Search
- Page Path
- HOME > Search
- Calcium & bone metabolism
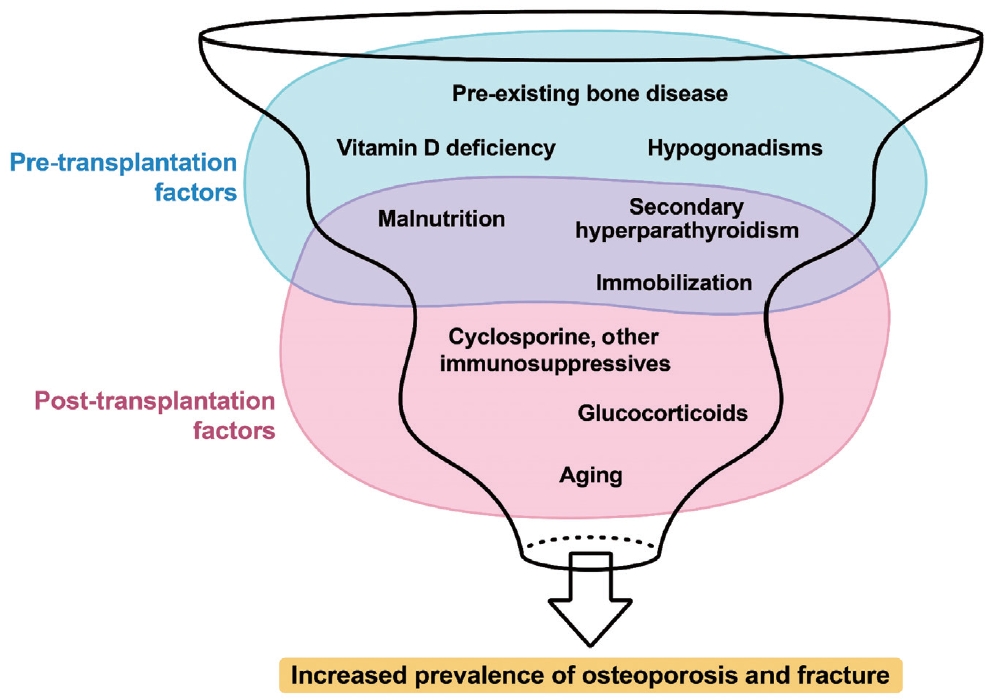
- Bone Loss after Solid Organ Transplantation: A Review of Organ-Specific Considerations
- Kyoung Jin Kim, Jeonghoon Ha, Sang Wan Kim, Jung-Eun Kim, Sihoon Lee, Han Seok Choi, Namki Hong, Sung Hye Kong, Seong Hee Ahn, So Young Park, Ki-Hyun Baek, on Behalf of Metabolic Bone Disease Study Group of Korean Endocrine Society
- Endocrinol Metab. 2024;39(2):267-282. Published online April 25, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1939
- 779 View
- 37 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - This review article investigates solid organ transplantation-induced osteoporosis, a critical yet often overlooked issue, emphasizing its significance in post-transplant care. The initial sections provide a comprehensive understanding of the prevalence and multifactorial pathogenesis of transplantation osteoporosis, including factors such as deteriorating post-transplantation health, hormonal changes, and the impact of immunosuppressive medications. Furthermore, the review is dedicated to organ-specific considerations in transplantation osteoporosis, with separate analyses for kidney, liver, heart, and lung transplantations. Each section elucidates the unique challenges and management strategies pertinent to transplantation osteoporosis in relation to each organ type, highlighting the necessity of an organ-specific approach to fully understand the diverse manifestations and implications of transplantation osteoporosis. This review underscores the importance of this topic in transplant medicine, aiming to enhance awareness and knowledge among clinicians and researchers. By comprehensively examining transplantation osteoporosis, this study contributes to the development of improved management and care strategies, ultimately leading to improved patient outcomes in this vulnerable group. This detailed review serves as an essential resource for those involved in the complex multidisciplinary care of transplant recipients.

- Diabetes, obesity and metabolism
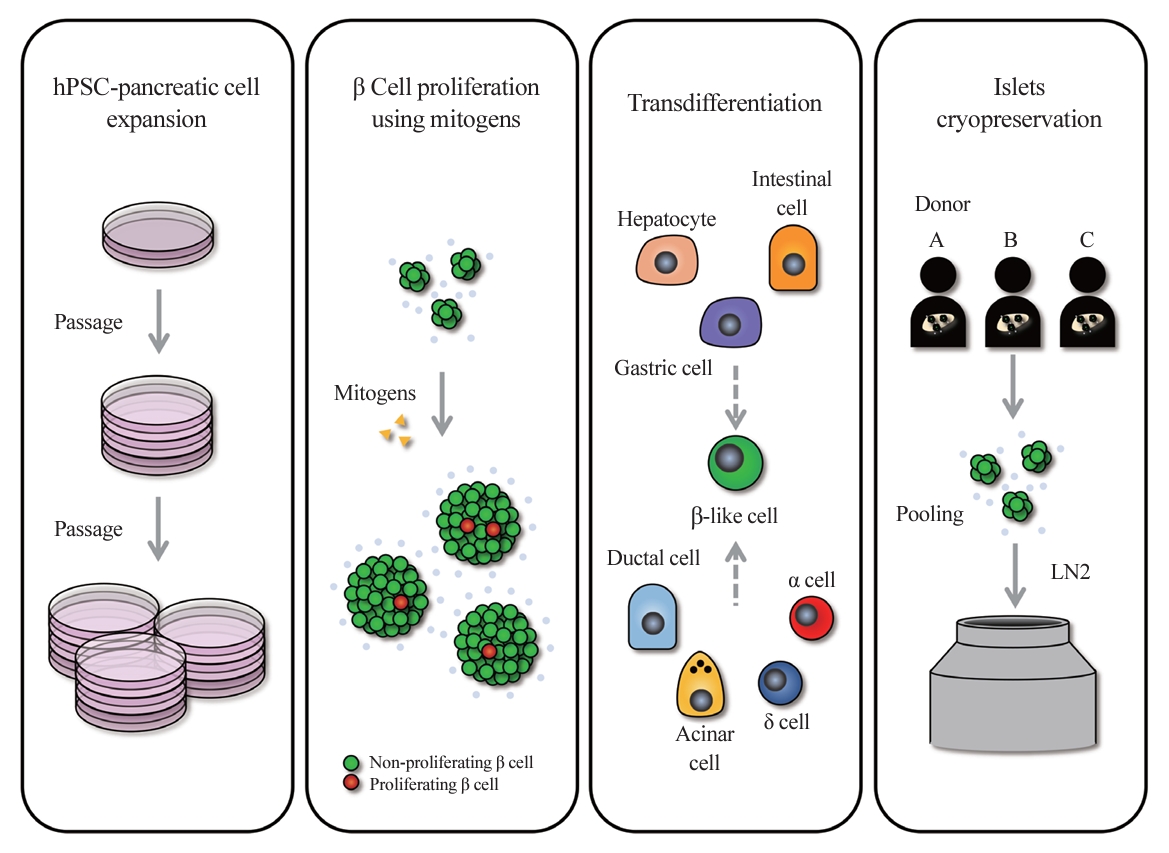
- Scaling Insulin-Producing Cells by Multiple Strategies
- Jinhyuk Choi, Fritz Cayabyab, Harvey Perez, Eiji Yoshihara
- Endocrinol Metab. 2024;39(2):191-205. Published online April 4, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1910
- 1,618 View
