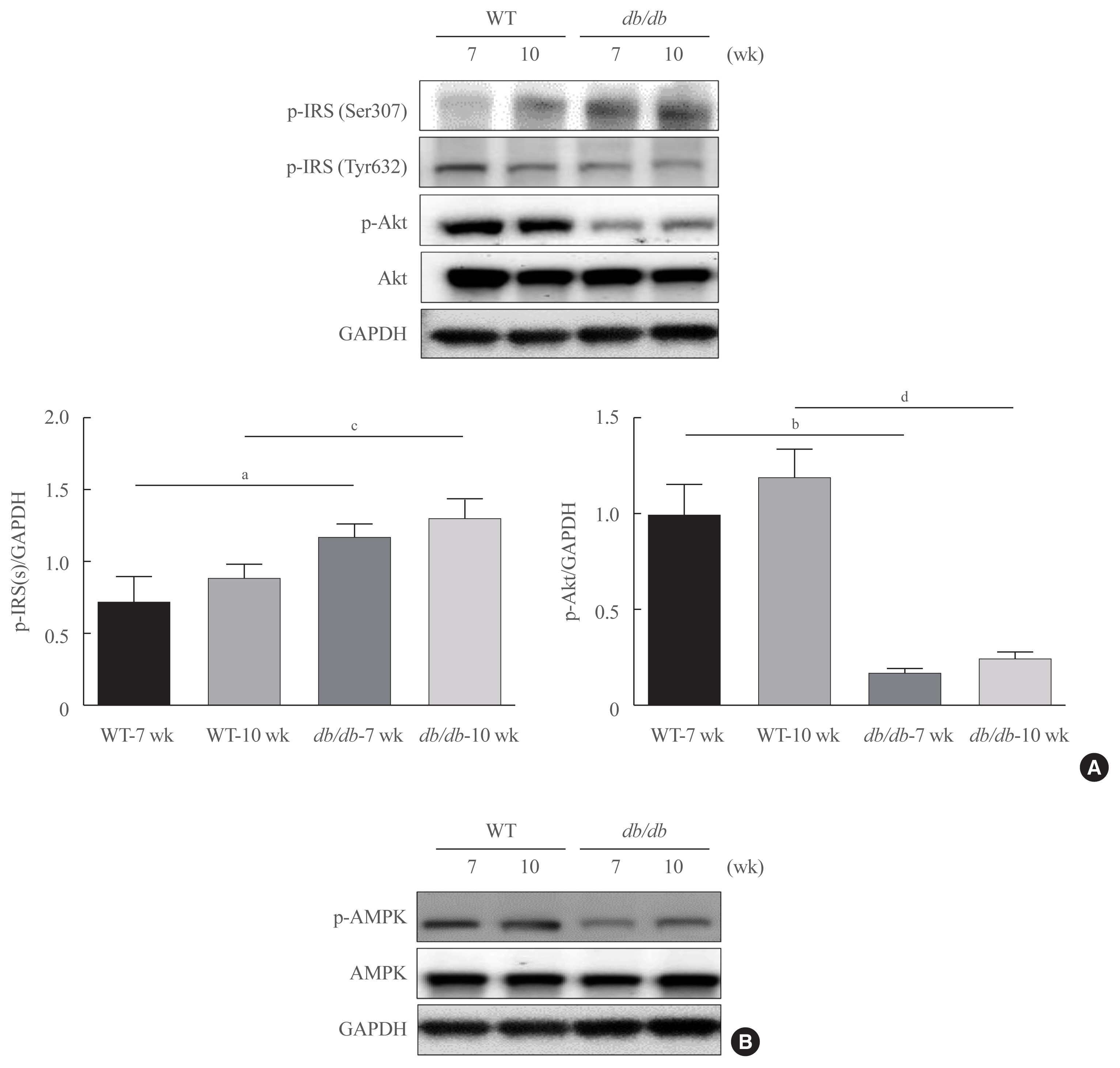
- Dae Hyun Kim, Ye Ra Kim, EunJin Bang, Sugyeong Ha, Sang Gyun Noh, Byeong Moo Kim, Seong Ho Jeong, Hee Jin Jung, Ji Young Lee, Hae Young Chung
- Endocrinol Metab. 2021;36(1):171-184. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.850
- 4,631 View
- 135 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Protease-activated protein-2 (PAR2) has been reported to regulate hepatic insulin resistance condition in type 2 diabetes mice. However, the mechanism of lipid metabolism through PAR2 in obesity mice have not yet been examined. In liver, Forkhead box O1 (FoxO1) activity induces peroxisome proliferator-activated receptor γ (PPARγ), leading to accumulation of lipids and hyperlipidemia. Hyperlipidemia significantly influence hepatic steatoses, but the mechanisms underlying PAR2 signaling are complex and have not yet been elucidated.
