Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Inhibition of Fatty Acid β-Oxidation by Fatty Acid Binding Protein 4 Induces Ferroptosis in HK2 Cells Under High Glucose Conditions
- Jiasi Chen, Keping Wu, Yan Lei, Mingcheng Huang, Lokyu Cheng, Hui Guan, Jiawen Lin, Ming Zhong, Xiaohua Wang, Zhihua Zheng
- Endocrinol Metab. 2023;38(2):226-244. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1604
- 3,607 View
- 179 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
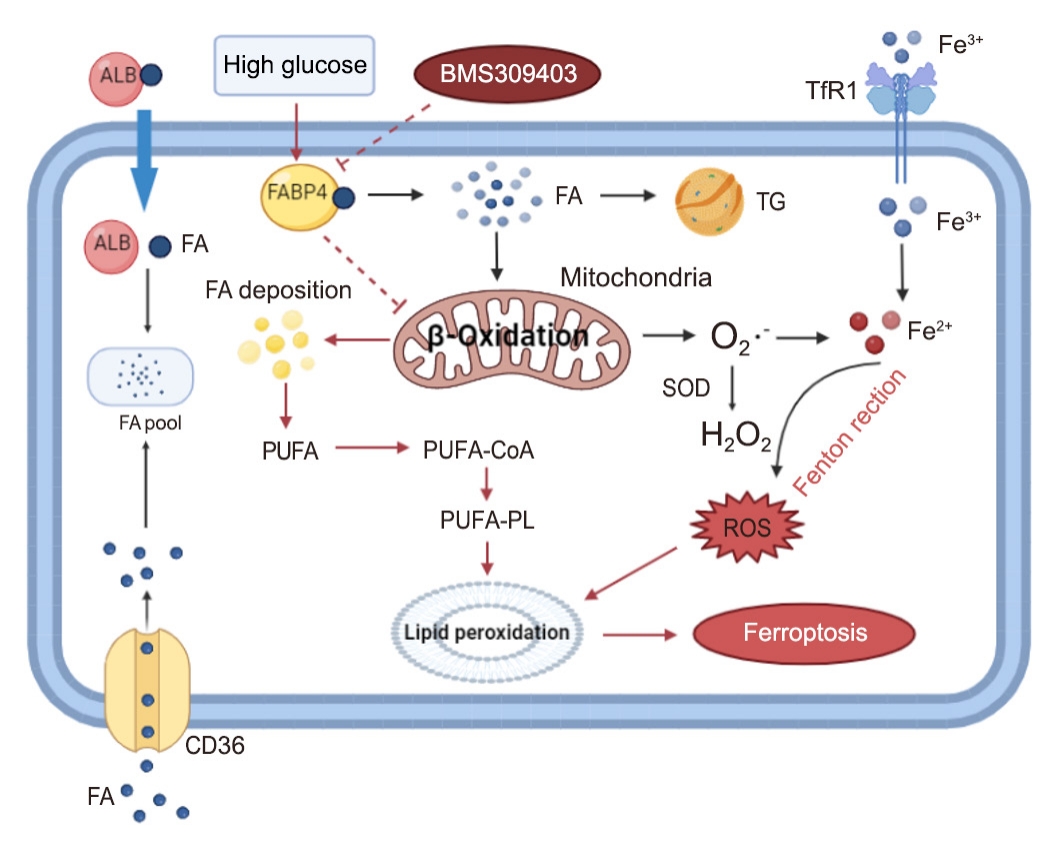
Ferroptosis, which is caused by an iron-dependent accumulation of lipid hydroperoxides, is a type of cell death linked to diabetic kidney disease (DKD). Previous research has shown that fatty acid binding protein 4 (FABP4) is involved in the regulation of ferroptosis in diabetic retinopathy. The present study was constructed to explore the role of FABP4 in the regulation of ferroptosis in DKD.
Methods
We first detected the expression of FABP4 and proteins related to ferroptosis in renal biopsies of patients with DKD. Then, we used a FABP4 inhibitor and small interfering RNA to investigate the role of FABP4 in ferroptosis induced by high glucose in human renal proximal tubular epithelial (HG-HK2) cells.
