Search
- Page Path
- HOME > Search
- Miscellaneous
- COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
- Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim, the Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2021;36(4):757-765. Published online August 17, 2021
- DOI: https://doi.org/10.3803/EnM.2021.404
- 10,404 View
- 419 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Since the first outbreak of coronavirus disease 2019 (COVID-19), ongoing efforts have been made to discover an efficacious vaccine against COVID-19 to combat the pandemic. In most countries, both mRNA and DNA vaccines have been administered, and their side effects have also been reported. The clinical course of COVID-19 and the effects of vaccination against COVID-19 are both influenced by patients’ health status and involve a systemic physiological response. In view of the systemic function of endocrine hormones, endocrine disorders themselves and the therapeutics used to treat them can influence the outcomes of vaccination for COVID-19. However, there are very limited data to support the development of clinical guidelines for patients with specific medical backgrounds based on large clinical trials. In the current severe circumstances of the COVID-19 pandemic, position statements made by clinical specialists are essential to provide appropriate recommendations based on both medical evidence and clinical experiences. As endocrinologists, we would like to present the medical background of COVID-19 vaccination, as well as precautions to prevent the side effects of COVID-19 vaccination in patients with specific endocrine disorders, including adrenal insufficiency, diabetes mellitus, osteoporosis, autoimmune thyroid disease, hypogonadism, and pituitary disorders.
-
Citations
Citations to this article as recorded by- COVID-19 mRNA vaccine may trigger subacute thyroiditis
Mehmet Sözen, Ömercan Topaloğlu, Berrin Çetinarslan, Alev Selek, Zeynep Cantürk, Emre Gezer, Damla Köksalan, Taner Bayraktaroğlu
Human Vaccines & Immunotherapeutics.2024; 17(12): 5120. CrossRef - The role of co-morbidities in the development of an AEFI after COVID-19 vaccination in a large prospective cohort with patient-reported outcomes in the Netherlands
C. Ouaddouh, J.W. Duijster, T. Lieber, F.P.A.M. van Hunsel
Expert Opinion on Drug Safety.2024; 23(3): 323. CrossRef - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
Hyeyeon Moon, Sunghwan Suh, Mi Kyoung Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Prior immunization status of COVID-19 patients and disease severity: A multicenter retrospective cohort study assessing the different types of immunity
Javaria Aslam, Faisal Shahzad Khan, Muhammad Talha Haris, Hewad Hewadmal, Maryam Khalid, Mohammad Y. Alshahrani, Qurrat-ul-ain Aslam, Irrum Aneela, Urooj Zafar
Vaccine.2023; 41(2): 598. CrossRef - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(2): 253. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Inactivated SARS-CoV-2 vaccination does not disturb the clinical course of Graves’ disease: An observational cohort study
Shichen Xu, Huixin Yu, Xian Cheng, Jing Wu, Jiandong Bao, Li Zhang
Vaccine.2023; 41(38): 5648. CrossRef - Adrenal Crisis Associated With COVID-19 Vaccination in Patients With Adrenal Insufficiency
Yukako Kurematsu, Takako Mohri, Sadanori Okada, Yutaka Takahashi
JCEM Case Reports.2023;[Epub] CrossRef - Adverse Events Associated with COVID-19 Vaccination in Adolescents with Endocrinological Disorders: A Cross-Sectional Study
İbrahim Mert Erbaş, İrem Ceren Erbaş, Gözde Akın Kağızmanlı, Kübra Yüksek Acinikli, Özge Besci, Korcan Demir, Ece Böber, Nurşen Belet, Ayhan Abacı
Journal of Clinical Research in Pediatric Endocrinology.2023; 15(3): 248. CrossRef - Neue Aspekte der Glukokortikoidsubstitution bei Nebennierenrindeninsuffizienz
Tina Kienitz, Gesine Meyer
Der Internist.2022; 63(1): 12. CrossRef - Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence
Rimesh Pal, Ameya Joshi, Sanjay K. Bhadada, Mainak Banerjee, Suresh Vaikkakara, Satinath Mukhopadhyay
Endocrine Practice.2022; 28(4): 425. CrossRef - Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study
Xi Xiong, Carlos King Ho Wong, Ivan Chi Ho Au, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Kristy Tsz Kwan Lau, Chi Ho Lee, Yu Cho Woo, David Tak Wai Lui, Ian Chi Kei Wong
Thyroid.2022; 32(5): 505. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism
Nikolina Markovic, Anila Faizan, Chirag Boradia, Sridhar Nambi
AACE Clinical Case Reports.2022; 8(4): 171. CrossRef - The Effect of Inactivated SARS-CoV-2 Vaccines on TRAB in Graves’ Disease
LingHong Huang, ZhengRong Jiang, JingXiong Zhou, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Osteoporosis in Patients With Respiratory Diseases
Yue Ma, Shui Qiu, Renyi Zhou
Frontiers in Physiology.2022;[Epub] CrossRef - Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Ach Taieb, El Euch Mounira
Vaccines.2022; 10(12): 2004. CrossRef - Forty Years Together, New Leap Forward! The 40th Anniversary of the Korean Endocrine Society
Jong Chul Won, Ki-Hyun Baek
Endocrinology and Metabolism.2022; 37(6): 851. CrossRef - No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine
Tania Pilli, Cristina Dalmiglio, Gilda Dalmazio, Alfonso Sagnella, Raffaella Forleo, Lucia Brilli, Fabio Maino, Cristina Ciuoli, Maria Grazia Castagna
European Journal of Endocrinology.2022; 187(1): K7. CrossRef - Diabetes and COVID-19 Vaccination
Hae Dong Choi, Jun Sung Moon
The Journal of Korean Diabetes.2021; 22(4): 221. CrossRef
- COVID-19 mRNA vaccine may trigger subacute thyroiditis

- Hypothalamus and Pituitary gland
- Current National and International Guidelines for the Management of Male Hypogonadism: Helping Clinicians to Navigate Variation in Diagnostic Criteria and Treatment Recommendations
- Ahmed Al-Sharefi, Richard Quinton
- Endocrinol Metab. 2020;35(3):526-540. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.760
- 9,522 View
- 505 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
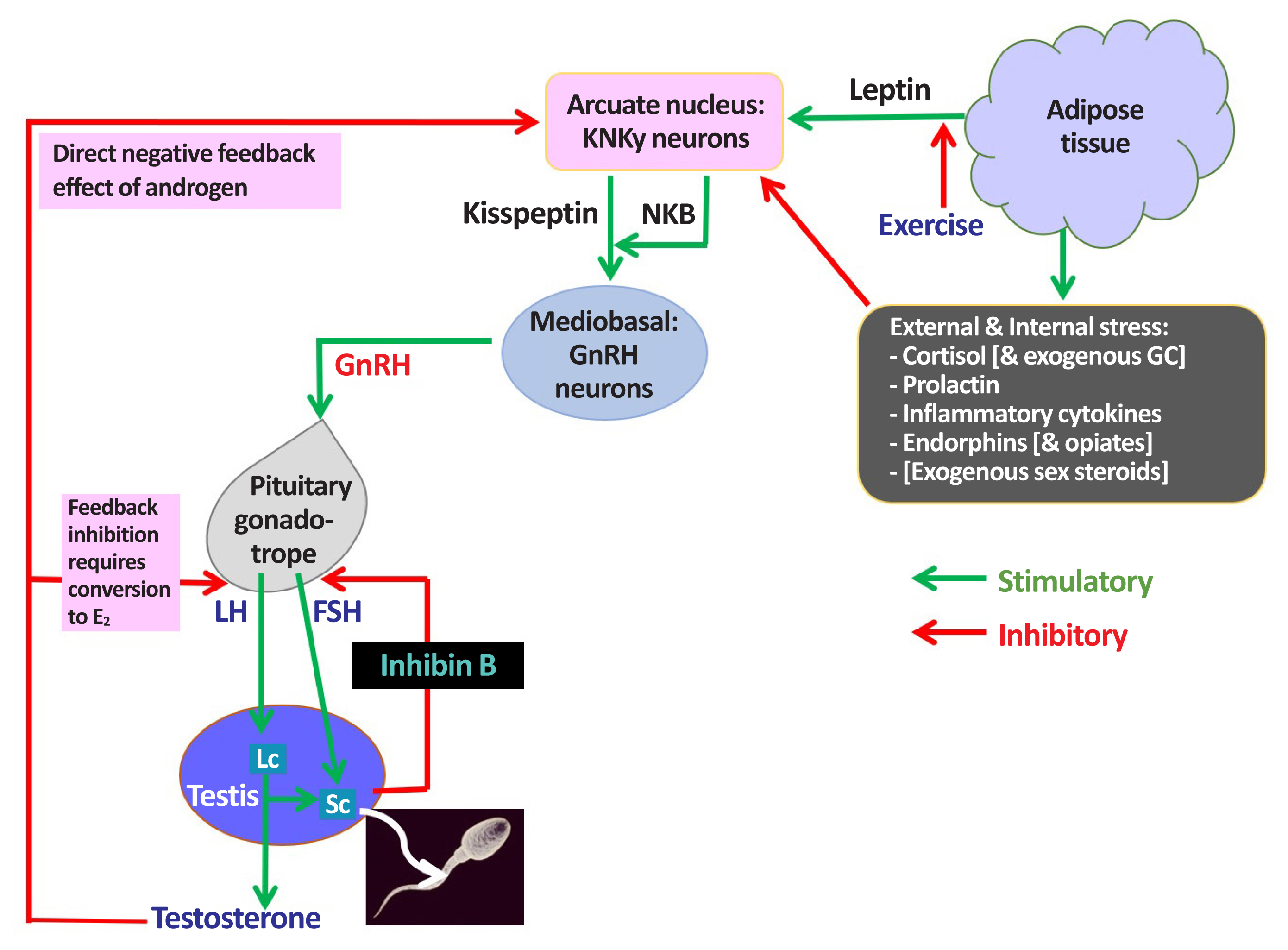
ePub - Male hypogonadism—rebadged by some as testosterone deficiency syndrome—is a clinical and biochemical diagnosis of increasing worldwide interest. Organic male hypogonadism—usually permanent—is well-established, but aging men may also exhibit lower serum testosterone levels; principally due to burden of extra-gonadal comorbidities such as obesity, diabetes and metabolic syndrome, but with an underlying intact hypothalamo-pituitary-testicular (HPT) axis capable of springing back into operation once comorbidities are addressed. Despite encouraging observational data and plausible theoretical underpinning, evidence for efficacy and safety of testosterone in this “aging” group of men is lacking; addressing comorbid illnesses remains the key priority instead. Nevertheless, in recent years, accumulation of misleading information online has triggered a global tsunami of testosterone prescriptions. Despite this, many men with organic hypogonadism remain undiagnosed or untreated; many more face a diagnostic odyssey before achieving care by the appropriate specialist. As testosterone therapy is not without risk several clinical practice guidelines have been published specialist societies to guide physicians on best practice. However, these are heterogeneous in key areas, reflecting divergent approaches to the same evidence basis. Herein, we navigate the major clinical practice guidelines on male hypogonadism and test their respective recommendations against current best evidence.
-
Citations
Citations to this article as recorded by- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India
Sanjay Kalra, Jubbin Jacob, A. G. Unnikrishnan, Ganapathi Bantwal, Abhay Sahoo, Rakesh Sahay, Sushil Jindal, Madhu Sudan Agrawal, Nitin Kapoor, Banshi Saboo, Mangesh Tiwaskar, Kapil Kochhar, Henrik Falhammar
International Journal of Endocrinology.2023; 2023: 1. CrossRef - Management Outcomes in Males With Hypogonadotropic Hypogonadism Treated With Gonadotropins
Bahaa O Sahib, Ibrahim H Hussein, Nassar T Alibrahim, Abbas A Mansour
Cureus.2023;[Epub] CrossRef - The Association between Inflammation, Testosterone and SHBG in men: A cross‐sectional Multi‐Ethnic Study of Atherosclerosis
Amar Osmancevic, Bledar Daka, Erin D. Michos, Penelope Trimpou, Matthew Allison
Clinical Endocrinology.2023; 99(2): 190. CrossRef - The Illusory Case for Treatment of an Invented Disease
David J. Handelsman
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Chronic Heart Failure Complicated with Type 2 Diabetes Mellitus on Cognitive Function in the Elderly
Yang Liu, Rui Meng, Jianzeng Dong, Xiaonan Xi
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Different Factors Are Associated With Sex Hormones and Leydig Cell Function in Israelis and Palestinians in Jerusalem
Guy Vishnevsky, Ronit Sinnreich, Hisham Nassar, Dafna Merom, Maya Ish-Shalom, Jeremy D. Kark, Hagai Levine
American Journal of Men's Health.2022; 16(4): 155798832211060. CrossRef - Association of rs9939609 polymorphism in the FTO gene with features of androgen status in men
S. V. Yankovskaya, K. I. Mosalev, I. D. Ivanov, B. B. Pinkhasov, V. G. Selyatitskaya
Сибирский научный медицинский журнал.2022; 42(2): 18. CrossRef - Clinical and pharmacological basis of the use of testosterone drugs for hormonal replacement therapy for hypogonadism in men
N. I. Volkova, A. V. Safronenko, E. V. Gantsgorn, Yu. S. Degtyareva
Obesity and metabolism.2022; 19(2): 233. CrossRef - Monitoring and Management of Bardet-Biedl Syndrome: What the Multi-Disciplinary Team Can Do
Lavinia Caba, Laura Florea, Elena Emanuela Braha, Valeriu Vasile Lupu, Eusebiu Vlad Gorduza
Journal of Multidisciplinary Healthcare.2022; Volume 15: 2153. CrossRef - Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones
Sara Arefhosseini, Mehrangiz Ebrahimi-Mameghani, Farzad Najafipour, Helda Tutunchi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men
Bruno Lunenfeld, George Mskhalaya, Michael Zitzmann, Giovanni Corona, Stefan Arver, Svetlana Kalinchenko, Yuliya Tishova, Abraham Morgentaler
The Aging Male.2021; 24(1): 119. CrossRef
- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India

- The Responses of Pituitary Hormones to the Combined Pituitary Stimulation Test in Hypogonadotropic Hypogonadism.
