Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Scaling Insulin-Producing Cells by Multiple Strategies
- Jinhyuk Choi, Fritz Cayabyab, Harvey Perez, Eiji Yoshihara
- Endocrinol Metab. 2024;39(2):191-205. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1910
- 1,286 View
- 72 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
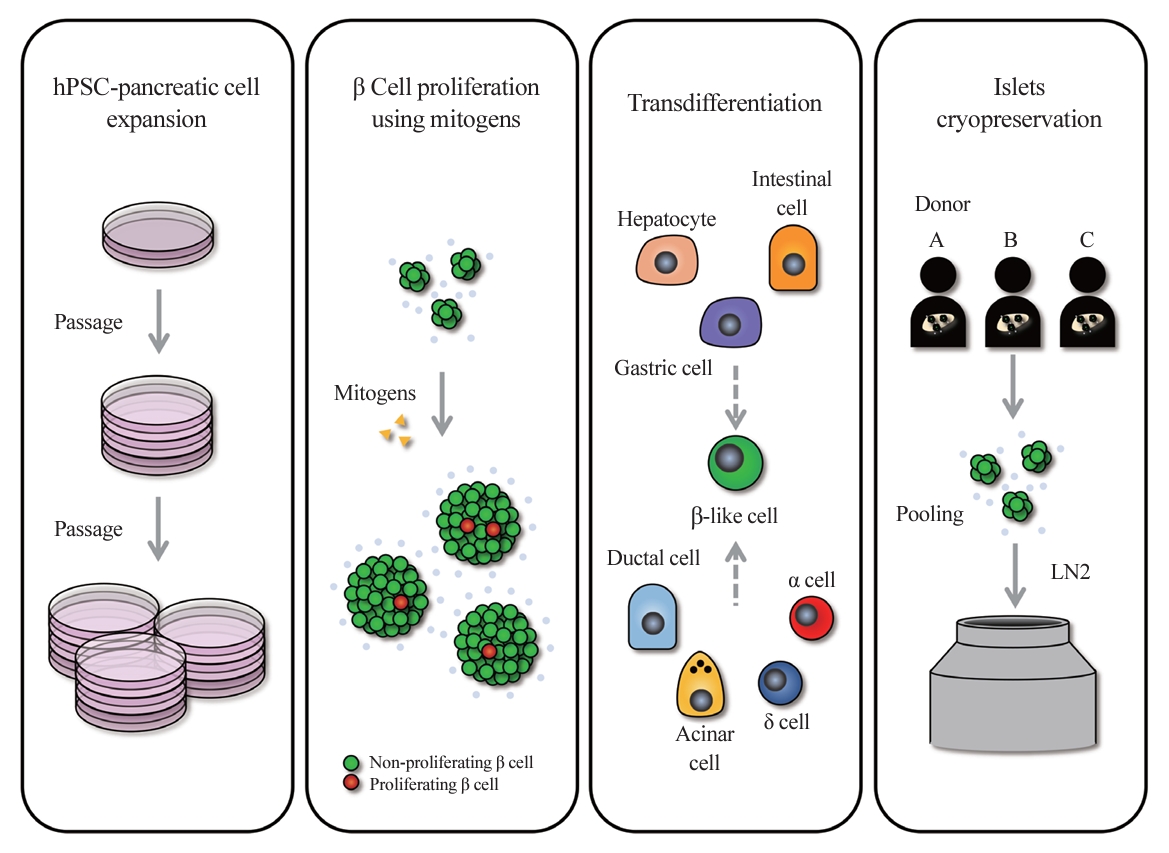
ePub - In the quest to combat insulin-dependent diabetes mellitus (IDDM), allogenic pancreatic islet cell therapy sourced from deceased donors represents a significant therapeutic advance. However, the applicability of this approach is hampered by donor scarcity and the demand for sustained immunosuppression. Human induced pluripotent stem cells are a game-changing resource for generating synthetic functional insulin-producing β cells. In addition, novel methodologies allow the direct expansion of pancreatic progenitors and mature β cells, thereby circumventing prolonged differentiation. Nevertheless, achieving practical reproducibility and scalability presents a substantial challenge for this technology. As these innovative approaches become more prominent, it is crucial to thoroughly evaluate existing expansion techniques with an emphasis on their optimization and scalability. This manuscript delineates these cutting-edge advancements, offers a critical analysis of the prevailing strategies, and underscores pivotal challenges, including cost-efficiency and logistical issues. Our insights provide a roadmap, elucidating both the promises and the imperatives in harnessing the potential of these cellular therapies for IDDM.