Results
In kidney biopsies of DKD patients, the expression of FABP4 was elevated, whereas carnitine palmitoyltransferase-1A (CP-T1A), glutathione peroxidase 4, ferritin heavy chain, and ferritin light chain showed reduced expression. In HG-HK2 cells, the induction of ferroptosis was accompanied by an increase in FABP4. Inhibition of FABP4 in HG-HK2 cells changed the redox state, sup-pressing the production of reactive oxygen species, ferrous iron (Fe2+), and malondialdehyde, increasing superoxide dismutase, and reversing ferroptosis-associated mitochondrial damage. The inhibition of FABP4 also increased the expression of CPT1A, reversed lipid deposition, and restored impaired fatty acid β-oxidation. In addition, the inhibition of CPT1A could induce ferroptosis in HK2 cells.
Conclusion
Our results suggest that FABP4 mediates ferroptosis in HG-HK2 cells by inhibiting fatty acid β-oxidation. -
Citations
Citations to this article as recorded by- Fatty Acid Binding Protein-4 Silencing Inhibits Ferroptosis to Alleviate Lipopolysaccharide-induced Injury of Renal Tubular Epithelial Cells by Blocking Janus Kinase 2/Signal Transducer and Activator of Transcription 3 Signaling
Suo Xu, Jiye Luo, Yanli Wang, Xiaobing Chen
Chinese Journal of Physiology.2024; 67(1): 47. CrossRef - Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications
Zhili Wu, Yanru Zhu, Wenchao Liu, Balamuralikrishnan Balasubramanian, Xiao Xu, Junhu Yao, Xinjian Lei
Antioxidants.2024; 13(3): 352. CrossRef - Mechanisms and regulations of ferroptosis
Xu-Dong Zhang, Zhong-Yuan Liu, Mao-Sen Wang, Yu-Xiang Guo, Xiang-Kun Wang, Kai Luo, Shuai Huang, Ren-Feng Li
Frontiers in Immunology.2023;[Epub] CrossRef - Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases
Yumin Wang, Jing Hu, Shuang Wu, Joshua S. Fleishman, Yulin Li, Yinshi Xu, Wailong Zou, Jinhua Wang, Yukuan Feng, Jichao Chen, Hongquan Wang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef
- Fatty Acid Binding Protein-4 Silencing Inhibits Ferroptosis to Alleviate Lipopolysaccharide-induced Injury of Renal Tubular Epithelial Cells by Blocking Janus Kinase 2/Signal Transducer and Activator of Transcription 3 Signaling

- Ghrelin Inhibits Oligodendrocyte Cell Death by Attenuating Microglial Activation
- Jee Youn Lee, Tae Young Yune
- Endocrinol Metab. 2014;29(3):371-378. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.371
- 4,304 View
- 30 Download
- 25 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Recently, we reported the antiapoptotic effect of ghrelin in spinal cord injury-induced apoptotic cell death of oligodendrocytes. However, how ghrelin inhibits oligodendrocytes apoptosis, is still unknown. Therefore, in the present study, we examined whether ghrelin inhibits microglia activation and thereby inhibits oligodendrocyte apoptosis.
Methods Using total cell extracts prepared from BV-2 cells activated by lipopolysaccharide (LPS) with or without ghrelin, the levels of p-p38 phosphor-p38 mitogen-activated protein kinase (p-p38MAPK), phospho-c-Jun N-terminal kinase (pJNK), p-c-Jun, and pro-nerve growth factor (proNGF) were examined by Western blot analysis. Reactive oxygen species (ROS) production was investigated by using dichlorodihydrofluorescein diacetate. To examine the effect of ghrelin on oligodendrocyte cell death, oligodendrocytes were cocultured in transwell chambers of 24-well plates with LPS-stimulated BV-2 cells. After 48 hours incubation, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and terminal deoxynucleotidyl transferase 2'-deoxyuridine, 5'-triphosphate nick end labeling staining were assessed.
Results Ghrelin treatment significantly decreased levels of p-p38MAPK, p-JNK, p-c-Jun, and proNGF in LPS-stimulated BV-2 cells. ROS production increased in LPS-stimulated BV-2 cells was also significantly inhibited by ghrelin treatment. In addition, ghrelin significantly inhibited oligodendrocyte cell death when cocultured with LPS-stimulated BV-2 cells.
Conclusion Ghrelin inhibits oligodendrocyte cell death by decreasing proNGF and ROS production as well as p38MAPK and JNK activation in activated microglia as an anti-inflammatory hormone.