- In Myung Yang, Jeong Taek Woo, Sung Woon Kim, Jin Woo Kim, Young Seol Kim, Young Kil Choi, Eun Kyung Park, Kyu Jeong Ahn
- J Korean Endocr Soc. 1994;9(2):93-107. Published online November 6, 2019
- 1,157 View
- 29 Download
-
 Abstract
Abstract
 PDF
PDF - To classify the causes of hypogonadotropic hypogonadism in Korean patients, and to improve the endocrinologic evaluation for the disease, we retrospectively studied the clinical findings and result of combined pituitary stimulation test in 35 patients with hypogonadotropic hypogonadism. The following results were obtained.1) The ratio of male to female was 1.3:1, and the 50% of male patients was under 20 years of age and the 20% of female patients in 30th decades. 2) The chief complaints of male patients on the admission were the failure of secondary sexual characteristics(95.0%) and loss of hair(5.0%), those of female patients were amenorrhea(46.7%), infertility(26.7%), failure of secondary characteristics(13.3%) and loss of hair(13.3%). 3) The causes of male hypogonadotropic hypogonadism were craniopharyngioma(35.0%), idiopathic(30.0%), Kallmann's syndrome(15.0%), pituitary adenoma(10.0%) and germinoma(5.0%), and those of female hypogonadotropic hypogonadism were prolactinoma(13.3%), Sheehan's syndrome(26.6%), pituitary adenoma(6.7%), tuberculous granuloma(6.7%), germinoma(6.7%), idiopathic hypogonadotropic hypogonadism(40.0%).4) The responses of LH and FSH to GnRH test were absent or markedly blunted in diffuse pituitary diseases such as pituitary tuberculous granuloma, pituitary macroadenomas, Sheehan's syndrome. However those were also absent or blunted in Cushing's disease and hypothalamic disease such as Kallmann's syndrome, germinoma, craniopharyngioma, idiopathic hypogonadotropic hypogonadism. 5) The responses of LH, FSH increased after repeated injection of GnRH in a patient with germinoma. 6) In diffuse destructive pituitary diseases such as Sheehan's syndrome, nonfunctioning macroadenomas, tuberculous granuloma, large prolactinoma, the combined deficiency of pituitary hormones other than gonadotropins was observed. 7) In many cases with hypothalamic diseases, the combined defects of pituitary hormone response were also seen.These data suggest that GnRH test is not always useful to localize the lesion between pituitary and hypothalamus, and combined pituitary stimulation test revealed defects of pituitary hormones other than gonadotropin in various hypothalamic diseases.Therefore repeated GnRH test would be useful for the differential diagnosis, and CRH test and GRH test would be necessary to demonstrate whether pituitary abnormality is present.

- Adrenal gland
- Congenital Hypogonadotropic Hypogonadism and Kallmann Syndrome: Past, Present, and Future
- Soo-Hyun Kim
- Endocrinol Metab. 2015;30(4):456-466. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.456
- 7,831 View
- 122 Download
- 71 Web of Science
- 67 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The proper development and coordination of the hypothalamic-pituitary-gonadal (HPG) axis are essential for normal reproductive competence. The key factor that regulates the function of the HPG axis is gonadotrophin-releasing hormone (GnRH). Timely release of GnRH is critical for the onset of puberty and subsequent sexual maturation. Misregulation in this system can result in delayed or absent puberty and infertility. Congenital hypogonadotropic hypogonadism (CHH) and Kallmann syndrome (KS) are genetic disorders that are rooted in a GnRH deficiency but often accompanied by a variety of non-reproductive phenotypes such as the loss of the sense of smell and defects of the skeleton, eye, ear, kidney, and heart. Recent progress in DNA sequencing technology has produced a wealth of information regarding the genetic makeup of CHH and KS patients and revealed the resilient yet complex nature of the human reproductive neuroendocrine system. Further research on the molecular basis of the disease and the diverse signal pathways involved will aid in improving the diagnosis, treatment, and management of CHH and KS patients as well as in developing more precise genetic screening and counseling regime.
-
Citations
Citations to this article as recorded by- In vitro modeling of cranial placode differentiation: Recent advances, challenges, and perspectives
Casey Griffin, Jean-Pierre Saint-Jeannet
Developmental Biology.2024; 506: 20. CrossRef - Etiology of Male Infertility: an Update
Indrashis Bhattacharya, Souvik Sen Sharma, Subeer S. Majumdar
Reproductive Sciences.2024; 31(4): 942. CrossRef - Clinical phenotype of a Kallmann syndrome patient with IL17RD and CPEB4 variants
Jianmei Zhang, Suhong Yang, Yan Zhang, Fei Liu, Lili Hao, Lianshu Han
Frontiers in Endocrinology.2024;[Epub] CrossRef - Unusual coexistence of restrictive heart disease and Kallmann syndrome: a case report
Ghali Bennani, Soukaina Zahri, Mohamed Khaldi, Ghali Benouna, Abdenasser Drighil, Rachida Habbal
The Egyptian Heart Journal.2024;[Epub] CrossRef - Effects of Exercise on Testosterone and Implications of Drug Abuse: A Review
Brendan Perreault, Nikki Hammond, Panayotis K. Thanos
Clinical Neuropharmacology.2023; 46(3): 112. CrossRef - A functional spectrum of PROKR2 mutations identified in isolated hypogonadotropic hypogonadism
Xinying Wang, Danna Chen, Yaguang Zhao, Meichao Men, Zhiheng Chen, Fang Jiang, Ruizhi Zheng, Maria I Stamou, Lacey Plummer, Ravikumar Balasubramanian, Jia-Da Li
Human Molecular Genetics.2023; 32(10): 1722. CrossRef - RNF216 affects the stability of STAU2 in the hypothalamus
Han Yang, Yong Zhu, Xin Li, Zuiming Jiang, Wenting Dai
Development, Growth & Differentiation.2023; 65(7): 408. CrossRef - Two Sisters with Kallmann Syndrome, Gonadal Dysgenesis, and Multiple Neuromuscular and Endocrine Disorders: Report of Two Cases with Description of an Unusual Association
Marta Camacho, Camil Castelo-Branco
Reproductive Sciences.2022; 29(10): 2859. CrossRef - A Molecular Analysis of Neural Olfactory Placode Differentiation in Human Pluripotent Stem Cells
Rebecca L. Bricker, Uchit Bhaskar, Rossella Titone, Melanie A. Carless, Tiziano Barberi
Stem Cells and Development.2022; 31(17-18): 507. CrossRef - The use of stimulation tests for the differential diagnosis of delayed puberty in boys. How to increase the specificity of the method?