Methods
To examine the modulatory action of FoxO1 and its altered interaction with PPARγ, we utilized db/db mice and PAR2-knockout (KO) mice administered with high-fat diet (HFD).
Results
Here, we demonstrated that PAR2 was overexpressed and regulated downstream gene expressions in db/db but not in db+ mice. The interaction between PAR2/β-arrestin and Akt was also greater in db/db mice. The Akt inhibition increased FoxO1 activity and subsequently PPARγ gene in the livers that led to hepatic lipid accumulation. Our data showed that FoxO1 was negatively controlled by Akt signaling and consequently, the activity of a major lipogenesis-associated transcription factors such as PPARγ increased, leading to hepatic lipid accumulation through the PAR2 pathway under hyperglycemic conditions in mice. Furthermore, the association between PPARγ and FoxO1 was increased in hepatic steatosis condition in db/db mice. However, HFD-fed PAR2-KO mice showed suppressed FoxO1-induced hepatic lipid accumulation compared with HFD-fed control groups.
Conclusion
Collectively, our results provide evidence that the interaction of FoxO1 with PPARγ promotes hepatic steatosis in mice. This might be due to defects in PAR2/β-arrestin-mediated Akt signaling in diabetic and HFD-fed mice. -
Citations
Citations to this article as recorded by- Biochanin‐A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF‐β1 and PAR‐2 genes expression in kidney tissues of STZ‐induced diabetic rats
Jamal Amri, Mona Alaee, Rasool Babaei, Zahra Salemi, Reza Meshkani, Ali Ghazavi, Ahmad Akbari, Mehdi Salehi
Biotechnology and Applied Biochemistry.2022; 69(5): 2112. CrossRef - Delineation of the healthy rabbit liver by immunohistochemistry – A technical note
Gabriella Meier Bürgisser, Olivera Evrova, Dorothea M. Heuberger, Julia Rieber, Pietro Giovanoli, Maurizio Calcagni, Johanna Buschmann
Acta Histochemica.2021; 123(7): 151795. CrossRef
- Biochanin‐A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF‐β1 and PAR‐2 genes expression in kidney tissues of STZ‐induced diabetic rats

- Miscellaneous
- Human Immunodeficiency Virus Infection and the Endocrine System
- Dana Zaid, Yona Greenman
- Endocrinol Metab. 2019;34(2):95-105. Published online May 20, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.2.95
- 6,798 View
- 179 Download
- 16 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub In the current era of effective antiretroviral therapies (ARTs), human immunodeficiency virus (HIV) infection became a chronic disorder that requires long term follow-up. Among other medical issues, these patients may develop endocrine problems, specific to HIV infection and its treatment. The purpose of this review is to give an overview of common endocrine complications associated with HIV infection, and to propose diagnostic and therapeutic strategies. HIV can affect the endocrine system at several levels. Adrenal and gonadal dysfunction, osteoporosis with increased fracture risk, dyslipidemia with increased cardiovascular risk, are some of the endocrine disorders prevalent in HIV-infected patients that may negatively influence quality of life, and increase morbidity and mortality. While ARTs have dramatically increased life expectancy in the HIV-infected population, they are not devoid of adverse effects, including endocrine dysfunction. Physicians caring for HIV-infected patients should be knowledgeable and exercise a high index of suspicion for the diagnosis of endocrine abnormalities, and in particular be aware of those that can be life threatening. Endocrine evaluation should follow the same strategies as in the general population, including prevention, early detection, and treatment.