-
Citations
Citations to this article as recorded by- Ghrelin Represses Thymic Stromal Lymphopoietin Gene Expression through Activation of Glucocorticoid Receptor and Protein Kinase C Delta in Inflamed Skin Keratinocytes
Hayan Jeong, Hyo-Jin Chong, Jangho So, Yejin Jo, Tae-Young Yune, Bong-Gun Ju
International Journal of Molecular Sciences.2022; 23(7): 3977. CrossRef - Inflammation: A Target for Treatment in Spinal Cord Injury
Ximena Freyermuth-Trujillo, Julia J. Segura-Uribe, Hermelinda Salgado-Ceballos, Carlos E. Orozco-Barrios, Angélica Coyoy-Salgado
Cells.2022; 11(17): 2692. CrossRef - The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases
Cristina Russo, Maria Stella Valle, Antonella Russo, Lucia Malaguarnera
International Journal of Molecular Sciences.2022; 23(21): 13432. CrossRef - Early low-dose ghrelin intervention via miniosmotic pumps could protect against the progressive dopaminergic neuron loss in Parkinson's disease mice
Lingling Jiao, Xixun Du, Fengju Jia, Yong Li, Dexiao Zhu, Tinging Tang, Qian Jiao, Hong Jiang
Neurobiology of Aging.2021; 101: 70. CrossRef - Ghrelin-Mediated Regeneration and Plasticity After Nervous System Injury
Irina Stoyanova, David Lutz
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Microglial Lipid Biology in the Hypothalamic Regulation of Metabolic Homeostasis
Andrew Folick, Suneil K. Koliwad, Martin Valdearcos
Frontiers in Endocrinology.2021;[Epub] CrossRef - Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer's and Parkinson's Disease
Niklas Reich, Christian Hölscher
Frontiers in Neuroscience.2020;[Epub] CrossRef - Effects of Ghrelin on the Apoptosis of Rheumatoid Arthritis Fibroblast-Like Synoviocyte MH7A Cells
Junxian Ma, Xinbo Wang, Tingting Lv, Jie Liu, Ying Ren, Jinshan Zhang, Yan Zhang
Biological and Pharmaceutical Bulletin.2019; 42(2): 158. CrossRef - Direct and indirect effects of lipids on microglia function
Q. Leyrolle, S. Layé, A. Nadjar
Neuroscience Letters.2019; 708: 134348. CrossRef - Dopamine neuronal protection in the mouse Substantia nigra by GHSR is independent of electric activity
Bernardo Stutz, Carole Nasrallah, Mariana Nigro, Daniel Curry, Zhong-Wu Liu, Xiao-Bing Gao, John D. Elsworth, Liat Mintz, Tamas L. Horvath
Molecular Metabolism.2019; 24: 120. CrossRef - MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer’s Disease
Yu-on Jeong, Soo Shin, Jun Park, Bo Ku, Ji Song, Jwa-Jin Kim, Seong Jeon, Sang Lee, Minho Moon
International Journal of Molecular Sciences.2018; 19(6): 1800. CrossRef - Involvement of Astrocytes in Mediating the Central Effects of Ghrelin
Laura Frago, Julie Chowen
International Journal of Molecular Sciences.2017; 18(3): 536. CrossRef - The neurological effects of ghrelin in brain diseases: Beyond metabolic functions
Qian Jiao, Xixun Du, Yong Li, Bing Gong, Limin Shi, Tingting Tang, Hong Jiang
Neuroscience & Biobehavioral Reviews.2017; 73: 98. CrossRef - Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases
Lila Carniglia, Delia Ramírez, Daniela Durand, Julieta Saba, Juan Turati, Carla Caruso, Teresa N. Scimonelli, Mercedes Lasaga
Mediators of Inflammation.2017; 2017: 1. CrossRef - Non-Neuronal Cells in the Hypothalamic Adaptation to Metabolic Signals
Alejandra Freire-Regatillo, Pilar Argente-Arizón, Jesús Argente, Luis Miguel García-Segura, Julie A. Chowen
Frontiers in Endocrinology.2017;[Epub] CrossRef - The Ghrelin/GOAT System Regulates Obesity-Induced Inflammation in Male Mice
Rebecca E. Harvey, Victor G. Howard, Moyra B. Lemus, Tara Jois, Zane B. Andrews, Mark W. Sleeman
Endocrinology.2017; 158(7): 2179. CrossRef - Central Modulation of Neuroinflammation by Neuropeptides and Energy-Sensing Hormones during Obesity
Roger Maldonado-Ruiz, Lizeth Fuentes-Mera, Alberto Camacho
BioMed Research International.2017; 2017: 1. CrossRef - Lifestyle Shapes the Dialogue between Environment, Microglia, and Adult Neurogenesis