- 82 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - In the quest to combat insulin-dependent diabetes mellitus (IDDM), allogenic pancreatic islet cell therapy sourced from deceased donors represents a significant therapeutic advance. However, the applicability of this approach is hampered by donor scarcity and the demand for sustained immunosuppression. Human induced pluripotent stem cells are a game-changing resource for generating synthetic functional insulin-producing β cells. In addition, novel methodologies allow the direct expansion of pancreatic progenitors and mature β cells, thereby circumventing prolonged differentiation. Nevertheless, achieving practical reproducibility and scalability presents a substantial challenge for this technology. As these innovative approaches become more prominent, it is crucial to thoroughly evaluate existing expansion techniques with an emphasis on their optimization and scalability. This manuscript delineates these cutting-edge advancements, offers a critical analysis of the prevailing strategies, and underscores pivotal challenges, including cost-efficiency and logistical issues. Our insights provide a roadmap, elucidating both the promises and the imperatives in harnessing the potential of these cellular therapies for IDDM.

- Diabetes, Obesity and Metabolism
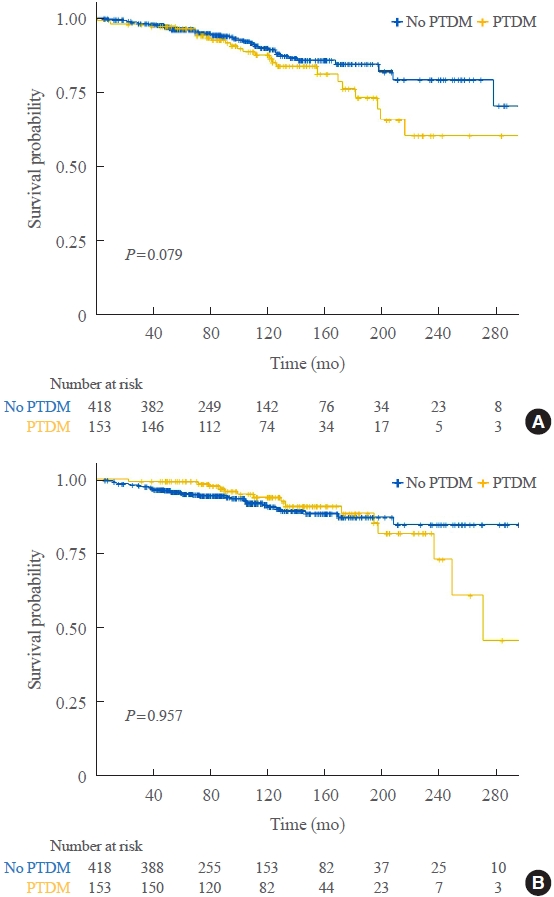
- Impact of Post-Transplant Diabetes Mellitus on Survival and Cardiovascular Events in Kidney Transplant Recipients
- Ja Young Jeon, Shin Han-Bit, Bum Hee Park, Nami Lee, Hae Jin Kim, Dae Jung Kim, Kwan-Woo Lee, Seung Jin Han
- Endocrinol Metab. 2023;38(1):139-145. Published online February 6, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1594
- 1,719 View
- 121 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Post-transplant diabetes mellitus (PTDM) is a risk factor for poor outcomes after kidney transplantation (KT). However, the outcomes of KT have improved recently. Therefore, we investigated whether PTDM is still a risk factor for mortality, major atherosclerotic cardiovascular events (MACEs), and graft failure in KT recipients.
Methods
We studied a retrospective cohort of KT recipients (between 1994 and 2017) at a single tertiary center, and compared the rates of death, MACEs, overall graft failure, and death-censored graft failure after KT between patients with and without PTDM using Kaplan-Meier analysis and a Cox proportional hazard model.
Results
Of 571 KT recipients, 153 (26.8%) were diagnosed with PTDM. The mean follow-up duration was 9.6 years. In the Kaplan- Meier analysis, the PTDM group did not have a significantly increased risk of death or four-point MACE compared with the non-diabetes mellitus group (log-rank test, P=0.957 and P=0.079, respectively). Multivariate Cox proportional hazard models showed that PTDM did not have a negative impact on death or four-point MACE (P=0.137 and P=0.181, respectively). In addition, PTDM was not significantly associated with overall or death-censored graft failure. However, patients with a long duration of PTDM had a higher incidence of four-point MACE.