Irina Yu. Ioffe, Yulia L. Skorodok, Elena V. Plotnikova, Irena I. Nagornaya, Ksenia O. Nagovitsyna, Lyudmila A. Jelenina
Pediatrician (St. Petersburg).2022; 13(3): 15. CrossRef - The diagnostic value of the olfactory evaluation for congenital hypogonadotropic hypogonadism
Bingqing Yu, Kepu Chen, Jiangfeng Mao, Bo Hou, Hui You, Xi Wang, Min Nie, Qibin Huang, Rui Zhang, Yiyi Zhu, Bang Sun, Feng Feng, Wen Zhou, Xueyan Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Genetic analysis of failed male puberty using whole exome sequencing
Maleeha Akram, David J. Handelsman, Mazhar Qayyum, Marina Kennerson, Sania Rauf, Shahid Ahmed, Osama Ishtiaq, Muhammad Ismail, Qaisar Mansoor, Afzaal Ahmed Naseem, Syed Shakeel Raza Rizvi
Journal of Pediatric Endocrinology and Metabolism.2022; 35(11): 1410. CrossRef - Genetics of congenital olfactory dysfunction: a systematic review of the literature
Matthias Deller, Janine Gellrich, Elisabeth C Lohrer, Valentin A Schriever
Chemical Senses.2022;[Epub] CrossRef - Testicular ultrasound in a patient with Kallmann syndrome: A case report
Daniela Donat, Sonja Lukac, Ivana Bajkin, Ivana Vorgucin, Viktor Till, Sladjana Zagorac
Medicinski pregled.2022; 75(7-8): 247. CrossRef - Prevalence and associated phenotypes of DUSP6, IL17RD and SPRY4 variants in a large Chinese cohort with isolated hypogonadotropic hypogonadism
Meichao Men, Xinying Wang, Jiayu Wu, Wang Zeng, Fang Jiang, Ruizhi Zheng, Jia-Da Li
Journal of Medical Genetics.2021; 58(1): 66. CrossRef - Conditional Fgfr1 Deletion in GnRH Neurons Leads to Minor Disruptions in the Reproductive Axis of Male and Female Mice
Cynthia Dela Cruz, Cassandra A. Horton, Kelsey N. Sanders, Nathan D. Andersen, Pei-San Tsai
Frontiers in Endocrinology.2021;[Epub] CrossRef - A case report of congenital idiopathic hypogonadotropic hypogonadism caused by novel mutation of GNRHR gene
Liping Wang, Weisheng Lin, Xiaohong Li, Lijuan Zhang, Kai Wang, Xiaoli Cui, Shanmei Tang, Guangguang Fang, Yan Tan, Xuelai Wang, Chuan Chen, Chuanchun Yang, Huiru Tang
Medicine.2021; 100(5): e24007. CrossRef - RNF216 regulates meiosis and PKA stability in the testes
Dengfeng Li, Fangfang Li, Lanlan Meng, Huafang Wei, Qianjun Zhang, Fang Jiang, Dan‐Na Chen, Wei Li, Yue‐Qiu Tan, Jia‐Da Li
The FASEB Journal.2021;[Epub] CrossRef - Congenital Hypogonadotropic Hypogonadism with Early-Onset Coronary Artery Disease
Akira Takashima, Shusuke Yagi, Koji Yamaguchi, Kiyoe Kurahashi, Yuko Kojima, Robert Zheng, Takayuki Ise, Kenya Kusunose, Sumiko Yoshida, Hirotsugu Yamada, Takeshi Soeki, Tetsuzo Wakatsuki, Ken-ichi Aihara, Masashi Akaike, Masataka Sata
The Journal of Medical Investigation.2021; 68(1.2): 189. CrossRef - The Differential Roles for Neurodevelopmental and Neuroendocrine Genes in Shaping GnRH Neuron Physiology and Deficiency
Roberto Oleari, Valentina Massa, Anna Cariboni, Antonella Lettieri
International Journal of Molecular Sciences.2021; 22(17): 9425. CrossRef - Analysis of PLXNA1, NRP1, and NRP2 variants in a cohort of patients with isolated hypogonadotropic hypogonadism
Meichao Men, Dan‐Na Chen, Jia‐Da Li, Xinying Wang, Wang Zeng, Fang Jiang, Ruizhi Zheng, Wenting Dai
Molecular Genetics & Genomic Medicine.2021;[Epub] CrossRef - A Novel SEMA3G Mutation in Two Siblings Affected by Syndromic GnRH Deficiency
Roberto Oleari, Valentina André, Antonella Lettieri, Sophia Tahir, Lise Roth, Alyssa Paganoni, Ivano Eberini, Chiara Parravicini, Valeria Scagliotti, Ludovica Cotellessa, Francesco Bedogni, Lisa Benedetta De Martini, Maria Vittoria Corridori, Simona Gulli
Neuroendocrinology.2021; 111(5): 421. CrossRef - A novel heterozygous intron mutation in SEMA7A causing kallmann syndrome in a female
Yongting Zhao, Fan Yang, Lili Qiu, Lihong Wang, Hui Che
Gynecological Endocrinology.2020; 36(3): 218. CrossRef - Phenotypic Spectrum of Idiopathic Hypogonadotropic Hypogonadism Patients With CHD7 Variants From a Large Chinese Cohort
Jia-Da Li, Jiayu Wu, Yaguang Zhao, Xinying Wang, Fang Jiang, Qiao Hou, Dan-Na Chen, Ruizhi Zheng, Renhe Yu, Wei Zhou, Meichao Men
The Journal of Clinical Endocrinology & Metabolism.2020; 105(5): 1515. CrossRef - Genotypic and phenotypic spectra of FGFR1, FGF8, and FGF17 mutations in a Chinese cohort with idiopathic hypogonadotropic hypogonadism
Meichao Men, Jiayu Wu, Yaguang Zhao, Xiaoliang Xing, Fang Jiang, Ruizhi Zheng, Jia-Da Li
Fertility and Sterility.2020; 113(1): 158. CrossRef - Posttranslational Modification Defects in Fibroblast Growth Factor Receptor 1 as a Reason for Normosmic Isolated Hypogonadotropic Hypogonadism
Hui Ying, Yan Sun, Huixiao Wu, Wenyu Jia, Qingbo Guan, Zhao He, Ling Gao, Jiajun Zhao, Yiming Ji, Guimei Li, Chao Xu, Fabio Altieri
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef - CHD7 missense variants and clinical characteristics of Chinese males with infertility
Leilei Li, Ruixue Wang, Yang Yu, Hongguo Zhang, Yuting Jiang, Xiao Yang, Ruizhi Liu
Molecular Genetics & Genomic Medicine.2020;[Epub] CrossRef - Lifestyle, metabolic disorders and male hypogonadism – A one-way ticket?
Luís Crisóstomo, Sara C. Pereira, Mariana P. Monteiro, João F. Raposo, Pedro F. Oliveira, Marco G. Alves
Molecular and Cellular Endocrinology.2020; 516: 110945. CrossRef - GnRH Deficient Patients With Congenital Hypogonadotropic Hypogonadism: Novel Genetic Findings in ANOS1, RNF216, WDR11, FGFR1, CHD7, and POLR3A Genes in a Case Series and Review of the Literature
Vassos Neocleous, Pavlos Fanis, Meropi Toumba, George A. Tanteles, Melpo Schiza, Feride Cinarli, Nicolas C. Nicolaides, Anastasis Oulas, George M. Spyrou, Christos S. Mantzoros, Dimitrios Vlachakis, Nicos Skordis, Leonidas A. Phylactou
Frontiers in Endocrinology.2020;[Epub] CrossRef - Eje hipotálamo hipofisario. Fisiología y patología
M. Araujo-Castro, E. Pascual-Corrales, A.E. Ortiz-Flores, H.F. Escobar-Morreale
Medicine - Programa de Formación Médica Continuada Acreditado.2020; 13(15): 846. CrossRef - WD40-Repeat Proteins in Ciliopathies and Congenital Disorders of Endocrine System
Yeonjoo Kim, Soo-Hyun Kim
Endocrinology and Metabolism.2020; 35(3): 494. CrossRef - Eight rare urinary disorders in a patient with Kallmann syndrome
Huining Tian, Zi Yan, You Lv, Lin Sun, Xiaokun Gang, Guixia Wang
Medicine.2020; 99(43): e22936. CrossRef - Delayed and Precocious Puberty: Genetic Underpinnings and Treatments
Anisha Gohil, Erica A. Eugster
Endocrinology and Metabolism Clinics of North America.2020; 49(4): 741. CrossRef - Disorders of Sex Development: Classification, Review, and Impact on Fertility
Pedro Acién, Maribel Acién
Journal of Clinical Medicine.2020; 9(11): 3555. CrossRef - A nonsense variant in FGFR1: a rare cause of combined pituitary hormone deficiency
İbrahim Mert Erbaş, Ahu Paketçi, Sezer Acar, Leman Damla Kotan, Korcan Demir, Ayhan Abacı, Ece Böber
Journal of Pediatric Endocrinology and Metabolism.2020; 33(12): 1613. CrossRef - Kallman syndrome and central non-obstructive azoospermia
Sameer Thakker, Jesse Persily, Bobby B. Najari
Best Practice & Research Clinical Endocrinology & Metabolism.2020; 34(6): 101475. CrossRef - RNF216 Regulates the Migration of Immortalized GnRH Neurons by Suppressing Beclin1-Mediated Autophagy
Fangfang Li, Dengfeng Li, Huadie Liu, Bei-Bei Cao, Fang Jiang, Dan-Na Chen, Jia-Da Li