Jorge Valero, Iñaki Paris, Amanda Sierra
ACS Chemical Neuroscience.2016; 7(4): 442. CrossRef - Signaling of ghrelin and its functional receptor, the growth hormone secretagogue receptor, promote tumor growth in glioblastomas
Yousuke Okada, Yasuo Sugita, Koichi Ohshima, Motohiro Morioka, Satoru Komaki, Junko Miyoshi, Hideyuki Abe
Neuropathology.2016; 36(6): 535. CrossRef - Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson's Disease
Jacqueline A. Bayliss, Moyra B. Lemus, Romana Stark, Vanessa V. Santos, Aiysha Thompson, Daniel J. Rees, Sandra Galic, John D. Elsworth, Bruce E. Kemp, Jeffrey S. Davies, Zane B. Andrews
The Journal of Neuroscience.2016; 36(10): 3049. CrossRef - MMP-3 secreted from endothelial cells of blood vessels after spinal cord injury activates microglia, leading to oligodendrocyte cell death
Jee Y. Lee, Hae Y. Choi, Tae Y. Yune
Neurobiology of Disease.2015; 82: 141. CrossRef - Role of Non-Neuronal Cells in Body Weight and Appetite Control
Pilar Argente-Arizón, Alejandra Freire-Regatillo, Jesús Argente, Julie A. Chowen
Frontiers in Endocrinology.2015;[Epub] CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Ghrelin Represses Thymic Stromal Lymphopoietin Gene Expression through Activation of Glucocorticoid Receptor and Protein Kinase C Delta in Inflamed Skin Keratinocytes

- Adrenal gland
- Role of Reactive Oxygen Species in Hypothalamic Regulation of Energy Metabolism
- Sabrina Diano
- Endocrinol Metab. 2013;28(1):3-5. Published online March 25, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.1.3
- 3,947 View
- 43 Download
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader To understand the etiology of metabolic disorders, including obesity and type II diabetes, it is essential to gain better insight into how stored and available energy sources are monitored by the central nervous system. In particular, a comprehension of the fine cellular interplay and intracellular mechanisms that enable appropriate hypothalamic and consequent endocrine and behavioral responses to both circulating hormonal and nutrient signals remains elusive. Recent data, including those from our laboratories, raised the notion that reactive oxygen species (ROS) generation is not merely a by-product of substrate oxidation, but it plays a crucial role in modulating cellular responses involved in the regulation of energy metabolism. These review summarizes the published recent data on the effect of ROS levels in the regulation of neuronal function, including that of hypothalamic melanocortin neurons, pro-opiomelanocortin and neuropeptide Y-/agouti related peptide-neurons, in the modulation of food intake.
-
Citations
Citations to this article as recorded by- Regulation of hypothalamic reactive oxygen species and feeding behavior by phosphorylation of the beta 2 thyroid hormone receptor isoform
Svetlana Minakhina, Sun Young Kim, Fredric E. Wondisford
Scientific Reports.2024;[Epub] CrossRef - Overexpression of PpmTERF18 enhances the antioxidant capacity of peach fruit to alleviate oxidative damage
Xiaoshan Guo, Guangqin Jing, Shuhua Zhu, Jianrong Feng, Dandan Huang
Scientia Horticulturae.2023; 318: 112123. CrossRef - Insights into the promising prospect of pharmacological approaches targeting mitochondrial dysfunction in major human diseases: At a glance
Md.Mominur Rahman, Md.Taslim Sarker, Sabbir Ahmed, Md.Nur Uddin, Md.Shariful Islam, Md.Rezaul Islam, Shanto Das, Nobendu Mukherjee, Hassan A. Hemeg, Abdur Rauf, Bimal Kumar Ghimire, Muthu Thiruvengadam
Process Biochemistry.2023; 132: 41. CrossRef - Structure-based analysis and rational design of human peroxiredoxin-1's C-terminus-derived peptides to target sulfiredoxin-1 in pancreatic cancer
Xiaoqiong Wu, Rongyuan Qiu, Wei Yi, Juan Chen, Zhou Zhang, Ji Zhang, Zhiyuan Zhu