Conclusion
Patient survival and MACEs were comparable between groups with and without PTDM. However, PTDM patients with long duration diabetes were at higher risk of cardiovascular disease. -
Citations
Citations to this article as recorded by- Effect of post-transplant diabetes mellitus on cardiovascular events and mortality: a single‐center retrospective cohort study
Uğur Ünlütürk, Tolga Yıldırım, Merve Savaş, Seda Hanife Oğuz, Büşra Fırlatan, Deniz Yüce, Nesrin Damla Karakaplan, Cemile Selimova, Rahmi Yılmaz, Yunus Erdem, Miyase Bayraktar
Endocrine.2024;[Epub] CrossRef - Prevalence of new-onset diabetes mellitus after kidney transplantation: a systematic review and meta-analysis
Qiufeng Du, Tao Li, Xiaodong Yi, Shuang Song, Jing Kang, Yunlan Jiang
Acta Diabetologica.2024;[Epub] CrossRef - Safety and efficacy of semaglutide in post kidney transplant patients with type 2 diabetes or Post-Transplant diabetes
Moeber Mohammed Mahzari, Omar Buraykan Alluhayyan, Mahdi Hamad Almutairi, Mohammed Abdullah Bayounis, Yazeed Hasan Alrayani, Amir A. Omair, Awad Saad Alshahrani
Journal of Clinical & Translational Endocrinology.2024; 36: 100343. CrossRef
- Effect of post-transplant diabetes mellitus on cardiovascular events and mortality: a single‐center retrospective cohort study

- Calcium & Bone Metabolism
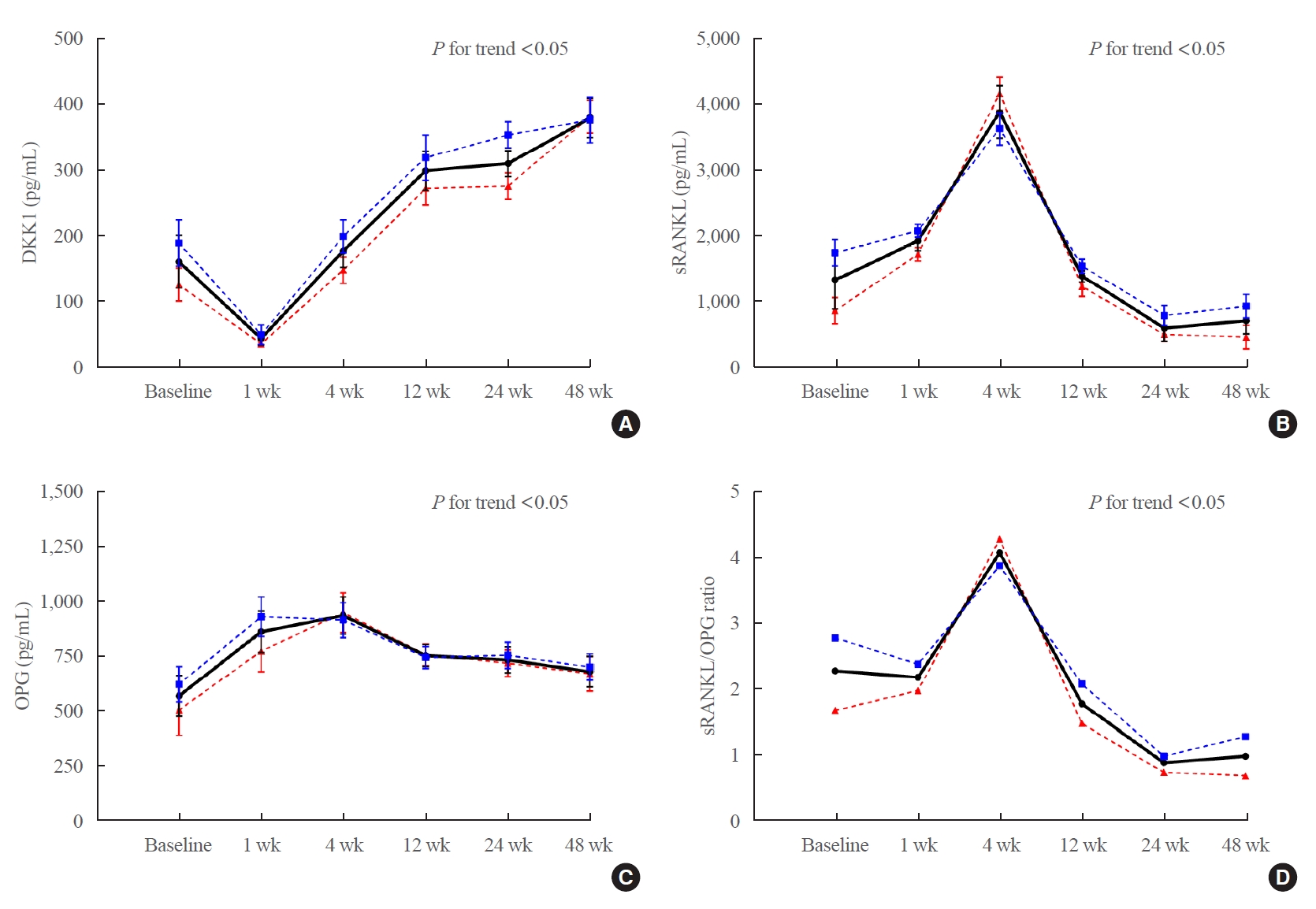
- Changes in Serum Dickkopf-1, RANK Ligand, Osteoprotegerin, and Bone Mineral Density after Allogeneic Hematopoietic Stem Cell Transplantation Treatment
- Eunhee Jang, Jeonghoon Ha, Ki-Hyun Baek, Moo Il Kang
- Endocrinol Metab. 2021;36(6):1211-1218. Published online December 8, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1248
- 3,119 View
- 104 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Dickkopf-1 (DKK1) regulates bone formation by inhibiting canonical Wnt/β-catenin pathway signaling, and indirectly enhances osteoclastic activity by altering the expression ratio of receptor activator of nuclear factor-κB ligand (RANKL) relative to osteoprotegerin (OPG). However, it is difficult to explain continued bone loss after allogeneic stem cell transplantation (allo-SCT) in terms of changes in only RANKL and OPG. Few studies have evaluated changes in DKK1 after allo-SCT.
Methods
We prospectively enrolled 36 patients with hematologic malignancies who were scheduled for allo-SCT treatment. Serum DKK1, OPG, and RANKL levels were measured before (baseline), and at 1, 4, 12, 24, and 48 weeks after allo-SCT treatment. Bone mineral density (BMD) was assessed using dual-energy X-ray absorptiometry before (baseline) and 24 and 48 weeks after allo-SCT treatment.
Results
After allo-SCT treatment, the DKK1 level decreased rapidly, returned to baseline during the first 4 weeks, and remained elevated for 48 weeks (P<0.0001 for changes observed over time). The serum RANKL/OPG ratio peaked at 4 weeks and then declined (P<0.001 for changes observed over time). BMD decreased relative to the baseline at all timepoints during the study period, and the lumbar spine in female patients had the largest decline (–11.3%±1.6% relative to the baseline at 48 weeks, P<0.05).