- Diabetes, Obesity and Metabolism
- Human Tissue-Engineered Skeletal Muscle: A Tool for Metabolic Research
- Ji-Hoon Kim, Seung-Min Yu, Jang Won Son
- Endocrinol Metab. 2022;37(3):408-414. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.302
- 4,057 View
- 163 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
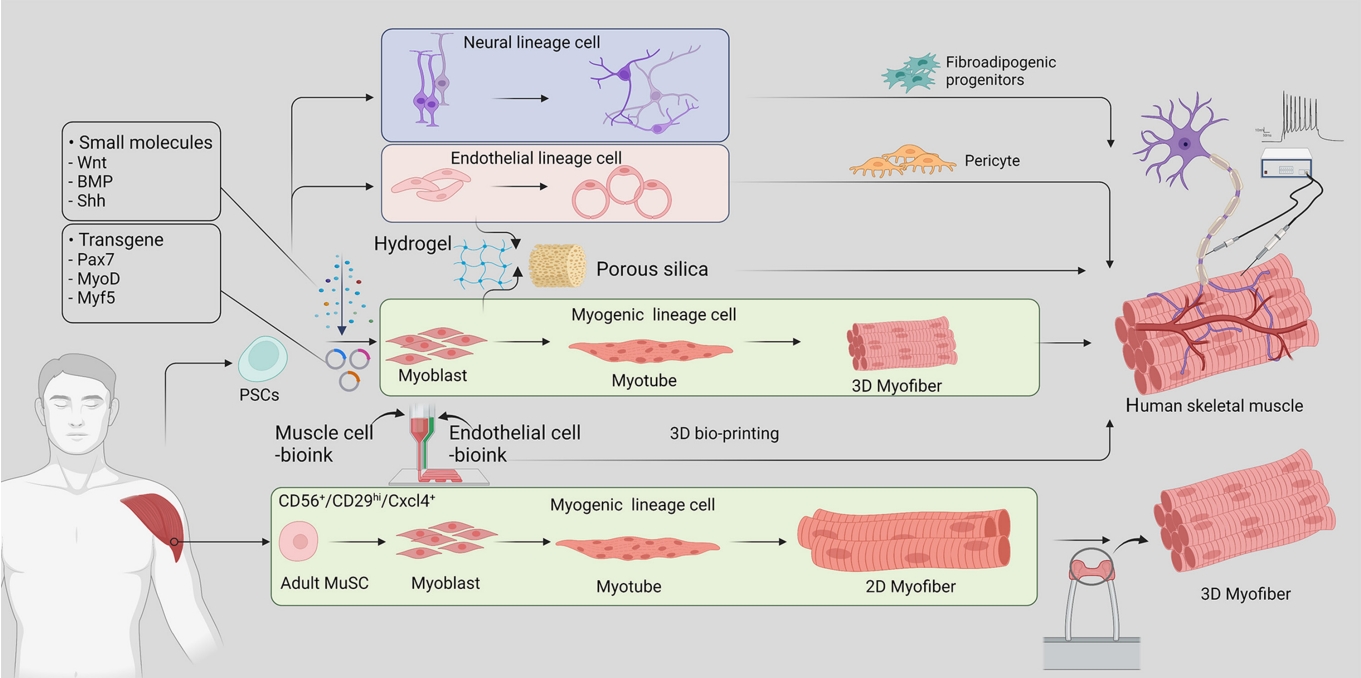
ePub - Skeletal muscle is now regarded as an endocrine organ based on its secretion of myokines and exerkines, which, in response to metabolic stimuli, regulate the crosstalk between the skeletal muscle and other metabolic organs in terms of systemic energy homeostasis. This conceptual basis of skeletal muscle as a metabolically active organ has provided insights into the potential role of physical inactivity and conditions altering muscle quality and quantity in the development of multiple metabolic disorders, including insulin resistance, obesity, and diabetes. Therefore, it is important to understand human muscle physiology more deeply in relation to the pathophysiology of metabolic diseases. Since monolayer cell lines or animal models used in conventional research differ from the pathophysiological features of the human body, there is increasing need for more physiologically relevant in vitro models of human skeletal muscle. Here, we introduce recent studies on in vitro models of human skeletal muscle generated from adult myogenic progenitors or pluripotent stem cells and summarize recent progress in the development of three-dimensional (3D) bioartificial muscle, which mimics the physiological complexity of native skeletal muscle tissue in terms of maturation and functionality. We then discuss the future of skeletal muscle 3D-organoid culture technology in the field of metabolic research for studying pathological mechanisms and developing personalized therapeutic strategies.