Biophysical Chemistry.2022; 288: 106857. CrossRef - Drp1 is required for AgRP neuronal activity and feeding
Sungho Jin, Nal Ae Yoon, Zhong-Wu Liu, Jae Eun Song, Tamas L Horvath, Jung Dae Kim, Sabrina Diano
eLife.2021;[Epub] CrossRef - Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction
Shuai Wang, Kuntan Wu, Dongfang Xue, Cong Zhang, Shahid Ali Rajput, Desheng Qi
Food and Chemical Toxicology.2021; 153: 112214. CrossRef - Redox Signaling from and to Peroxisomes: Progress, Challenges, and Prospects
Marc Fransen, Celien Lismont
Antioxidants & Redox Signaling.2019; 30(1): 95. CrossRef - The melanocortin pathway and control of appetite-progress and therapeutic implications
Giulia Baldini, Kevin D Phelan
Journal of Endocrinology.2019; 241(1): R1. CrossRef - Melanocortin Receptor 4 Signaling Regulates Vertebrate Limb Regeneration
Mengshi Zhang, Youwei Chen, Hanqian Xu, Li Yang, Feng Yuan, Lei Li, Ying Xu, Ying Chen, Chao Zhang, Gufa Lin
Developmental Cell.2018; 46(4): 397. CrossRef - Hypothalamic Mitochondrial Dysfunction as a Target in Obesity and Metabolic Disease
Juan Cunarro, Sabela Casado, Javier Lugilde, Sulay Tovar
Frontiers in Endocrinology.2018;[Epub] CrossRef - Role of oxidative stress and antioxidants in daily nutrition and human health
Geir Bjørklund, Salvatore Chirumbolo
Nutrition.2017; 33: 311. CrossRef - DRP1 Suppresses Leptin and Glucose Sensing of POMC Neurons
Anna Santoro, Michela Campolo, Chen Liu, Hiromi Sesaki, Rosaria Meli, Zhong-Wu Liu, Jung Dae Kim, Sabrina Diano
Cell Metabolism.2017; 25(3): 647. CrossRef - Administration of a leptin antagonist during the neonatal leptin surge induces alterations in the redox and inflammatory state in peripubertal /adolescent rats
Virginia Mela, Oskarina Hernandez, Caroline Hunsche, Francisca Diaz, Julie A. Chowen, Mónica De la Fuente
Molecular and Cellular Endocrinology.2017; 454: 125. CrossRef - Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells
Hye-Jin Jung, Seung-Soon Im, Dae-Kyu Song, Jae-Hoon Bae
BMB Reports.2017; 50(6): 323. CrossRef - Trimetazidine attenuates pressure overload-induced early cardiac energy dysfunction via regulation of neuropeptide Y system in a rat model of abdominal aortic constriction
Ailan Chen, Wanglin Li, Xinyu Chen, Yuechun Shen, Wenjun Dai, Qi Dong, Xinchun Li, Caiwen Ou, Minsheng Chen
BMC Cardiovascular Disorders.2016;[Epub] CrossRef - Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages
Seung-Jae Kim, Ji-Young Cha, Hye Suk Kang, Jae-Ho Lee, Ji Yoon Lee, Jae-Hyung Park, Jae-Hoon Bae, Dae-Kyu Song, Seung-Soon Im
BMB Reports.2016; 49(5): 276. CrossRef - The sulfiredoxin–peroxiredoxin (Srx–Prx) axis in cell signal transduction and cancer development
Murli Mishra, Hong Jiang, Lisha Wu, Hedy A. Chawsheen, Qiou Wei
Cancer Letters.2015; 366(2): 150. CrossRef - Effects of methanolic extracts of edible plants on RAGE in high-glucose-induced human endothelial cells
Mizue Okada, Yoshinori Okada
Bio-Medical Materials and Engineering.2015; 25(3): 257. CrossRef - Reactive oxygen species are physiological mediators of the noradrenergic signaling pathway in the mouse supraoptic nucleus
Ronald St-Louis, Caroline Parmentier, Valérie Grange-Messent, Sakina Mhaouty-Kodja, Hélène Hardin-Pouzet
Free Radical Biology and Medicine.2014; 71: 231. CrossRef - Reactive oxygen species play a role in muscle wasting during thyrotoxicosis
Sara Santos Bernardes, Flávia Alessandra Guarnier, Poliana Camila Marinello, André Armani, Andréa Name Colado Simão, Rubens Cecchini, Alessandra Lourenço Cecchini
Cell and Tissue Research.2014; 357(3): 803. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef
- Regulation of hypothalamic reactive oxygen species and feeding behavior by phosphorylation of the beta 2 thyroid hormone receptor isoform


 KES
KES

 First
First Prev
Prev