Conclusion
Serum DKK1 levels rapidly decreased at 1 week and then continued to increase for 48 weeks; bone mass decreased for 48 weeks following engraftment in patients treated with allo-SCT, suggesting that DKK1-mediated inhibition of osteoblast differentiation plays a role in bone loss in patients undergoing allo-SCT. -
Citations
Citations to this article as recorded by- Fracture risk and assessment in adults with cancer
Carrie Ye, William D. Leslie
Osteoporosis International.2023; 34(3): 449. CrossRef - Short-Term Impact of Hematopoietic Stem Cell Transplantation in Leukemia Patients on Bone Bio Markers, Electrolytes and Blood Profile
Rhythm Joshi, Zehva Khan, Aakriti Garg, Dinesh Bhurani, Nidhi B Agarwal, Ubada Aqeel, Mohd Ashif Khan
OBM Transplantation.2023; 07(02): 1. CrossRef
- Fracture risk and assessment in adults with cancer

- Endocrine Research
- Suppression of Fibrotic Reactions of Chitosan-Alginate Microcapsules Containing Porcine Islets by Dexamethasone Surface Coating
- Min Jung Kim, Heon-Seok Park, Ji-Won Kim, Eun-Young Lee, Marie Rhee, Young-Hye You, Gilson Khang, Chung-Gyu Park, Kun-Ho Yoon
- Endocrinol Metab. 2021;36(1):146-156. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.879

- 5,951 View
- 155 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The microencapsulation is an ideal solution to overcome immune rejection without immunosuppressive treatment. Poor biocompatibility and small molecular antigens secreted from encapsulated islets induce fibrosis infiltration. Therefore, the aims of this study were to improve the biocompatibility of microcapsules by dexamethasone coating and to verify its effect after xenogeneic transplantation in a streptozotocin-induced diabetes mice.
Methods
Dexamethasone 21-phosphate (Dexa) was dissolved in 1% chitosan and was cross-linked with the alginate microcapsule surface. Insulin secretion and viability assays were performed 14 days after microencapsulation. Dexa-containing chitosan-coated alginate (Dexa-chitosan) or alginate microencapsulated porcine islets were transplanted into diabetic mice. The fibrosis infiltration score was calculated from the harvested microcapsules. The harvested microcapsules were stained with trichrome and for insulin and macrophages.
Results
No significant differences in glucose-stimulated insulin secretion and islet viability were noted among naked, alginate, and Dexa-chitosan microencapsulated islets. After transplantation of microencapsulated porcine islets, nonfasting blood glucose were normalized in both the Dexa-chitosan and alginate groups until 231 days. The average glucose after transplantation were lower in the Dexa-chitosan group than the alginate group. Pericapsular fibrosis and inflammatory cell infiltration of microcapsules were significantly reduced in Dexa-chitosan compared with alginate microcapsules. Dithizone and insulin were positive in Dexa-chitosan capsules. Although fibrosis and macrophage infiltration was noted on the surface, some alginate microcapsules were stained with insulin.
Conclusion
Dexa coating on microcapsules significantly suppressed the fibrotic reaction on the capsule surface after transplantation of xenogenic islets containing microcapsules without any harmful effects on the function and survival of the islets. -
Citations
Citations to this article as recorded by- Engineering superstable islets-laden chitosan microgels with carboxymethyl cellulose coating for long-term blood glucose regulation in vivo
Haofei Li, Weijun He, Qi Feng, Junlin Chen, Xinbin Xu, Chuhan Lv, Changchun Zhu, Hua Dong
Carbohydrate Polymers.2024; 323: 121425. CrossRef - Investigation of encapsulation of pancreatic beta cells and curcumin within alginate microcapsules
Zahra Hosseinzadeh, Iran Alemzadeh, Manouchehr Vossoughi
The Canadian Journal of Chemical Engineering.2024; 102(2): 561. CrossRef - Advancements in innate immune regulation strategies in islet transplantation
Kehang Duan, Jiao Liu, Jian Zhang, Tongjia Chu, Huan Liu, Fengxiang Lou, Ziyu Liu, Bing Gao, Shixiong Wei, Feng Wei
Frontiers in Immunology.2024;[Epub] CrossRef - A Case for Material Stiffness as a Design Parameter in Encapsulated Islet Transplantation
Courtney D. Johnson, Helim Aranda-Espinoza, John P. Fisher
Tissue Engineering Part B: Reviews.2023; 29(4): 334. CrossRef - Improved membrane stability of alginate-chitosan microcapsules by crosslinking with tannic acid
Li Chen, Fang Jiang, Haidan Xu, Yaoyao Fan, Cunbin Du
Biotechnology Letters.2023; 45(8): 1039. CrossRef - Advances in alginate encapsulation of pancreatic islets for immunoprotection in type 1 diabetes
Dinesh Chaudhary, Tiep Tien Nguyen, Simmyung Yook, Jee-Heon Jeong
Journal of Pharmaceutical Investigation.2023; 53(5): 601. CrossRef - Emerging strategies for beta cell transplantation to treat diabetes
Jesus Paez-Mayorga, Izeia Lukin, Dwaine Emerich, Paul de Vos, Gorka Orive, Alessandro Grattoni
Trends in Pharmacological Sciences.2022; 43(3): 221. CrossRef - Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications
Wenyan Li, Xuejiao Lei, Hua Feng, Bingyun Li, Jiming Kong, Malcolm Xing
Pharmaceutics.2022; 14(2): 297. CrossRef - β cell replacement therapy for the cure of diabetes
Joonyub Lee, Kun‐Ho Yoon
Journal of Diabetes Investigation.2022; 13(11): 1798. CrossRef - Modern pancreatic islet encapsulation technologies for the treatment of type 1 diabetes
P. S. Ermakova, E. I. Cherkasova, N. A. Lenshina, A. N. Konev, M. A. Batenkin, S. A. Chesnokov, D. M. Kuchin, E. V. Zagainova, V. E. Zagainov, A. V. Kashina
Russian Journal of Transplantology and Artificial Organs.2021; 23(4): 95. CrossRef
- Engineering superstable islets-laden chitosan microgels with carboxymethyl cellulose coating for long-term blood glucose regulation in vivo

- Clinical Study
- Insulin Secretion and Insulin Resistance Trajectories over 1 Year after Kidney Transplantation: A Multicenter Prospective Cohort Study
- Jun Bae Bang, Chang-Kwon Oh, Yu Seun Kim, Sung Hoon Kim, Hee Chul Yu, Chan-Duck Kim, Man Ki Ju, Byung Jun So, Sang Ho Lee, Sang Youb Han, Cheol Woong Jung, Joong Kyung Kim, Su Hyung Lee, Ja Young Jeon
- Endocrinol Metab. 2020;35(4):820-829. Published online November 18, 2020
- DOI: https://doi.org/10.3803/EnM.2020.743

- 5,072 View
- 120 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated the changing patterns of insulin secretion and resistance and risk factors contributing to the development of post-transplant diabetes mellitus (PTDM) in kidney recipients under tacrolimus-based immunosuppression regimen during 1 year after transplantation.