Frontiers in Endocrinology.2019;[Epub] CrossRef - Parallel Multi-Gene Panel Testing for Diagnosis of Idiopathic Hypogonadotropic Hypogonadism/Kallmann Syndrome
Manickavasagam Senthilraja, Aaron Chapla, Felix K. Jebasingh, Dukhabhandhu Naik, Thomas V. Paul, Nihal Thomas
Case Reports in Genetics.2019; 2019: 1. CrossRef - Screening for mutations in selected miRNA genes in hypogonadotropic hypogonadism patients
Anna-Pauliina Iivonen, Johanna Känsäkoski, Kirsi Vaaralahti, Taneli Raivio
Endocrine Connections.2019; 8(5): 506. CrossRef - Live birth in male de novo Kallmann syndrome after cross-generational genetic sequencing
Cindy Chan, Cheng-Wei Wang, Ching-Hui Chen, Chi-Huang Chen
Journal of Assisted Reproduction and Genetics.2019; 36(12): 2481. CrossRef - Is Hormonal Treatment of Congenital Undescended Testes Justified A Debate
Faruk Hadziselimovic
Sexual Development.2019; 13(1): 3. CrossRef - High frequency of CHD7 mutations in congenital hypogonadotropic hypogonadism
Catarina Inês Gonçalves, Filipa Marina Patriarca, José Maria Aragüés, Davide Carvalho, Fernando Fonseca, Sofia Martins, Olinda Marques, Bernardo Dias Pereira, José Martinez-de-Oliveira, Manuel Carlos Lemos
Scientific Reports.2019;[Epub] CrossRef - PROKR2mutations in idiopathic hypogonadotropic hypogonadism: selective disruption of the binding to aGα‐protein leads to biased signaling
Yaguang Zhao, Jiayu Wu, Hong Jia, Xinying Wang, Ruizhi Zheng, Fang Jiang, Dan-Na Chen, Zhiheng Chen, Jia-Da Li
The FASEB Journal.2019; 33(3): 4538. CrossRef - Congenital diaphragmatic hernia is associated with nonscrotal testes
Stan Janssen, Kim Heiwegen, Iris ALM van Rooij, Janielle van Alfen-van der Velden, Ivo de Blaauw, Sanne MBI Botden
Journal of Pediatric Surgery.2019; 54(3): 445. CrossRef - Cranial Pair 0: The Nervus Terminalis
Ángel Peña‐Melián, Juan Pablo Cabello‐de la Rosa, Maria José Gallardo‐Alcañiz, Julia Vaamonde‐Gamo, Fernanda Relea‐Calatayud, Lucía González‐López, Patricia Villanueva‐Anguita, Alicia Flores‐Cuadrado, Daniel Saiz‐Sánchez, Alino Martínez‐Marcos
The Anatomical Record.2019; 302(3): 394. CrossRef - Semaphorin Signaling in GnRH Neurons: From Development to Disease
Roberto Oleari, Antonella Lettieri, Alyssa Paganoni, Luca Zanieri, Anna Cariboni
Neuroendocrinology.2019; 109(3): 193. CrossRef - Whole Exome Sequencing Revealed a Novel Nonsense Variant in the GNRHR Gene Causing Normosmic Hypogonadotropic Hypogonadism in a Pakistani Family
Hafiz Muhammad Jafar Hussain, Ghulam Murtaza, Xiaohua Jiang, Ranjha Khan, Manan Khan, Mian Basit Shah Kakakhel, Teka Khan, Fazal Wahab, Huan Zhang, Yuanwei Zhang, Muhammad Bilal Khan, Parvez Ahmed, Hui Ma, Zhipeng Xu
Hormone Research in Paediatrics.2019; 91(1): 9. CrossRef - New intronic Fibroblast Growth Factor Receptor 1 (FGFR1) mutation leading to disrupted splicing and Kallmann syndrome
J Känsäkoski, K Vaaralahti, T Raivio
Human Reproduction.2018; 33(2): 328. CrossRef - Rare cause of manic period trigger in bipolar mood disorder: testosterone replacement
Gulcin Elboga, Zeynel Abidin Sayiner
BMJ Case Reports.2018; : bcr-2018-225108. CrossRef - WDR11‐mediated Hedgehog signalling defects underlie a new ciliopathy related to Kallmann syndrome
Yeon‐Joo Kim, Daniel PS Osborn, Ji‐Young Lee, Masatake Araki, Kimi Araki, Timothy Mohun, Johanna Känsäkoski, Nina Brandstack, Hyun‐Taek Kim, Francesc Miralles, Cheol‐Hee Kim, Nigel A Brown, Hyung‐Goo Kim, Juan Pedro Martinez‐Barbera, Paris Ataliotis, Tane
EMBO reports.2018; 19(2): 269. CrossRef - A rare ANOS1 variant in siblings with Kallmann syndrome identified by whole exome sequencing
D. M. Lopategui, A. J. Griswold, H. Arora, R. I. Clavijo, M. Tekin, R. Ramasamy
Andrology.2018; 6(1): 53. CrossRef - Investigation of common, low-frequency and rare genome-wide variation in anorexia nervosa
L M Huckins, K Hatzikotoulas, L Southam, L M Thornton, J Steinberg, F Aguilera-McKay, J Treasure, U Schmidt, C Gunasinghe, A Romero, C Curtis, D Rhodes, J Moens, G Kalsi, D Dempster, R Leung, A Keohane, R Burghardt, S Ehrlich, J Hebebrand, A Hinney, A Lud
Molecular Psychiatry.2018; 23(5): 1169. CrossRef - The hypothalamus-pituitary-gonad axis: Tales of mice and men
Athina Kaprara, Ilpo T. Huhtaniemi
Metabolism.2018; 86: 3. CrossRef - Assisted reproductive techniques with congenital hypogonadotropic hypogonadism patients: a systematic review and meta-analysis
Yinjie Gao, Bingqing Yu, Jiangfeng Mao, Xi Wang, Min Nie, Xueyan Wu
BMC Endocrine Disorders.2018;[Epub] CrossRef - A novel PNPLA6 compound heterozygous mutation identified in a Chinese patient with Boucher‑Neuh�user syndrome
Ruizhi Zheng, Yaguang Zhao, Jiayu Wu, Yuanmei Wang, Jian‑Ling Liu, Zhi‑Ling Zhou, Xiao‑Tao Zhou, Dan‑Na Chen, Wei‑Hua Liao, Jia‑Da Li
Molecular Medicine Reports.2018;[Epub] CrossRef - A dominant negative FGFR1 mutation identified in a Kallmann syndrome patient
Hunjin Luo, Ruizhi Zheng, Yaguang Zhao, Jiayu Wu, Jie Li, Fang Jiang, Dan-Na Chen, Xiao-Tao Zhou, Jia-Da Li
Gene.2017; 621: 1. CrossRef - Characterization of an X-chromosomal non-mosaic monosomy (59, X0) dairy heifer detected using routinely available single nucleotide polymorphism genotype data1
D. P. Berry, A. Wolfe, J. O'Donovan, N. Byrne, R. G. Sayers, K. G. Dodds, J. C. McEwan, R. E. O'Connor, M. McClure, D. C. Purfield
Journal of Animal Science.2017; 95(3): 1042. CrossRef - Genetic basis of eugonadal and hypogonadal female reproductive disorders
Tatiana Trofimova, Daria Lizneva, Larisa Suturina, Walidah Walker, Yen-Hao Chen, Ricardo Azziz, Lawrence C. Layman
Best Practice & Research Clinical Obstetrics & Gynaecology.2017; 44: 3. CrossRef - Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility
Kashan Ahmed, Mary P. LaPierre, Emanuel Gasser, Rémy Denzler, Yinjie Yang, Thomas Rülicke, Jukka Kero, Mathieu Latreille, Markus Stoffel
Journal of Clinical Investigation.2017; 127(3): 1061. CrossRef - Next-generation sequencing of patients with congenital anosmia
Anna Alkelai, Tsviya Olender, Catherine Dode, Sagit Shushan, Pavel Tatarskyy, Edna Furman-Haran, Valery Boyko, Ruth Gross-Isseroff, Matthew Halvorsen, Lior Greenbaum, Roni Milgrom, Kazuya Yamada, Ayumi Haneishi, Ilan Blau, Doron Lancet
European Journal of Human Genetics.2017; 25(12): 1377. CrossRef - Analysis of genetic and clinical characteristics of a Chinese Kallmann syndrome cohort with ANOS1 mutations
Min Nie, Hongli Xu, Rongrong Chen, Jiangfeng Mao, Xi Wang, Shuyu Xiong, Junjie Zheng, Bingqing Yu, Mingxuan Cui, Wanlu Ma, Qibin Huang, Hongbing Zhang, Xueyan Wu
European Journal of Endocrinology.2017; 177(4): 389. CrossRef - Kallmann syndrome in pediatric otorhinolaryngology practice – Case report and literature review
Karolina Dżaman, Karolina Zborowska – Piskadło, Mirosława Pietniczka – Załęska, Ireneusz Kantor
International Journal of Pediatric Otorhinolaryngology.2017; 100: 149. CrossRef - A heterozygous microdeletion of 20p12.2–3 encompassing PROKR2 and BMP2 in a patient with congenital hypopituitarism and growth hormone deficiency
Samuel J. H. Parsons, Neville B. Wright, Emma Burkitt‐Wright, Mars S. Skae, Phillip G. Murray
American Journal of Medical Genetics Part A.2017; 173(8): 2261. CrossRef - Arachnoid cyst: a further anomaly associated with Kallmann syndrome?