-
Citations
Citations to this article as recorded by- Tuberculosis and diabetes mellitus comorbidity in an adult Ugandan population
Davis Kibirige, Irene Andia-Biraro, Ronald Olum, Susan Adakun, Stella Zawedde-Muyanja, Christine Sekaggya-Wiltshire, Ivan Kimuli
BMC Infectious Diseases.2024;[Epub] CrossRef - Morphometric analysis of adrenal gland in people living with human immunodeficiency virus
Ayşe Gül Kabakcı, Ferit Kuşcu, Ferhat Can Pişkin, Yeşim Taşova, Memduha Gülhal Bozkır
Cukurova Medical Journal.2024; 49(1): 159. CrossRef - Viruses and Endocrine Diseases
Magloire Pandoua Nekoua, Cyril Debuysschere, Inès Vergez, Corentin Morvan, Chaldam Jespere Mbani, Famara Sane, Enagnon Kazali Alidjinou, Didier Hober
Microorganisms.2023; 11(2): 361. CrossRef - Assessment of adrenal cortex function in a group of HIV infected patients in sub-Saharan-Africa
Sida Ghislaine Biwole, Boli Anne Ongmeb, Etoga Martine Claude Etoa, Charly Feutseu, Yefou Mesmin Dehayem, Mekobe Francine Mendane, Samba Esther Mbono, Armel Quentin Essomba, Manga Jean Arnaud Ndi, Moor Vicky Ama, Eugene Sobngwi, Jean Claude Mbanya
Journal of Diabetes and Endocrinology.2023; 13(1): 1. CrossRef - Newly diagnosed type 1 diabetes mellitus in a human immunodeficiency virus-infected patient with antiretroviral therapy-induced immune reconstitution inflammatory syndrome: a case report
Min-ChunYeh, Han-Chuan Chuang, Shuen-Fu Weng, Chung-Huei Hsu, Chen-Ling Huang, Yu-Pei Lin, Yan-Yu Lin, Yu-Shan Hsieh
BMC Infectious Diseases.2023;[Epub] CrossRef - Allopregnanolone and neuroHIV: Potential benefits of neuroendocrine modulation in the era of antiretroviral therapy
Mohammed F. Salahuddin, Alaa N. Qrareya, Fakhri Mahdi, Emaya Moss, Nicholas S. Akins, Jing Li, Hoang V. Le, Jason J. Paris
Journal of Neuroendocrinology.2022;[Epub] CrossRef - HIV in Primary Care: Case Study of Common Chronic Comorbidities
Melody Wilkinson, Pam Biernacki, Joyce Knestrick
The Journal for Nurse Practitioners.2022; 18(5): 525. CrossRef - The crucial role of prolactin-lactogenic hormone in Covid-19
Hayder M. Al-Kuraishy, Ali I. Al-Gareeb, Monica Butnariu, Gaber El-Saber Batiha
Molecular and Cellular Biochemistry.2022; 477(5): 1381. CrossRef - Lower bone density and microarchitecture alterations in HIV‐infected Brazilian men aged 50 years and older are associated with estradiol levels
Felipe P. Oliveira, Luis F. C. Lima, Francisco de Paula Paranhos Neto, Laura M. C. de Mendonça, Annie Schtscherbyna, Luiz A. A. de Lima, Branca A. Fonseca, Miguel Madeira, Ronir R. Luiz, Leonardo V. Neto, Maria L. F. Farias, Elizabeth S. Machado
Clinical Endocrinology.2022; 97(1): 142. CrossRef - The impact of dolutegravir‐based combination antiretroviral therapy on the spermatozoa and fertility parameters of men living with human immunodeficiency virus
Edidiong N. Akang, Olufunke O. Dosumu, Ann A. Ogbenna, Utom‐obong U. Akpan, Jane C. Ezeukwu, Mayowa O. Odofin, Ademola A. Oremosu, Alani S. Akanmu
Andrologia.2022;[Epub] CrossRef - Toxic Metals and Non-Communicable Diseases in HIV Population: A Systematic Review
Opeyemi M. Folorunso, Chiara Frazzoli, Ifeyinwa Chijioke-Nwauche, Beatrice Bocca, Orish E. Orisakwe