Methods
This was a multicenter prospective cohort study. Of the 168 subjects enrolled in this study, we analyzed a total 87 kidney transplant recipients without diabetes which was assessed by oral glucose tolerance test before transplantation. We evaluated the incidence of PTDM and followed up the index of insulin secretion (insulinogenic index [IGI]) and resistance (homeostatic model assessment for insulin resistance [HOMA-IR]) at 3, 6, 9 months, and 1 year after transplantation by oral glucose tolerance test and diabetes treatment. We also assessed the risk factors for incident PTDM.
Results
PTDM developed in 23 of 87 subjects (26.4%) during 1 year after transplantation. More than half of total PTDM (56.5%) occurred in the first 3 months after transplantation. During 1 year after transplantation, insulin resistance (HOMA-IR) was increased in both PTDM and no PTDM group. In no PTDM group, the increase in insulin secretory function to overcome insulin resistance was also observed. However, PTDM group showed no increase in insulin secretion function (IGI). Old age, status of prediabetes and episode of acute rejection were significantly associated with the development of PTDM.
Conclusion
In tacrolimus-based immunosuppressive drugs regimen, impaired insulin secretory function for reduced insulin sensitivity contributed to the development of PTDM than insulin resistance during 1 year after transplantation. -
Citations
Citations to this article as recorded by- Prevalence of new-onset diabetes mellitus after kidney transplantation: a systematic review and meta-analysis
Qiufeng Du, Tao Li, Xiaodong Yi, Shuang Song, Jing Kang, Yunlan Jiang
Acta Diabetologica.2024;[Epub] CrossRef - Distúrbio do eixo hipotálamo-hipófise-gonadal e sua associação com resistência à insulina em receptores de transplante renal
Lourdes Balcázar-Hernández, Victoria Mendoza-Zubieta, Baldomero González-Virla, Brenda González-García, Mariana Osorio-Olvera, Jesús Ubaldo Peñaloza-Juarez, Irene Irisson-Mora, Martha Cruz-López, Raúl Rodríguez-Gómez, Ramón Espinoza-Pérez, Guadalupe Varga
Brazilian Journal of Nephrology.2023; 45(1): 77. CrossRef - Hypothalamic-pituitary-gonadal axis disturbance and its association with insulin resistance in kidney transplant recipients
Lourdes Balcázar-Hernández, Victoria Mendoza-Zubieta, Baldomero González-Virla, Brenda González-García, Mariana Osorio-Olvera, Jesús Ubaldo Peñaloza-Juarez, Irene Irisson-Mora, Martha Cruz-López, Raúl Rodríguez-Gómez, Ramón Espinoza-Pérez, Guadalupe Varga
Brazilian Journal of Nephrology.2023; 45(1): 77. CrossRef - Postoperative fasting plasma glucose and family history diabetes mellitus can predict post-transplantation diabetes mellitus in kidney transplant recipients
Le Wang, Jin Huang, Yajuan Li, Kewei Shi, Sai Gao, Wangcheng Zhao, Shanshan Zhang, Chenguang Ding, Wei Gao
Endocrine.2023; 81(1): 58. CrossRef - Changes in glucose metabolism among recipients with diabetes 1 year after kidney transplant: a multicenter 1-year prospective study
Jun Bae Bang, Chang-Kwon Oh, Yu Seun Kim, Sung Hoon Kim, Hee Chul Yu, Chan-Duck Kim, Man Ki Ju, Byung Jun So, Sang Ho Lee, Sang Youb Han, Cheol Woong Jung, Joong Kyung Kim, Hyung Joon Ahn, Su Hyung Lee, Ja Young Jeon
Frontiers in Endocrinology.2023;[Epub] CrossRef - Pretransplant evaluation and the risk of glucose metabolic alterations after renal transplantation: a prospective study
Arminda Fariña-Hernández, Domingo Marrero-Miranda, Estefania Perez-Carreño, Antonia De Vera-Gonzalez, Alejandra González, Cristian Acosta-Sorensen, Ana Elena Rodríguez-Rodríguez, Tatiana Collantes, Marta del Pino García, Ana Isabel Rodríguez-Muñoz, Carla
Nephrology Dialysis Transplantation.2022;[Epub] CrossRef
- Prevalence of new-onset diabetes mellitus after kidney transplantation: a systematic review and meta-analysis

- Stepwise Approach to Problematic Hypoglycemia in Korea: Educational, Technological, and Transplant Interventions
- Sang-Man Jin
- Endocrinol Metab. 2017;32(2):190-194. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.190
- 3,186 View
- 35 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Impaired awareness of hypoglycemia has been found to be prevalent in 20% to 40% of people with type 1 diabetes. If a similar prevalence exists in Koreans with type 1 diabetes, at a minimum, thousands of people with type 1 diabetes suffer at least one unpredicted episode of severe hypoglycemia per year in Korea. For patients with problematic hypoglycemia, an evidence-based stepwise approach was suggested in 2015. The first step is structured education regarding multiple daily injections of an insulin analog, and the second step is adding a technological intervention, such as continuous subcutaneous insulin infusion or real-time continuous glucose monitoring. The next step is a sensor-augmented pump, preferably with a low glucose suspension feature or very frequent contact, and the final step is islet or pancreas transplantation. In Korea, however, none of these treatments are reimbursed by the National Health Insurance, and thus have not been widely implemented. The low prevalence of type 1 diabetes means that Korean physicians are relatively unfamiliar with the new technologies in this field. Therefore, the roles of new technologies and pancreas or islet transplantation in the treatment of problematic hypoglycemia need to be defined in the current clinical setting of Korea.