-
Citations
Citations to this article as recorded by- Human‐based new approach methodologies to accelerate advances in nutrition research
Manuela Cassotta, Danila Cianciosi, Maria Elexpuru‐Zabaleta, Inaki Elio Pascual, Sandra Sumalla Cano, Francesca Giampieri, Maurizio Battino
Food Frontiers.2024;[Epub] CrossRef - Key indicators of beef safety and quality as important aspects of conservation
S. V. Furman, I. M. Sokulskyi, D. V. Lisohurska, O. V. Lisohurska, B. V. Gutyj
Ukrainian Journal of Veterinary and Agricultural Sciences.2024; 7(1): 68. CrossRef
- Human‐based new approach methodologies to accelerate advances in nutrition research

- Adrenal gland
- Embryonic Development and Adult Regeneration of the Adrenal Gland
- Ji-Hoon Kim, Man Ho Choi
- Endocrinol Metab. 2020;35(4):765-773. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.403

- 7,989 View
- 366 Download
- 15 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The adrenal gland plays a pivotal role in an organism’s health span by controlling the endocrine system. Decades of research on the adrenal gland have provided multiscale insights into the development and maintenance of this essential organ. A particularly interesting finding is that founder stem/progenitor cells participate in adrenocortical development and enable the adult adrenal cortex to regenerate itself in response to hormonal stress and injury. Since major advances have been made in understanding the dynamics of the developmental process and the remarkable regenerative capacity of the adrenal gland, understanding the mechanisms underlying adrenal development, maintenance, and regeneration will be of interest to basic and clinical researchers. Here, we introduce the developmental processes of the adrenal gland and discuss current knowledge regarding stem/progenitor cells that regulate adrenal cortex remodeling and regeneration. This review will provide insights into the fascinating ongoing research on the development and regeneration of the adrenal cortex.
-
Citations
Citations to this article as recorded by- Update on Adrenarche—Still a Mystery
Philipp Augsburger, Jani Liimatta, Christa E Flück
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Aging induces region-specific dysregulation of hormone synthesis in the primate adrenal gland
Qiaoran Wang, Xuebao Wang, Beibei Liu, Shuai Ma, Feng Zhang, Shuhui Sun, Yaobin Jing, Yanling Fan, Yingjie Ding, Muzhao Xiong, Jiaming Li, Qiaocheng Zhai, Yandong Zheng, Chengyu Liu, Gang Xu, Jiayin Yang, Si Wang, Jinlin Ye, Juan Carlos Izpisua Belmonte,
Nature Aging.2024; 4(3): 396. CrossRef - Adrenal Dysfunction in Mitochondrial Diseases
Madeleine Corkery-Hayward, Louise A. Metherell
International Journal of Molecular Sciences.2023; 24(2): 1126. CrossRef - Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system
L. Barrea, L. Verde, E. Camajani, A. S. Šojat, L. Marina, S. Savastano, A. Colao, M. Caprio, G. Muscogiuri
Journal of Endocrinological Investigation.2023; 46(8): 1509. CrossRef - Post‐hatching developmental changes in the adrenal gland of the Japanese quail (Coturnix coturnix japonica): Histological, immunohistochemical, and electron microscopic studies
Fatma M. Abdel‐maksoud, Saher Fadl, Ahmed Abou‐Elmagd, Abdelmohaimen M.M. Saleh
Microscopy Research and Technique.2023; 86(11): 1461. CrossRef - Interactive metabolic signatures of testicular testosterone with bilateral adrenalectomy in mice
Hae Lim Cho, Ji-Hoon Kim, Seuk-Min Ryu, Jongsung Noh, Sang Won Lee, Man Ho Choi