Luca Massimi, Alessandro Izzo, Giovanna Paternoster, Paolo Frassanito, Concezio Di Rocco
Child's Nervous System.2016; 32(9): 1607. CrossRef - What do we learn from the murineJacob/Nsmfgene knockout for human disease?
Christina Spilker, Katarzyna M. Grochowska, Michael R. Kreutz
Rare Diseases.2016; 4(1): e1241361. CrossRef - Sexuality and quality of life in congenital hypogonadisms
María Fernanda Garrido Oyarzún, Camil Castelo-Branco
Gynecological Endocrinology.2016; 32(12): 947. CrossRef - Gene Expression Changes Underlying Idiopathic Central Hypogonadism in Cryptorchidism with Defective Mini-Puberty
Faruk Hadziselimovic, Katharina Gegenschatz-Schmid, Gilvydas Verkauskas, Maria J. Docampo-Garcia, Philippe Demougin, Vytautas Bilius, Dalius Malcius, Darius Dasevicius, Michael B. Stadtler
Sexual Development.2016; 10(3): 136. CrossRef
- In vitro modeling of cranial placode differentiation: Recent advances, challenges, and perspectives

- Obesity and Metabolism
- Low Serum Testosterone Concentrations in Hospitalized Men with Poorly Controlled Type 2 Diabetes
- Kyung-Soo Kim, San-Ha Kang, Moon-Jong Kim, Soo-Kyung Kim, Yoo-Lee Kim, Won-Keun Park, Seok Won Park, Yong-Wook Cho
- Endocrinol Metab. 2014;29(4):574-578. Published online December 29, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.4.574
- 3,357 View
- 31 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Our aim was to examine whether serum testosterone concentrations are in fact low in hospitalized men with poorly controlled type 2 diabetes compared with healthy men. In this study, 79 men aged 40 years or older (41 healthy men and 38 men with type 2 diabetes) were included. Total testosterone and sex hormone-binding globulin levels were measured. The average duration of diagnosed diabetes was 10.8 years and the mean glycated hemoglobin value was 10.8%. Total testosterone concentrations were lower in men with type 2 diabetes than in healthy men, after adjusting for age and body mass index (3.83±0.32 ng/mL vs. 5.63±0.31 ng/mL,
P <0.001). In conclusion, this study shows that serum testosterone concentrations are lower in hospitalized men with poorly controlled type 2 diabetes than in healthy men. Therefore, men with poorly controlled type 2 diabetes should undergo further assessment for hypogonadism.-
Citations
Citations to this article as recorded by- Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α
Duaa Bakhshwin, Khadija Abdul Jalil Faddladdeen, Soad Shaker Ali, Samar Mohammed Alsaggaf, Nasra Naeim Ayuob
Molecules.2022; 27(3): 1027. CrossRef - Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis
Jianzhong Zhang, Xiao Li, Zhonglin Cai, Hongjun Li, Bin Yang
The Aging Male.2020; 23(5): 607. CrossRef - Momordica charantia Extract Protects against Diabetes-Related Spermatogenic Dysfunction in Male Rats: Molecular and Biochemical Study
Gamal A. Soliman, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Hassan N. Althurwi, Reham M. Abd-Elsalam, Faisal F. Albaqami, Maged S. Abdel-Kader
Molecules.2020; 25(22): 5255. CrossRef - Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study
Gamal A. Soliman, Abdulaziz S. Saeedan, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Reham M. Abd-Elsalam, Maged S. Abdel-Kader
Saudi Pharmaceutical Journal.2019; 27(3): 326. CrossRef - Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review
Jianzhong Zhang, Bin Yang, Wenhui Xiao, Xiao Li, Hongjun Li
World Journal of Urology.2018; 36(8): 1315. CrossRef - RAS and sex differences in diabetic nephropathy
Sergi Clotet, Marta Riera, Julio Pascual, María José Soler
American Journal of Physiology-Renal Physiology.2016; 310(10): F945. CrossRef
- Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α

- Bone Metabolism
- Testosterone Replacement and Bone Mineral Density in Male Pituitary Tumor Patients
- Min Jeong Lee, Hyoung Kyu Ryu, So-Yeon An, Ja Young Jeon, Ji In Lee, Yoon-Sok Chung
- Endocrinol Metab. 2014;29(1):48-53. Published online March 14, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.1.48
- 3,522 View
- 33 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Hypopituitarism is associated with osteoporosis and osteopenia especially when hypogonadotropic hypogonadism is present. Despite hypopituitarism being an important cause of secondary osteoporosis, osteoporosis in patients receiving surgery for pituitary tumors in Korea has not been studied. In this study, we evaluated the effects of testosterone replacement therapy (TRT) on bone mineral density (BMD) in postoperative hypogonadal patients with pituitary tumors.
Methods To examine the effect of TRT on BMD, we performed a retrospective observational study in 21 postoperative male patients who underwent pituitary tumor surgery between 2003 and 2012 at the Ajou University Hospital. Testosterone was replaced in postoperative hypogonadal patients by regular intramuscular injection, daily oral medication, or application of transdermal gel. BMD (g/cm2) measurements of central skeletal sites (lumbar spine, femoral neck, and total femur) were obtained using dual-energy X-ray absorptiometry (GE Lunar). For lumbar spine BMD, L1 to L4 values were chosen for analysis. Femur neck and total femur were also analyzed.