-
Citations
Citations to this article as recorded by- Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross-sectional analysis of baseline data from the PR-IAH study
Naoki Sakane, Ken Kato, Sonyun Hata, Erika Nishimura, Rika Araki, Kunichi kouyama, Masako Hatao, Yuka Matoba, Yuichi Matsushita, Masayuki Domichi, Akiko Suganuma, Seiko Sakane, Takashi Murata, Fei Ling Wu
Diabetology & Metabolic Syndrome.2023;[Epub] CrossRef - A 4-Week, Two-Center, Open-Label, Single-Arm Study to Evaluate the Safety and Efficacy of EOPatch in Well-Controlled Type 1 Diabetes Mellitus
Jiyun Park, Nammi Park, Sangjin Han, You-Bin Lee, Gyuri Kim, Sang-Man Jin, Woo Je Lee, Jae Hyeon Kim
Diabetes & Metabolism Journal.2022; 46(6): 941. CrossRef - Impaired hypoglycemia awareness in diabetes: epidemiology, mechanisms and therapeutic approaches
Vadim V. Klimontov
Diabetes mellitus.2019; 21(6): 513. CrossRef
- Protective and risk factors of impaired awareness of hypoglycemia in patients with type 1 diabetes: a cross-sectional analysis of baseline data from the PR-IAH study

- Clinical Study
- Effects of Dipeptidyl Peptidase-4 Inhibitors on Hyperglycemia and Blood Cyclosporine Levels in Renal Transplant Patients with Diabetes: A Pilot Study
- Jaehyun Bae, Min Jung Lee, Eun Yeong Choe, Chang Hee Jung, Hye Jin Wang, Myoung Soo Kim, Yu Seun Kim, Joong-Yeol Park, Eun Seok Kang
- Endocrinol Metab. 2016;31(1):161-167. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.161
- 5,786 View
- 58 Download
- 21 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The use of dipeptidyl peptidase-4 (DPP-4) inhibitors is increasing among renal transplant patients with diabetes. However, the glucose-lowering efficacies of various DPP-4 inhibitors and their effects on blood cyclosporine levels have not been fully investigated. We compared the glucose-lowering efficacies of DPP 4 inhibitors and evaluate their effects on the blood levels of cyclosporine in renal transplant recipients with diabetes.
Methods Sixty-five renal allograft recipients who received treatment with DPP-4 inhibitors (vildagliptin, sitagliptin, or linagliptin) following kidney transplant were enrolled. The glucose-lowering efficacies of the DPP-4 inhibitors were compared according to the changes in the hemoglobin A1c (HbA1c) levels after 3 months of treatment. Changes in the trough levels of the cyclosporine were also assessed 2 months after treatment with each DPP-4 inhibitor.
Results HbA1c significantly decreased in the linagliptin group in comparison with other DPP-4 inhibitors (vildagliptin –0.38%±1.03%, sitagliptin –0.53%±0.95%, and linagliptin –1.40±1.34;
P =0.016). Cyclosporine trough levels were significantly increased in the sitagliptin group compared with vildagliptin group (30.62±81.70 ng/mL vs. –24.22±53.54 ng/mL,P =0.036). Cyclosporine trough levels were minimally changed in patients with linagliptin.Conclusion Linagliptin demonstrates superior glucose-lowering efficacy and minimal effect on cyclosporine trough levels in comparison with other DPP-4 inhibitors in kidney transplant patients with diabetes.
-
Citations
Citations to this article as recorded by- Diabetic Kidney Disease in Post-Kidney Transplant Patients
Ngoc-Yen T. Pham, Diego Cruz, Luis Madera-Marin, Raja Ravender, Pablo Garcia
Journal of Clinical Medicine.2024; 13(3): 793. CrossRef - International consensus on post-transplantation diabetes mellitus
Adnan Sharif, Harini Chakkera, Aiko P J de Vries, Kathrin Eller, Martina Guthoff, Maria C Haller, Mads Hornum, Espen Nordheim, Alexandra Kautzky-Willer, Michael Krebs, Aleksandra Kukla, Amelie Kurnikowski, Elisabeth Schwaiger, Nuria Montero, Julio Pascual
Nephrology Dialysis Transplantation.2024; 39(3): 531. CrossRef - Metabolic Disorders in Liver Transplant Recipients: The State of the Art
Filippo Gabrielli, Lucia Golfieri, Fabio Nascimbeni, Pietro Andreone, Stefano Gitto
Journal of Clinical Medicine.2024; 13(4): 1014. CrossRef - Diabetic Kidney Disease in Post-Transplant Diabetes Mellitus: Causes, Treatment and Outcomes
Lee-Moay Lim, Jer-Ming Chang, Hung-Tien Kuo
Biomedicines.2023; 11(2): 470. CrossRef - Sweet and simple as syrup: A review and guidance for use of novel antihyperglycemic agents for post‐transplant diabetes mellitus and type 2 diabetes mellitus after kidney transplantation
S. Elise Lawrence, Mary Moss Chandran, Jeong M. Park, Helen Sweiss, Thomas Jensen, Palak Choksi, Barrett Crowther
Clinical Transplantation.2023;[Epub] CrossRef - Interventions Against Posttransplantation Diabetes: A Scientific Rationale for Treatment Hierarchy Based on Literature Review
Adnan Sharif
Transplantation.2022; 106(12): 2301. CrossRef - Dipeptidyl Peptidase-4 Inhibitor Decreases Allograft Vasculopathy Via Regulating the Functions of Endothelial Progenitor Cells in Normoglycemic Rats
Feng-Yen Lin, Chun-Min Shih, Chun-Yao Huang, Yi-Tin Tsai, Shih-Hurng Loh, Chi-Yuan Li, Cheng-Yen Lin, Yi-Wen Lin, Chien-Sung Tsai
Cardiovascular Drugs and Therapy.2021; 35(6): 1111. CrossRef - Review of Newer Antidiabetic Agents for Diabetes Management in Kidney Transplant Recipients
Sonya Anderson, Laura Cotiguala, Sarah Tischer, Jeong Mi Park, Katie McMurry
Annals of Pharmacotherapy.2021; 55(4): 496. CrossRef - Incretin based therapies and SGLT-2 inhibitors in kidney transplant recipients with diabetes: A systematic review and meta-analysis
Dora Oikonomaki, Evangelia Dounousi, Anila Duni, Stefanos Roumeliotis, Vassilios Liakopoulos
