Search
- Page Path
- HOME > Search
- Miscellaneous
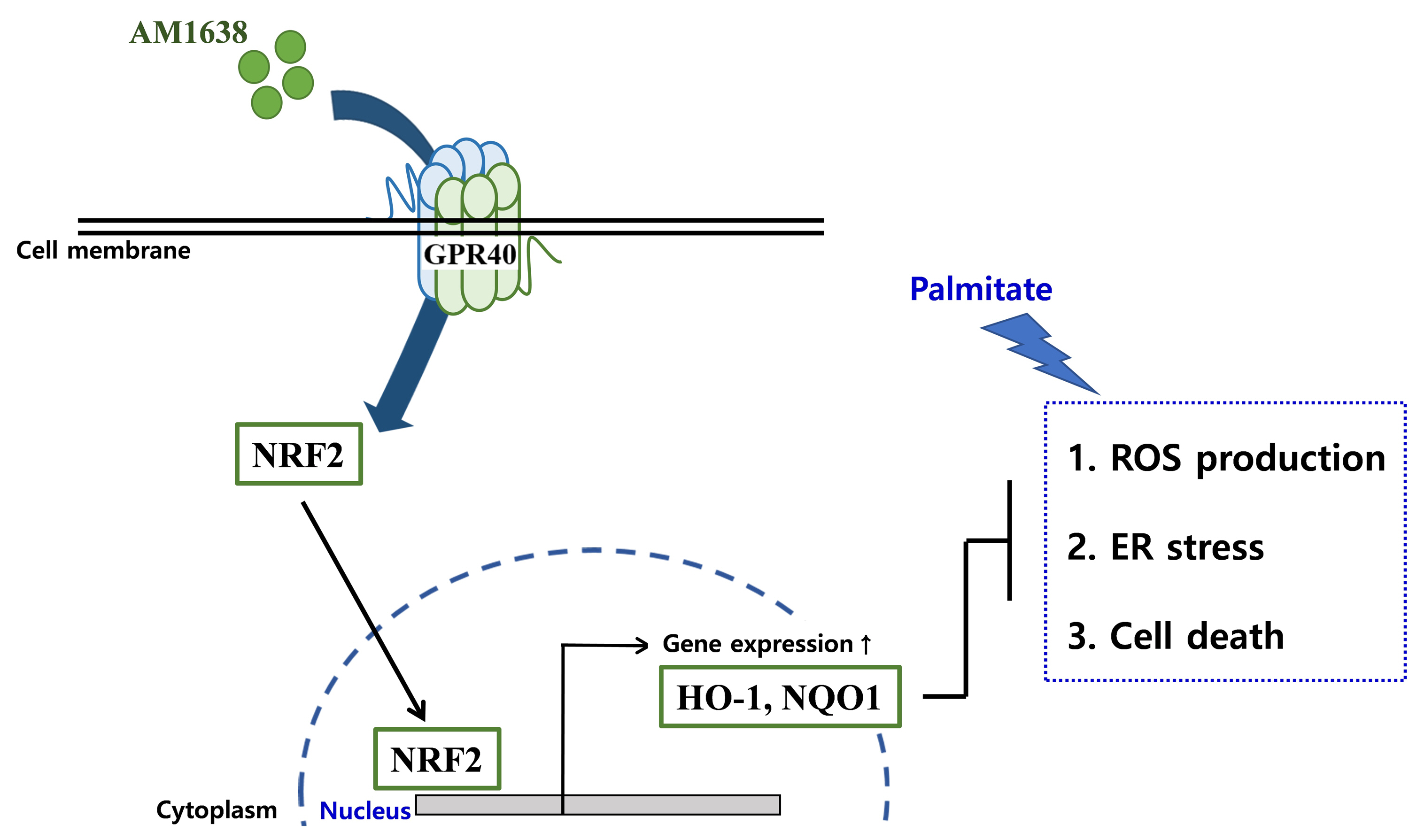
- AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Hwan-Jin Hwang, Joo Won Kim, SukHwan Yun, Min Jeong Park, Eyun Song, Sooyeon Jang, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
- Endocrinol Metab. 2023;38(6):760-769. Published online November 2, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1774
- 1,231 View
- 85 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
G protein-coupled receptor 40 (GPR40) is a key molecule in diabetes and fatty liver, but its role in endothelial dysfunction remains unclear. Our objective in this study was to determine whether GPR40 agonists protect endothelial cells against palmitatemediated oxidative stress.