Results During the follow-up period (mean, 56 months; range, 12 to 99 months) serum testosterone levels increased with the administration of TRT (
P =0.007). There was significant improvement (4.56%±9.81%) in the lumbar spine BMD compared to baseline BMD. There were no significant changes in the femur neck BMD or total femur BMD. We did not find any statistically significant relationships between changes in testosterone levels and BMD using Spearman correlation analysis.Conclusion Our results indicated that TRT used in the postoperative period for hypogonadal pituitary tumor surgery patients may have beneficial effects on the BMD of the spine.
-
Citations
Citations to this article as recorded by- Testosterone supplementation and bone parameters: a systematic review and meta-analysis study
G. Corona, W. Vena, A. Pizzocaro, V. A. Giagulli, D. Francomano, G. Rastrelli, G. Mazziotti, A. Aversa, A. M. Isidori, R. Pivonello, L. Vignozzi, E. Mannucci, M. Maggi, A. Ferlin
Journal of Endocrinological Investigation.2022; 45(5): 911. CrossRef - Physiological testosterone replacement effects on male aged rats with orchiectomy-induced osteoporosis in advanced stage: a tomographic and biomechanical pilot study
Vinícius de Paiva Gonçalves, Adriana Alicia Cabrera-Ortega, Jhonatan de Souza Carvalho, Dania Ramadan, Luís Carlos Spolidorio
The Aging Male.2021; 24(1): 139. CrossRef - Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy
Jia-Feng Chen, Pei-Wen Lin, Yi-Ru Tsai, Yi-Chien Yang, Hong-Yo Kang
Cells.2019; 8(11): 1318. CrossRef - Testosterone and male rejuvenation
Sevann Helo, Peyton Thomas, Nicholas N. Tadros
Panminerva Medica.2019;[Epub] CrossRef - Systemic Non-Reproductive Effects of Sex Steroids in Adult Males and Females
Syed Imran Ali Shah
Human Physiology.2018; 44(1): 83. CrossRef - Benefits and Health Implications of Testosterone Therapy in Men With Testosterone Deficiency
Abdulmaged M. Traish
Sexual Medicine Reviews.2018; 6(1): 86. CrossRef - Multiple Fractures in Patient with Graves' Disease Accompanied by Isolated Hypogonadotropic Hypogonadism
Hyon-Seung Yi, Ji Min Kim, Sang Hyeon Ju, Younghak Lee, Hyun Jin Kim, Koon Soon Kim
Journal of Bone Metabolism.2016; 23(1): 40. CrossRef - Severity and pattern of bone mineral loss in endocrine causes of osteoporosis as compared to age-related bone mineral loss
D Dutta, P Dharmshaktu, A Aggarwal, K Gaurav, R Bansal, N Devru, UC Garga, B Kulshreshtha
Journal of Postgraduate Medicine.2016; 62(3): 162. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Bone health in hypogonadal men
Michael S. Irwig
Current Opinion in Urology.2014; 24(6): 608. CrossRef - Testosterone Replacement Therapy and Bone Mineral Density in Men with Hypogonadism
Se Hwa Kim
Endocrinology and Metabolism.2014; 29(1): 30. CrossRef
- Testosterone supplementation and bone parameters: a systematic review and meta-analysis study

- Androgen Receptor Gene CAG Repeat Polymorphism and Effect of Testosterone Therapy in Hypogonadal Men in Korea.
- Min Joo Kim, Jin Taek Kim, Sun Wook Cho, Sang Wan Kim, Chan Soo Shin, Kyong Soo Park, Seong Yeon Kim
- Endocrinol Metab. 2011;26(3):225-231. Published online September 1, 2011
- DOI: https://doi.org/10.3803/EnM.2011.26.3.225
- 2,038 View
- 25 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
As the number of CAG repeats in the androgen receptor (AR) gene increases, transcriptional activities decrease and the effects of testosterone decline. In this study, we evaluated the importance of the CAG repeat polymorphism in regard to the effect/safety of testosterone therapy in hypogonadal Korean men. METHODS: The number of CAG repeats was determined in 42 hypogonadal men who underwent testosterone therapy for more than 24 months between December 1999 and August 2007. Body mass index, lean body mass, body fat, bone mineral density, type I collagen N-telopeptide (NTx), osteocalcin, lipid profile, hematocrit and PSA levels prior to and after 24 months of testosterone therapy were identified in our medical record review. RESULTS: Twenty-four months of testosterone therapy increased lean body mass, hematocrit, and PSA levels and reduced body fat, NTx, and HDL cholesterol levels. The mean number of CAG repeats in the AR gene was 23 +/- 3 (range, 15-29) in hypogonadal Korean men. The number of CAG repeats was not found to be associated with changes in lean body mass, body fat, NTx, HDL cholesterol, hematocrit, or PSA levels during testosterone therapy. CONCLUSIONS: No association between the number of CAG repeats in the AR gene and the effect/safety of testosterone therapy was detected in hypogonadal Korean men. -
Citations
Citations to this article as recorded by- Androgen Receptor CAG Repeat Length as a Risk Factor of Late-Onset Hypogonadism in a Korean Male Population
Jong Wook Kim, Young Dae Bae, Sun Tae Ahn, Jin Wook Kim, Je Jong Kim, Du Geon Moon
Sexual Medicine.2018; 6(3): 203. CrossRef - Positive Correlation between Androgen Receptor CAG Repeat Length and Metabolic Syndrome in a Korean Male Population
Jong Wook Kim, Young Dae Bae, Sun Tae Ahn, Jin Wook Kim, Je Jong Kim, Du Geon Moon
The World Journal of Men's Health.2018; 36(1): 73. CrossRef - Genome-Based Approaches in Endocrinology and Metabolism: From Clinical and Research Aspects
Sihoon Lee
Endocrinology and Metabolism.2011; 26(3): 208. CrossRef
- Androgen Receptor CAG Repeat Length as a Risk Factor of Late-Onset Hypogonadism in a Korean Male Population

- A Case of Ectopic Neurohypophysis Presenting with Hypogonadism.
- In Woon Baek, Ji Hyun Kim, Guk Jin Lee, Kyoung Eun Lee, Hae Lim Lee, Hye Won Lee, Nam Yong Kim, Yon Kwon Ihn, Seung Hyun Ko, Seung Hwan Lee, Je Ho Han
- Endocrinol Metab. 2011;26(1):67-71. Published online March 1, 2011
- DOI: https://doi.org/10.3803/EnM.2011.26.1.67
- 2,216 View
- 27 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Pituitary stalk interruption and ectopic neurohypophysis seen on magnetic resonance Imaging (MRI) are often associated with either isolated growth hormone (GH) deficiency or combined anterior pituitary hormone deficiency, but their pathogenesis is not clear and the clinical data regarding these anatomical defect is limited. We experienced a 23-year-old male with the absence of secondary sexual characteristics and this was accompanied with pituitary stalk dysgenesis and ectopic neurohypophysis. He received growth hormone for a year when he was 12 years old due to his short stature. Sella MRI showed no visible pituitary stalk with minimal high signal change, suggesting ectopic neurohypophysis. The combined pituitary stimulation test revealed blunted responses of growth hormone, follicle stimulating hormone and luteinizing hormone. For the hypogonadotropic hypogonadism, the patient was given testosterone intramuscularly and he gradually developed secondary sexual characteristics. We concluded that the hypogonadism and growth hormone deficiency in this patient was caused by hypopituitarism due to pituitary stalk dysgenesis and ecopic nuerohypophysis.