Diabetes Research and Clinical Practice.2021; 172: 108604. CrossRef - CD161a-positive natural killer (NK) cells and α-smooth muscle actin-positive myofibroblasts were upregulated by extrarenal DPP4 in a rat model of acute renal rejection
Franziska Schmid, Christina Mayer, Maike Büttner-Herold, Stephan von Hörsten, Kerstin Amann, Christoph Daniel
Diabetes Research and Clinical Practice.2021; 173: 108691. CrossRef - Current Pharmacological Intervention and Medical Management for Diabetic Kidney Transplant Recipients
Theerawut Klangjareonchai, Natsuki Eguchi, Ekamol Tantisattamo, Antoney J. Ferrey, Uttam Reddy, Donald C. Dafoe, Hirohito Ichii
Pharmaceutics.2021; 13(3): 413. CrossRef - Recent advances in new-onset diabetes mellitus after kidney transplantation
Tess Montada-Atin, G V Ramesh Prasad
World Journal of Diabetes.2021; 12(5): 541. CrossRef - Safety and Efficacy of Long-Term Administration of Dipeptidyl peptidase IV Inhibitors in Patients With New Onset Diabetes After Kidney Transplant
Adamantia Mpratsiakou, Marios Papasotiriou, Theodoros Ntrinias, Konstantinos Tsiotsios, Evangelos Papachristou, Dimitrios S. Goumenos
Experimental and Clinical Transplantation.2021; 19(5): 411. CrossRef - Medical management of metabolic and cardiovascular complications after liver transplantation
Chiara Becchetti, Melisa Dirchwolf, Vanessa Banz, Jean-François Dufour
World Journal of Gastroenterology.2020; 26(18): 2138. CrossRef - Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors in Kidney Transplant Recipients with Post-transplant Diabetes Mellitus (PTDM)- a Systematic Review and Meta-Analysis
Tarek Samy Abdelaziz, Ahmed Yamany Ali, Moataz Fatthy
Current Diabetes Reviews.2020; 16(6): 580. CrossRef - NAFLD and liver transplantation: Disease burden, current management and future challenges
Patrizia Burra, Chiara Becchetti, Giacomo Germani
JHEP Reports.2020; 2(6): 100192. CrossRef - Linagliptin plus insulin for hyperglycemia immediately after renal transplantation: A comparative study
Rodolfo Guardado-Mendoza, David Cázares-Sánchez, María Lola Evia-Viscarra, Lilia M. Jiménez-Ceja, Edgar G. Durán-Pérez, Alberto Aguilar-García
Diabetes Research and Clinical Practice.2019; 156: 107864. CrossRef - Post-Liver Transplantation Diabetes Mellitus: A Review of Relevance and Approach to Treatment
Maria J. Peláez-Jaramillo, Allison A. Cárdenas-Mojica, Paula V. Gaete, Carlos O. Mendivil
Diabetes Therapy.2018; 9(2): 521. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Drug–drug interactions between immunosuppressants and antidiabetic drugs in the treatment of post-transplant diabetes mellitus
Thomas Vanhove, Quinten Remijsen, Dirk Kuypers, Pieter Gillard
Transplantation Reviews.2017; 31(2): 69. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef
- Diabetic Kidney Disease in Post-Kidney Transplant Patients

- Contributing Factors to Different Natural Courses of Posttansplantation Diabetes Mellitus in Renal Allograft Recipients.
- Kyu Yeon Hur, Myoung Soo Kim, Jae Hyun Nam, Eun Seok Kang, Hyun Joo Lee, So Hun Kim, Bong Soo Cha, Chul Woo Ahn, Soon Il Kim, Yu Seun Kim, Hyun Chul Lee
- J Korean Endocr Soc. 2006;21(5):373-381. Published online October 1, 2006
- DOI: https://doi.org/10.3803/jkes.2006.21.5.373
- 2,036 View
- 22 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
New onset diabetes is a major complication after kidney transplantation. However, the natural course of posttransplantation diabetes mellitus (PTDM) remains unclear. The aim of this study was to demonstrate the detailed natural courses of PTDM according to the onset and persistency of hyperglycemia, and to investigate risk factors for development of different courses of PTDM in renal allograft recipients. METHODS: A total of 77 renal allograft recipients without previously known diabetes were enrolled and performed a serial 75 g oral glucose tolerance test at 0, 1, and 7 years after kidney transplantation. Patients were classified according to the onset and persistency of PTDM: early PTMD (E-PTDM), late PTDM (L-PTDM), persistent PTDM (P-PTDM), transient PTMD (T-PTDM), and non-PTDN (N-PTDM). RESULTS: The incidence of each group was as follows: E-PTDM, 39%; L-PTDM, 11.7%; P-PTDM, 23.4% T-PTDM, 15.6%; N-PTDM, 49.3%. Tacrolimus and female gender were associated with the development of E-PTDM. Among E-PTDM, age at transplantation was a high risk factor for the development of P-PTDM. Higher BMI at year1 was associated with the development of L-PTDM. CONCLUSION: Different risk factors were associated with various natural courses of PTDM. Since old age and female gender are not modifiable risk factors, it may be important to modify immunosuppressive therapy regimens for the prevention of E-PTDM and control of body weight for L-PTDM. -
Citations
Citations to this article as recorded by- Efficacy and Safety of Gemigliptin in Post-Transplant Patients With Type 2 Diabetes Mellitus
Jaehyun Bae, Youjin Kim, Yongin Cho, Minyoung Lee, Ji-Yeon Lee, Yong-ho Lee, Byung-Wan Lee, Bong-Soo Cha, Dong Jin Joo, Kyu Ha Huh, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Transplantation Proceedings.2019; 51(10): 3444. CrossRef - Post-transplantation Diabetes Mellitus
Kun-Ho Yoon
Journal of Korean Endocrine Society.2006; 21(5): 370. CrossRef
- Efficacy and Safety of Gemigliptin in Post-Transplant Patients With Type 2 Diabetes Mellitus

- Standardization of Isolation Procedure and Analysis of Variables on Successful Isolation of Islet from the Human Pancreas.