Methods
Human umbilical vein endothelial cells (HUVECs) were used to investigate effects of various GPR40 agonists on vascular endothelium.
Results
In HUVECs, AM1638, a GPR40-full agonist, enhanced nuclear factor erythroid 2–related factor 2 (NRF2) translocation to the nucleus and heme oxygenase-1 (HO-1) expression, which blocked palmitate-induced superoxide production. Those antioxidant effects were not detected after treatment with LY2922470 or TAK875, GPR40-partial agonists, suggesting that GPR40 regulates reactive oxygen species (ROS) removal in a ligand-dependent manner. We also found that palmitate-induced CCAAT/enhancer‐binding protein homologous protein expression; X-box binding protein-1 splicing, nuclear condensation, and fragmentation; and caspase-3 cleavage were all blocked in an NRF2-dependent manner after AM1638 treatment. Both LY2922470 and TAK875 also improved cell viability independent of the NRF2/ROS pathway by reducing palmitate-mediated endoplasmic reticulum stress and nuclear damage. GPR40 agonists thus have beneficial effects against palmitate in HUVECs. In particular, AM1638 reduced palmitate-induced superoxide production and cytotoxicity in an NRF2/HO-1 dependent manner.
Conclusion
GPR40 could be developed as a good therapeutic target to prevent or treat cardiovascular diseases such as atherosclerosis.

- Clinical Study
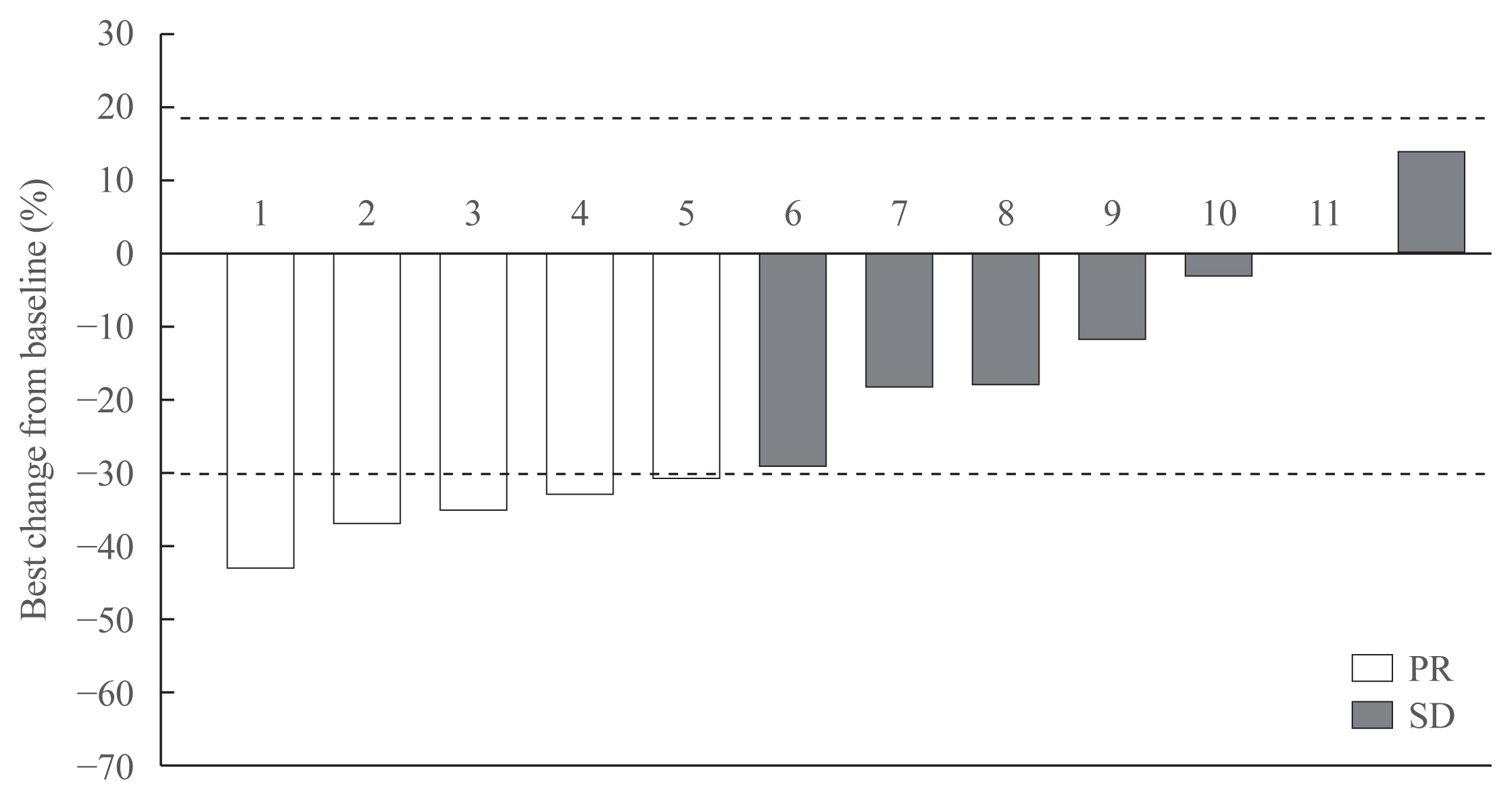
- Vandetanib for the Management of Advanced Medullary Thyroid Cancer: A Real-World Multicenter Experience
- Mijin Kim, Jee Hee Yoon, Jonghwa Ahn, Min Ji Jeon, Hee Kyung Kim, Dong Jun Lim, Ho-Cheol Kang, In Joo Kim, Young Kee Shong, Tae Yong Kim, Bo Hyun Kim
- Endocrinol Metab. 2020;35(3):587-594. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.687
- 5,670 View
- 147 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Vandetanib is the most widely used tyrosine kinase inhibitor for the treatment of patients with advanced medullary thyroid cancer (MTC). However, only limited data regarding its use outside clinical trials are available. We aimed to evaluate the efficacy and safety of vandetanib in patients with advanced MTC in routine clinical practice.
Methods
In this multicenter retrospective study, 12 patients with locally advanced or metastatic MTC treated with vandetanib at four tertiary hospitals were included. The primary outcome was the objective response rate (ORR) based on the Response Evaluation Criteria in Solid Tumors. The progression-free survival (PFS), overall survival (OS), and toxicities were also evaluated.
Results
Eleven patients (92%) had distant metastasis and 10 (83%) had disease progression at enrollment. Partial response was observed in five patients (ORR, 42%) and stable disease lasting ≥24 weeks was reported in an additional five patients (83%). During the median 31.7 months of follow-up, disease progression was seen in five patients (42%); of these, two died due to disease progression. The median PFS was 25.9 months, while the median OS was not reached. All patients experienced adverse events (AEs) which were generally consistent with the known safety profile of vandetanib. Vandetanib was discontinued in two patients due to skin toxicity.