The Journal of Steroid Biochemistry and Molecular Biology.2023; 231: 106333. CrossRef - Construction of a novel clinical nomogram to predict cancer-specific survival in patients with primary malignant adrenal tumors: a large population-based retrospective study
Mingzhen Li, Xiaoying Duan, Di You, Linlin Liu
Frontiers in Medicine.2023;[Epub] CrossRef - The anti-platelet drug cilostazol enhances heart rate and interrenal steroidogenesis and exerts a scant effect on innate immune responses in zebrafish
Wei-Chun Chang, Mei-Jen Chen, Chung-Der Hsiao, Rong-Ze Hu, Yu-Shan Huang, Yu-Fu Chen, Tsai-Hua Yang, Guan-Yi Tsai, Chih-Wei Chou, Ren-Shiang Chen, Yung-Jen Chuang, Yi-Wen Liu, Mohammed Fouad El Basuini
PLOS ONE.2023; 18(10): e0292858. CrossRef - Regulation of Morphogenetic Processes during Postnatal Development and Physiological Regeneration of the Adrenal Medulla
S. S. Obernikhin, N. V. Yaglova, E. P. Timokhina, S. V. Nazimova, V. V. Yaglov
Bulletin of Experimental Biology and Medicine.2023; 175(4): 549. CrossRef - Distinct HAND2/HAND2-AS1 Expression Levels May Fine-Tune Mesenchymal and Epithelial Cell Plasticity of Human Mesenchymal Stem Cells
Rachel Vazana-Netzarim, Yishay Elmalem, Shachar Sofer, Hod Bruck, Naama Danino, Udi Sarig
International Journal of Molecular Sciences.2023; 24(22): 16546. CrossRef - An Update on Genetics of Adrenal Gland and Associated Disorders
Chester Gauss, Dustin Rowland, Berrin Ergun-Longmire
Endocrines.2022; 3(2): 187. CrossRef - Immune dysfunction after spinal cord injury – A review of autonomic and neuroendocrine mechanisms
Kyleigh A. Rodgers, Kristina A. Kigerl, Jan M. Schwab, Phillip G. Popovich
Current Opinion in Pharmacology.2022; 64: 102230. CrossRef - Clinical and Technical Aspects in Free Cortisol Measurement
Man Ho Choi
Endocrinology and Metabolism.2022; 37(4): 599. CrossRef - Surrénalectomies
Isabelle Valin, Dan Rosenberg
Le Nouveau Praticien Vétérinaire canine & féline.2022; 19(82): 50. CrossRef - Adrenal medulla development and medullary-cortical interactions
Nicole Bechmann, Ilona Berger, Stefan R. Bornstein, Charlotte Steenblock
Molecular and Cellular Endocrinology.2021; 528: 111258. CrossRef - Theory: Treatments for Prolonged ICU Patients May Provide New Therapeutic Avenues for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Dominic Stanculescu, Lars Larsson, Jonas Bergquist
Frontiers in Medicine.2021;[Epub] CrossRef - Unravelling Polycystic Ovary Syndrome and Its Comorbidities
Kyung-Wook Kim
Journal of Obesity & Metabolic Syndrome.2021; 30(3): 209. CrossRef
- Update on Adrenarche—Still a Mystery

- Polarized and Stage-Dependent Distribution of Immunoreactivity for Novel PDZ-Binding Protein Preso1 in Adult Neurogenic Regions
- Eun Soo Lee, Woon Ryoung Kim, Younghwa Kim, Hyun Woo Lee, Hyun Kim, Woong Sun
- Endocrinol Metab. 2014;29(3):349-355. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.349
- 3,890 View
- 35 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Adult neural stem cells have the potential for self-renewal and differentiation into multiple cell lineages via symmetric or asymmetric cell division. Preso1 is a recently identified protein involved in the formation of dendritic spines and the promotion of axonal growth in developing neurons. Preso1 can also bind to cell polarity proteins, suggesting a potential role for Preso1 in asymmetric cell division.
Methods To investigate the distribution of Preso1, we performed immunohistochemistry with adult mouse brain slice. Also, polarized distribution of Preso1 was assessed by immunocytochemistry in cultured neural stem cells.