Medicina.2021; 57(5): 492. CrossRef - Propensity score matching evaluation of psychological stress and hair cortisol among people living with HIV in China
Xu Chen, Shuaifeng Liu, Chengbo Zeng, Xiaoming Li, Shan Qiao, Riying Lv, Zhiyong Shen
Scientific Reports.2021;[Epub] CrossRef - Adrenal insufficiency in HIV/AIDS: a review
Simon Mifsud, Zachary Gauci, Mark Gruppetta, Charles Mallia Azzopardi, Stephen Fava
Expert Review of Endocrinology & Metabolism.2021; 16(6): 351. CrossRef - REPRODUCTIVE DISORDERS AND THEIR PATHOGENETIC MECHANISMS IN WOMEN WITH HIV
O. Ya. Leshchenko, E. V. Genich
HIV Infection and Immunosuppressive Disorders.2020; 11(4): 20. CrossRef - A case of acanthosis nigricans in a HIV-infected patient
Alessandra Iacovelli, Ivano Mezzaroma, Marcello Di Paolo, Giuseppe Soda, Ludovica De Vincentiis, Paolo Palange
BMC Infectious Diseases.2020;[Epub] CrossRef - Clinically Relevant Interactions between Atypical Antipsychotics and Anti-Infective Agents
Edoardo Spina, Maria Antonietta Barbieri, Giuseppe Cicala, Jose de Leon
Pharmaceuticals.2020; 13(12): 439. CrossRef
- Tuberculosis and diabetes mellitus comorbidity in an adult Ugandan population

- One Family of Familial Combined hyperlipidemia Associated with Various Metabolic Abnormalities.
- Kwan Woo Lee, Sung Kyu Lee, Yun Suk Chung, Hyun Man Kim, Yoon Jung Kim, Eun Kyung Hong, Bong Nam Chae, Ji Won Park
- J Korean Endocr Soc. 1999;14(2):418-424. Published online January 1, 2001
- 1,062 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Familial combined hyperlipidemia is one af the manogenic disorders frequently found in humans and is seen in 0.5~2% of the general populatian, accounting for at least 10% of persons with pemature atlmmcletusis. The distinguishing feature of familial combined hyperlipidemia, in camparison with other single-gene abnarmalities of lipoprotein metabolism, is that not all affected members have the same plasma lipid phenotype; some individuals have an elevation of cholesterol concentration alane(type IIa lipoprotein pattern), while some athers have an elevation of triglyceride concentration alone(type IV pattem), and still others have elevations of both values(type IIb pattem). In any one persan, the lipid phenotype can change as a result of dietary or drug treatment. Familial combined hyperlipidemia should be suspected in those subjects with moderate hypertriglyceridemia and/or moderate hypercholestaolemia (lipoprotein types IIa, Ilb, IV), especially when premature coronary heart disease is evident in the family histary. Low plasma HDL-cholesterol, obesity, insulin resistance and hyperuricemia are often . Family members affected by familial combined hyperlipidemia should be identified and be treated, since tbe condition is associated with premature caronary heart diasease. We have found one family of familial combined hyperlipidemia with one member(case 1) associated with insulin resistance, hyperuricemia and gout, and another member(case 2) associated with diabetes mellitus and infertiTity.


 KES
KES

 First
First Prev
Prev