Conclusion
Consistent with the phase III trial, this study confirmed the efficacy of vandetanib for advanced MTC in terms of both ORR and PFS in the real-world setting. Vandetanib was well tolerated in the majority of patients, and there were no fatal AEs. -
Citations
Citations to this article as recorded by- Metastatic medullary thyroid carcinoma (MTC): disease course, treatment modalities and factors predisposing for drug resistance
Katerina Saltiki, George Simeakis, Olga Karapanou, Stavroula A. Paschou, Maria Alevizaki
Endocrine.2023; 80(3): 570. CrossRef - Initial Experiences of Selective RET Inhibitor Selpercatinib in Adults with Metastatic Differentiated Thyroid Carcinoma and Medullary Thyroid Carcinoma: Real-World Case Series in Korea
Han-Sang Baek, Jeonghoon Ha, Seunggyun Ha, Ja Seong Bae, Chan Kwon Jung, Dong-Jun Lim
Current Oncology.2023; 30(3): 3020. CrossRef - Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET
Nicolas Sahakian, Frédéric Castinetti, Pauline Romanet
Cancers.2023; 15(19): 4865. CrossRef - Sporadic Medullary Thyroid Carcinoma: Towards a Precision Medicine
Antonio Matrone, Carla Gambale, Alessandro Prete, Rossella Elisei
Frontiers in Endocrinology.2022;[Epub] CrossRef - Targeted therapy and drug resistance in thyroid cancer
Yujie Zhang, Zhichao Xing, Tianyou Liu, Minghai Tang, Li Mi, Jingqiang Zhu, Wenshuang Wu, Tao Wei
European Journal of Medicinal Chemistry.2022; 238: 114500. CrossRef - Daily Management of Patients on Multikinase Inhibitors’ Treatment
Carla Colombo, Simone De Leo, Matteo Trevisan, Noemi Giancola, Anna Scaltrito, Laura Fugazzola
Frontiers in Oncology.2022;[Epub] CrossRef - The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview
Cătălina Ionescu, Bogdan Oprea, Georgeta Ciobanu, Milena Georgescu, Ramona Bică, Garofiţa-Olivia Mateescu, Fidan Huseynova, Veronique Barragan-Montero
Medicina.2022; 58(7): 903. CrossRef - Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy
Tobiloba C. Elebiyo, Damilare Rotimi, Ikponmwosa O. Evbuomwan, Rotdelmwa Filibus Maimako, Matthew Iyobhebhe, Oluwafemi Adeleke Ojo, Olarewaju M. Oluba, Oluyomi S. Adeyemi
Cancer Treatment and Research Communications.2022; 32: 100620. CrossRef - Current Guidelines for Management of Medullary Thyroid Carcinoma
Mijin Kim, Bo Hyun Kim
Endocrinology and Metabolism.2021; 36(3): 514. CrossRef - Recent advances in precision medicine for the treatment of medullary thyroid cancer
Jolanta Krajewska, Aleksandra Kukulska, Malgorzata Oczko-Wojciechowska, Barbara Jarzab
Expert Review of Precision Medicine and Drug Development.2021; 6(5): 307. CrossRef - Functional evaluation of vandetanib metabolism by CYP3A4 variants and potential drug interactions in vitro
Mingming Han, Xiaodan Zhang, Zhize Ye, Jing Wang, Jianchang Qian, Guoxin Hu, Jianping Cai
Chemico-Biological Interactions.2021; 350: 109700. CrossRef - Nephrotoxicity in advanced thyroid cancer treated with tyrosine kinase inhibitors: An update
Alice Nervo, Francesca Retta, Alberto Ragni, Alessandro Piovesan, Alberto Mella, Luigi Biancone, Marco Manganaro, Marco Gallo, Emanuela Arvat
Critical Reviews in Oncology/Hematology.2021; 168: 103533. CrossRef
- Metastatic medullary thyroid carcinoma (MTC): disease course, treatment modalities and factors predisposing for drug resistance

- Diabetes
- Pioglitazone Attenuates Palmitate-Induced Inflammation and Endoplasmic Reticulum Stress in Pancreatic β-Cells
- Seok-Woo Hong, Jinmi Lee, Jung Hwan Cho, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
- Endocrinol Metab. 2018;33(1):105-113. Published online March 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.105
- 6,273 View
- 96 Download
- 19 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background The nuclear receptor peroxisome proliferator-activator gamma (PPARγ) is a useful therapeutic target for obesity and diabetes, but its role in protecting β-cell function and viability is unclear.