Results Immunoreactivity for Preso1 (Preso1-IR) was strong in the rostral migratory stream and subventricular zone, where proliferating transit-amplifying cells and neuroblasts are prevalent. In cultured neural stem cells, Preso1-IR was unequally distributed in the cell cytosol. We also observed the distribution of Preso1 in the subgranular zone of the hippocampal dentate gyrus, another neurogenic region in the adult brain. Interestingly, Preso1-IR was transiently observed in the nuclei of doublecortin-expressing neuroblasts immediately after asymmetric cell division.
Conclusion Our study demonstrated that Preso1 is asymmetrically distributed in the cytosol and nuclei of neural stem/progenitor cells in the adult brain, and may play a significant role in cell differentiation via association with cell polarity machinery.
-
Citations
Citations to this article as recorded by- FERM domain–containing proteins are active components of the cell nucleus
Péter Borkúti, Ildikó Kristó, Anikó Szabó, Zoltán Kovács, Péter Vilmos
Life Science Alliance.2024; 7(4): e202302489. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- FERM domain–containing proteins are active components of the cell nucleus

- Functional Role of Parkin against Oxidative Stress in Neural Cells
- Minyoung Hwang, Ja-Myong Lee, Younghwa Kim, Dongho Geum
- Endocrinol Metab. 2014;29(1):62-69. Published online March 14, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.1.62
- 3,602 View
- 28 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Parkinson disease (PD) is caused by selective cell death of dopaminergic neurons in the substantia nigra. An early onset form of PD, autosomal recessive juvenile parkinsonism has been associated with a mutation in the parkin gene. The function of parkin is known to remove misfolding proteins and protect cell death. We aimed to investigate the role of parkin against oxidative stress in neuronal cells.
Methods Parkin knockout embryonic stem cells (PKO ES cells) were differentiated into neurons by adherent monolayer culture method. Oxidative stress was induced by the treatment of 1-methyl-4-phenylpyridinium (MPP+) in neurons derived from wild type and PKO ES cells, and cell viability was examined by MTT assay. After exposure to MPP+, Tuj1-positive cell population was compared between PKO and wild type cells by fluorescence activated cell sorter (FACS) analysis. The activated caspase3 protein level was also measured by Western blot analysis, FACS and immunocytochemistry.
Results There was no difference in the efficiency of neuronal differentiation between wild type and PKO ES cells. After exposure to MPP+, no significant differences were found in cell viability and Tuj1-positive cell population between the two groups determined by MTT assay and FACS analysis, respectively. The activated caspase3 protein levels examined by Western blot analysis, FACS and immunocytochemistry were not changed in PKO cells compared with those of wild type cells after MPP+ treatment.
Conclusion These results suggest that PKO neuronal cells including dopaminergic neurons are not sensitive to caspase3-dependent cell death pathway during the response against MPP+-induced oxidative stress.
-
Citations
Citations to this article as recorded by- A Modified Differentiation Protocol In Vitro to Generate Dopaminergic Neurons from Pluripotent Stem Cells
Nianping Zhang, Xudong Zhang, Zhaoli Yan, Ronghui Li, Song Xue, Dahong Long
Journal of Biomaterials and Tissue Engineering.2023; 13(10): 1017. CrossRef - miR-146b-5p promotes the neural conversion of pluripotent stem cells by targeting Smad4
Nianping Zhang, Ying Lyu, Xuebing Pan, Liping Xu, Aiguo Xuan, Xiaosong He, Wandan Huang, Dahong Long
International Journal of Molecular Medicine.2017; 40(3): 814. CrossRef - Increased susceptibility to fundus camera-delivered light-induced retinal degeneration in mice deficient in oxidative stress response proteins
Yi Ding, Bogale Aredo, Xin Zhong, Cynthia X. Zhao, Rafael L. Ufret-Vincenty
Experimental Eye Research.2017; 159: 58. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Neural stem cells in Parkinson’s disease: a role for neurogenesis defects in onset and progression
Jaclyn Nicole Le Grand, Laura Gonzalez-Cano, Maria Angeliki Pavlou, Jens C. Schwamborn
Cellular and Molecular Life Sciences.2015; 72(4): 773. CrossRef
- A Modified Differentiation Protocol In Vitro to Generate Dopaminergic Neurons from Pluripotent Stem Cells


 KES
KES

 First
First Prev
Prev