-
Citations
Citations to this article as recorded by- MRI of ectopic posterior pituitary gland with dysgenesis of pituitary stalk in a patient with hypogonadotropic hypogonadism
Ashim Kumar Lahiri, Ramanivas Sundareyan, David Jenkins, Anjumara Nilak
Radiology Case Reports.2018; 13(4): 764. CrossRef - Hypothalamic Hypopituitarism Caused by Pituitary Stalk Dysgenesis
Seong-Ju Lee, Hye-Jin Yoon, A-Reum Cho, Yoo-Jin Um, Keun-Young Park, Dong-Mee Lim, Byung-Joon Kim
Korean Journal of Medicine.2013; 85(4): 420. CrossRef
- MRI of ectopic posterior pituitary gland with dysgenesis of pituitary stalk in a patient with hypogonadotropic hypogonadism

- Primary Hypogonadism Associated with Ankylosing Spondylitis.
- Byoung Yeon Jun, Guk Jin Lee, Ji Hyun Kim, Jung Min Lee, Sang Ah Chang
- J Korean Endocr Soc. 2008;23(5):352-357. Published online October 1, 2008
- DOI: https://doi.org/10.3803/jkes.2008.23.5.352
- 1,817 View
- 24 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Male patients with hypogonadism have an increased risk of developing rheumatic diseases. Most causes of hypogonadism related with rheumatic disease are karyotype abnormality such as Klinefelter's syndrome or Turner's syndrome and gonadal dysgenesis. A 24-year-old year male was admitted for pain of both hip joints that had worsened over 2 months. He had hip joint involvement from ankylosing spondylitis and did not show secondary sex characteristics. His sex hormones and gonadotropins levels indicated hypergonadotropic hypogonadism. The karyotype was 46 XY, and there was no obvious cause of hypogonadism. Here we report on clinical features of this first Korean case of primary hypogonadism accompanying ankylosing spondylitis.
-
Citations
Citations to this article as recorded by- Ankylosing spondylitis associated with balanced reciprocal X-1 translocation
Young Hoon Kim, Jung Ouk Lee
Yeungnam University Journal of Medicine.2017; 34(1): 80. CrossRef - A Case of Klinefelter's Syndrome Accompanying with Polymyositis
Min Kyu Lee, Byung Sik Kim, Suk Hyun Jung, Gun Hwa Lee, Jin Ok Kim, Dong Hwi Rim, Yu Hwa Lee, Woong Jun Kim, So-Young Bang, Hye-Soon Lee
Journal of Rheumatic Diseases.2012; 19(3): 152. CrossRef
- Ankylosing spondylitis associated with balanced reciprocal X-1 translocation

- A Case of Patient with Opioid-Induced Adrenocortical Insufficiency and Hypogonadism.
- Hai Jin Kim, Chul Sik Kim, Jong Suk Park, Jina Park, Eun Seok Kang, Chul Woo Ahn, Bong Soo Cha, Sung Kil Lim, Kyung Rae Kim, Hyun Chul Lee
- J Korean Endocr Soc. 2006;21(3):257-260. Published online June 1, 2006
- DOI: https://doi.org/10.3803/jkes.2006.21.3.257
- 1,816 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Opioids are known to decrease plasma cortisol and testosterone level in human and other mammals. Nowadays, opioid use is exponentially increasing, but little is known about its side effects. With the help of progressive human science, we can habit longer life and as result, are becoming more avid for healthy life. In this respect, analgesics play important role in maintaining good and healthy quality of life. For this reason, it is important to fully understand its side effects and handle it with special precaution. We are reporting a 22-year-old male who had been taken opioid analgesic for more than six years to relieve chronic, intractable headache. Then, his hormone test revealed hypogonadotropic hypogonadism combined with hypoadrenocorticotropic hypoadrenalism but showed no definite clinical features except for sexual frigidity. After two years of oxycodon discontinuation, we reevaluated that his hormone test, and all other laboratory tests returned to the normal range.

- Relationship between Adiponectin, Leptin and Body Fat in Men with Hypogonadism Before and After Testosterone Treatment.
- Sang Wan Kim, Joon Ku Kang, Do Joon Park, Chan Soo Shin, Kyung Soo Park, Seong Yeon Kim, Bo Youn Cho, Hong Kyu Lee
- J Korean Endocr Soc. 2004;19(5):473-484. Published online October 1, 2004
- 1,110 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Testosterone replacement therapy in men with hypogonadism improves sexual function, decreases body fat, and increases the mass and function of lean muscle. These beneficial effects of testosterone replacement therapy are accompanied by slight lowering of the high density lipoprotein (HDL) cholesterol levels, increase in the hematocrit/hemoglobin ratio and size of the prostate gland. It is presently unknown whether the effect of testosterone on body fat could also reduce the risk of atherosclerotic disease associated with obesity. We investigated the relationship between body fat and blood leptin and adiponectin levels to elucidate the effect of testosterone on body fat metabolism, as well as the effect of testosterone on lipid and bone metabolism. METHODS: We selected 28 men, who were hypogonadal (mean serum testosterone+/-SD, 22.3+/-35.3 ng/dL) due to an organic disease, and them with oral testosterone (testosterone undecanoate) for 12 months. We measured the body composition, serum leptin, plasma adiponectin, biochemical bone markers, bone mineral density, prostate-specific antigen, and serum lipids before and 3, 6 and 12 months after treatment. We analyzed the relationship between body fat and blood leptin and adiponectin levels. RESULTS: The mean serum testosterone concentration reached the subnormal range after 6 months of treatment, which remained for the duration of treatment. The fat mass decreased and muscle mass increased, not within the first 6 months, but principally within 12 months (p<0.05). Although the decrease in the serum leptin level was not statistically significant, there were positive correlations between the leptin level and fat mass before and after 6 months of treatment (p<0.05). The plasma adiponectin did not increase or correlate with body fat parameters. The bone mineral densities of the lumbar spine (L2-L4) and femoral neck did not increased, but the serum osteocalcin and urine N-telopeptide were significantly decreased (p<0.05 and <0.01, respectively). The HDL-cholesterol decreased, principally within the first 6 months (p<0.01), but the total and LDL cholesterols, and the triglycerides remained unchanged during the course of treatment. There was also no change in prostate-specific antigen. CONCLUSION: Twelve months of oral testosterone replacement in men with hypogonadism improved body composition and bone metabolism, but demonstrated subnormal serum testosterone levels, had no effect on the leptin and adiponectin levels and decrease in HDL-cholesterol levels. It will be necessary to examine the long-term effects of testosterone replacement on the incidence of cardiovascular events as well as cardiovascular risk factors in men with hypogonadism

- A Case of Type II Autoimmune Polyglandular Syndrome: Acute adrenal crisis presented as the first manifestation of Addison's disease in a patient with diabetic ketoacidosis and hypgonadism.
- Young Sook Lee, Jong Min Lee, Hyun Ok Park, Sung Kyu Park, Sung Ro Yoon, Seok Young Kim, Bong Yeon Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- J Korean Endocr Soc. 1998;13(1):115-120. Published online January 1, 2001
- 1,021 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Type II autoimmune polyglandular syndrome typically presents in adulthood. Insulin dependent diabetes mellitus and thyroid dysfunction are the most frequent manifestations. Addison's disease is the third major endocrine component of this disorder. In this report, we described a thirty-two year-old male patient who had hypogonadism, insulin dependent diabetes mellitus, and mild Addison's disease presenting its first manifestation as an acute adrenal crisis due to diabetic ketoacidosis. The ACTH concentration will be elevated early in the course of Addisons disease even before a significant reduction in the basal cortisol level or its response to exogenous ACTH occurs. Therefore, plasma ACTH measurements serve as a valuable screening study for Addisons disease.


 KES
KES

 First
First Prev
Prev