Methods To identify the potential functions of PPARγ in β-cells, we treated mouse insulinoma 6 (MIN6) cells with the PPARγ agonist pioglitazone in conditions of lipotoxicity, endoplasmic reticulum (ER) stress, and inflammation.
Results Palmitate-treated cells incubated with pioglitazone exhibited significant improvements in glucose-stimulated insulin secretion and the repression of apoptosis, as shown by decreased caspase-3 cleavage and poly (adenosine diphosphate [ADP]-ribose) polymerase activity. Pioglitazone also reversed the palmitate-induced expression of inflammatory cytokines (tumor necrosis factor α, interleukin 6 [IL-6], and IL-1β) and ER stress markers (phosphor-eukaryotic translation initiation factor 2α, glucose-regulated protein 78 [GRP78], cleaved-activating transcription factor 6 [ATF6], and C/EBP homologous protein [CHOP]), and pioglitazone significantly attenuated inflammation and ER stress in lipopolysaccharide- or tunicamycin-treated MIN6 cells. The protective effect of pioglitazone was also tested in pancreatic islets from high-fat-fed KK-Ay mice administered 0.02% (wt/wt) pioglitazone or vehicle for 6 weeks. Pioglitazone remarkably reduced the expression of ATF6α, GRP78, and monocyte chemoattractant protein-1, prevented α-cell infiltration into the pancreatic islets, and upregulated glucose transporter 2 (Glut2) expression in β-cells. Moreover, the preservation of β-cells by pioglitazone was accompanied by a significant reduction of blood glucose levels.
Conclusion Altogether, these results support the proposal that PPARγ agonists not only suppress insulin resistance, but also prevent β-cell impairment via protection against ER stress and inflammation. The activation of PPARγ might be a new therapeutic approach for improving β-cell survival and insulin secretion in patients with diabetes mellitus
-
Citations
Citations to this article as recorded by- Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson’s
Nancy Ahuja, Shalini Gupta, Rashmi Arora, Ella Bhagyaraj, Drishti Tiwari, Sumit Kumar, Pawan Gupta
Life Science Alliance.2024; 7(7): e202302416. CrossRef - Prosthetic vascular grafts engineered to combat calcification: Progress and future directions
Taylor K. Brown, Sara Alharbi, Karen J. Ho, Bin Jiang
Biotechnology and Bioengineering.2023; 120(4): 953. CrossRef - Obesity, diabetes mellitus, and cardiometabolic risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023
Harold Edward Bays, Shagun Bindlish, Tiffany Lowe Clayton
Obesity Pillars.2023; 5: 100056. CrossRef - Metformin promotes osteogenic differentiation and prevents hyperglycaemia-induced osteoporosis by suppressing PPARγ expression
Lifeng Zheng, Ximei Shen, Yun Xie, Hong Lian, Sunjie Yan, Shizhong Wang
Acta Biochimica et Biophysica Sinica.2023; 55(3): 394. CrossRef - Peroxisome proliferator-activated receptors as targets to treat metabolic diseases: Focus on the adipose tissue, liver, and pancreas
Henrique Souza-Tavares, Carolline Santos Miranda, Isabela Macedo Lopes Vasques-Monteiro, Cristian Sandoval, Daiana Araujo Santana-Oliveira, Flavia Maria Silva-Veiga, Aline Fernandes-da-Silva, Vanessa Souza-Mello
World Journal of Gastroenterology.2023; 29(26): 4136. CrossRef - Nicotinamide N-methyltransferase upregulation contributes to palmitate-elicited peroxisome proliferator-activated receptor transactivation in hepatocytes
Qing Song, Jun Wang, Alexandra Griffiths, Samuel Man Lee, Iredia D. Iyamu, Rong Huang, Jose Cordoba-Chacon, Zhenyuan Song
American Journal of Physiology-Cell Physiology.2023; 325(1): C29. CrossRef - The global perspective on peroxisome proliferator-activated receptor γ (PPARγ) in ectopic fat deposition: A review
Yanhao Qiu, Mailin Gan, Xingyu Wang, Tianci Liao, Qiuyang Chen, Yuhang Lei, Lei Chen, Jinyong Wang, Ye Zhao, Lili Niu, Yan Wang, Shunhua Zhang, Li Zhu, Linyuan Shen
International Journal of Biological Macromolecules.2023; 253: 127042. CrossRef - Chemical inducer of regucalcin attenuates lipopolysaccharide‐induced inflammatory responses in pancreatic MIN6 β‐cells and RAW264.7 macrophages
Tomiyasu Murata, Kazunori Hashimoto, Susumu Kohno, Chiaki Takahashi, Masayoshi Yamaguchi, Chihiro Ito, Itoigawa Masataka, Roji Kojima, Kiyomi Hikita, Norio Kaneda
FEBS Open Bio.2022; 12(1): 175. CrossRef - Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
Seok-Woo Hong, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 57. CrossRef - Analysis of changes in the proteomic profile of porcine corpus luteum during different stages of the oestrous cycle: effects of PPAR gamma ligands
Zuzanna Kunicka, Karol Mierzejewski, Aleksandra Kurzyńska, Robert Stryiński, Jesús Mateos, Mónica Carrera, Monika Golubska, Iwona Bogacka, Xiaolong Wang
Reproduction, Fertility and Development.2022; 34(11): 776. CrossRef - Activation of PPARγ Protects Obese Mice from Acute Lung Injury by Inhibiting Endoplasmic Reticulum Stress and Promoting Mitochondrial Biogenesis
Yin Tang, Ke Wei, Ling Liu, Jingyue Ma, Siqi Wu, Wenjing Tang, Stéphane Mandard
PPAR Research.2022; 2022: 1. CrossRef - Effect of Pioglitazone on endoplasmic reticulum stress regarding in situ perfusion rat model
Vivien Telek, Luca Erlitz, Ibitamuno Caleb, Tibor Nagy, Mónika Vecsernyés, Bálint Balogh, György Sétáló, Péter Hardi, Gábor Jancsó, Ildikó Takács
Clinical Hemorheology and Microcirculation.2021; 79(2): 311. CrossRef - Inflammation in Metabolic Diseases and Insulin Resistance
Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2021; 3(2): 31. CrossRef - Current Status of Endoplasmic Reticulum Stress in Type II Diabetes
Sagir Mustapha, Mustapha Mohammed, Ahmad Khusairi Azemi, Abubakar Ibrahim Jatau, Aishatu Shehu, Lukman Mustapha, Ibrahim Muazzamu Aliyu, Rabi’u Nuhu Danraka, Abdulbasit Amin, Auwal Adam Bala, Wan Amir Nizam Wan Ahmad, Aida Hanum Ghulam Rasool, Mohd Rais M
Molecules.2021; 26(14): 4362. CrossRef - JunD Regulates Pancreatic β-Cells Function by Altering Lipid Accumulation
Kexin Wang, Yixin Cui, Peng Lin, Zhina Yao, Yu Sun
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial
Giuseppe Della Pepa, Marco Russo, Marilena Vitale, Fabrizia Carli, Claudia Vetrani, Maria Masulli, Gabriele Riccardi, Olga Vaccaro, Amalia Gastaldelli, Angela A. Rivellese, Lutgarda Bozzetto
Diabetes Research and Clinical Practice.2021; 178: 108984. CrossRef - Radioprotective Effect of Pioglitazone Against Genotoxicity Induced by Ionizing Radiation in Healthy Human Lymphocytes
Roya Kazemi, Seyed J. Hosseinimehr
Cardiovascular & Hematological Agents in Medicinal Chemistry .2021; 19(1): 72. CrossRef - Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes
Maria Lytrivi, Anne-Laure Castell, Vincent Poitout, Miriam Cnop
Journal of Molecular Biology.2020; 432(5): 1514. CrossRef - Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress
Ke Chen, Hu Hua, Ziyang Zhu, Tong Wu, Zhanjun Jia, Qianqi Liu
Apoptosis.2020; 25(3-4): 192. CrossRef - Mechanisms of impaired pancreatic β‑cell function in high‑fat diet‑induced obese mice: The role of endoplasmic reticulum stress
Xiaoqing Yi, Xuan Cai, Sisi Wang, Yanfeng Xiao
Molecular Medicine Reports.2020;[Epub] CrossRef - Docosahexaenoic and Eicosapentaenoic Acids Prevent Altered-Muc2 Secretion Induced by Palmitic Acid by Alleviating Endoplasmic Reticulum Stress in LS174T Goblet Cells
Quentin Escoula, Sandrine Bellenger, Michel Narce, Jérôme Bellenger
Nutrients.2019; 11(9): 2179. CrossRef - PPAR-γ agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats
Eman Soliman, Shereen F. Behairy, Nabila N. El-maraghy, Shimaa M. Elshazly
Life Sciences.2019; 239: 117047. CrossRef - Changes of MODY signal pathway genes in the endoplasmic reticulum stress in INS-1-3 cells
Yanan Dong, Shirui Li, Wenhui Zhao, Yanlei Wang, Tingting Ge, Jianzhong Xiao, Yukun Li, Herve Le Stunff
PLOS ONE.2018; 13(6): e0198614. CrossRef
- Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson’s

- Antibody-dependent Cell-mediated Cytotoxitity as a Prognostic Indicator in the Medical Treatment of Graves' Disease.
- Kwan Woo Lee, Young Goo Shin, Hye Rim Ro, Sung Kyu Lee, Yun Suk Chung, Hyun Man Kim, Yoon Jung Kim, Eun Kyung Hong, Bong Nam Chae
- J Korean Endocr Soc. 1998;13(4):554-562. Published online January 1, 2001
- 1,056 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The several forms of treatment of Graves disease-thyroidectomy, antithyroid drugs and radioiodide therapy-are in wide use now. But which therapy is best is a matter of debate. Some authors reported that in patients who underwent thyroidectomy, higher titers of serum antimicrosomal antibody were associated with 1) higher formation rates of germinal centers, 2) more lymphocyte infiltration in the thyroid tissue, 3) higher incidence of hypothyroidism, and 4) lower incidence of recurrence. We were interested in the relationship of thyroid autoantibody titers, ADCC(antibody-dependent cell-mediated cytotoxicity) activity and the clinical response to antithyroid medication. METHODS: We measured ADCC activities from patients in Graves disease(n-48), Hashimoto thyroiditis(n=17) and normal control(n=9). The patients of Graves disease were followed up for more than 1 year, and they were grouped into A(n=17, well responsed group to antithyroid medication) and B(n=31, poorly responsed group). We examined ADCC activities of patients' sera by chromium release assay. RESULTS: 1) Mean age of patients with Graves disease was 34.4210.4 years and 15 patients were male(31%). 2) Results of thyroid function tests of the Graves' patients were T 585.9 +/- 255.3 ng/dL, T4 21.3 +/- 12.2 mg/dL, TSH 0.11 +/- 0.06mIU/mL. Concentrations of antimicrosomal antibody, antithyroglobulin antibody and thyrotropin binding inhibitory immunoglobulin were 1279.1 +/- 1486.7 IU/mL, 488.1 +/- 751.1 IU/mL, and 38.5 +/- 33.4U/L respectively. 3) There was no significant difference between levels of thyroid hormones or concentrations of thyroid autoantibodies and ADCC activities in graves patients. 4) The ADCC activity of the Graves patient group(24.49%) was significantly higher than that of the normal control group(3.76%), and significantly lower than that of the Hashimotos thyroiditis group(36.34%). 5) There was no significant difference in ADCC activity between group A(18.24 +/- 13.44%) and B(27.91 +20.02%). CONCLUSION: From this results, we suggested that ADCC activity seems to be no value as a prognostic factor in predicting the response to antithyroid drugs in Graves disease patients. But, further studies, larger number of patients and long-term follow up, are needed.


 KES
KES

 First
First Prev
Prev