- Song Cheol Kim, Duck Jong Han, Ik Hee Kim, Yoo Me We, Yang Hee Kim, Jin Hee Kim, Ji He Back, Dong Gyun Lim
- J Korean Endocr Soc. 2006;21(1):22-31. Published online February 1, 2006
- DOI: https://doi.org/10.3803/jkes.2006.21.1.22
- 1,628 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Identifying the donor and isolation-related factors during the islet isolation would be greatly helpful to improve the result of human islet isolation for successful clinical islet transplantation. METHODS: Sixty-nine pancreata from cadaveric donors were isolated with standard protocol and analyzed to identify the donor factors and isolation variables for successful isolation. Islet isolations recovered > or = 100,000 Islet Equivalent (IEQ, n=53) were compared to islet mass less than 100,000 IEQ (n=16). RESULTS: The mean islet recovery was 216.0 x 10(3) +/- 173.7 x 10(3) (IEQ) before purification and 130.6 x 10(3) +/- 140.2 x 10(3) (IEQ) after purification. Mean purity was 54 +/- 31%. Mean age of donor was 31.2 +/- 13.2 year and mean cold ischemic time was 6.9 +/- 6.2 hour. Quality of isolated islets was acceptable in terms of bacterial culture, viability and secretory function in vitro and in vivo. In univariate analysis on successful isolation, status of pancreas was the only significant factor and sex, duration of collagenase expansion and digestion time were marginal factors. Stepwise multivariate logistic regression analysis showed donor sex, status of pancreas and digestion time were significant factors for the successful islet isolation. CONCLUSION: This study confirms some donor factors and variables in isolation process can influence the ability to obtain the successful isolation of human islet. Enough experiences and pertinent review of donor and isolation factors can make islet isolation successful, supporting the clinical islet transplantation without spending of cost.

- The Changes in the Serum RANKL and OPG levels after Bone Marrow Transplantation: Association with Bone Mineral Metabolism.
- Hyun Jung Tae, Ki Hyun Baek, Eun Sook Oh, Ki Won Oh, Won Young Lee, Hye Soo Kim, Je Ho Han, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang, Choon Choo Kim, Moo Il Kang
- J Korean Endocr Soc. 2005;20(1):40-51. Published online February 1, 2005
- DOI: https://doi.org/10.3803/jkes.2005.20.1.40
- 1,663 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The loss of bone mass is usually detected after bone marrow transplantation(BMT), particularly during the early post-transplant period. We recently reported that enhanced bone resorption following BMT was related to both the steroid dose and increase in IL-6. It was also suggested damage of the marrow microenvironment due to myeloablation and changes in bone growth factors contribute to post-BMT bone loss. Recently, the interactions of OPG and RANKL have been reported to be crucial in osteoclastogenesis and therefore in bone homeostasis. There are few data on the changes in RANKL/OPG status during the post-BMT period. This study investigated the changes in the levels of RANKL and OPG during the post-BMT period, and also assessed whether the changes in these cytokine levels actually influenced bone turnover and post-BMT bone loss. METHODS: We prospectively investigated 110 patients undergoing allogenic BMT and analyzed 36 (32.4+/-1.3 years, 17 men and 19 women) where DEXA was performed before and 1 year after the BMT. The serum bone turnover marker levels were measured before and 1, 2, 3, 4 and 12 wks, 6 Ms, and 1 yr after the BMT. The serum sRANKL and OPG levels were measured in all patients before and 1, 3 and 12 wks after the BMT. RESULTS: The mean bone losses in the lumbar spine and total proximal femur, which were calculated as the percent change from the baseline to 1 yr, were 5.2(P<0.01) and 11.6%(P<0.01), respectively. The mean serum ICTP, a bone resorption marker, increased progressively until 3 and 6 months after the BMT, but decreased gradually thereafter, reaching the basal values after 1 year. The serum osteocalcin levels decreased progressively until 3 wks after the BMT, then increased transiently at 3 and 6 Ms, but returned to the basal level by 1 yr. The serum sRANKL and OPG levels had increased significantly by weeks 1 and 3 compared with the baseline(P<0.01), but decreased at 3 months. The sRANKL/OPG ratio increased progressively until 3 weeks, but then decreased to the basal values. During the observation period, the percent changes from the baseline in the serum RANKL levels and RANKL/OPG ratio showed positive correlations with the percent changes from the baseline serum ICTP levels. Patients with higher RANKL levels and RANKL/OPG ratio during the early post-BMT period lost more bone mass at the lumbar spine. CONCLUSION: In conclusion, dynamic changes in the sRANKL and OPG levels were observed during the immediate post-BMT period, which were related to a decrease in bone formation and loss of L-spine BMD during the year following the BMT. Taken together, these results suggest that increased sRANKL levels and sRANKL/OPG ratios could be involved in a negative balance in bone metabolism following BMT.

- The Changes of Serum Growth Factors after Hematopoietic Stem Cell Transplantation: Impact on Bone Mineral Metabolism.
- Ki Hyun Baek, Eun Sook Oh, Ki Won Oh, Won Young Lee, Hye Soo Kim, Soon Yong Kwon, Je Ho Han, Moo Il Kang, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang, Choon Choo Kim
- J Korean Endocr Soc. 2002;17(5):664-674. Published online October 1, 2002
- 1,102 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
A loss of bone mass is usually detected after a bone marrow transplantation (BMT), especially during the early post-transplant period. We recently reported that enhanced bone resorption following a BMT was related to both the steroid dose and the increase in IL-6. We also suggested damage to the marrow stromal microenvironment, by myoablation, partly explains the impaired bone formation following a BMT. It is well known that some growth factors play important role in bone growth and osteogenesis. However, the pathogenetic role of bone growth factors in post-BMT bone loss is unknown and data on the changes in the growth factors, in accordance with bone turnover markers and bone mineral density (BMD) changes are scarce. We investigated changes in bone growth factors such as IGF-I (Insulin-like growth factor-I), fibroblast growth factor-2 (FGF-2) and Macrophage colony stimulating factor (M-CSF), during the post-BMT period, and assessed whether the growth factor changes influenced the bone turnover and post-BMT bone loss. The present study is the first prospective study to describe the changes in bone growth factors following a BMT. METHODS: We prospectively investigated 110 patients undergoing a BMT, and analyzed 36 patients (32.4+/-1.3 years, 17 men and 19 women) whose BMDs were measured before, and 1 year after, the BMT. The serum biochemical markers of bone turnover were measured before, 1, 2, 3 and 4 weeks, 3 and 6 months, and 1 year, after the BMT. The serum FGF-2, IGF-I and M-CSF levels were measured before and 1 and 3 weeks, and 3 months after the BMT. The correlation between the changes of growth factors and various bone parameters was analyzed. RESULTS: The mean bone losses in the lumbar spine and total proximal femur, calculated as the percentage change from the baseline to the level at 1 year, were 5.2 (p<0.05) and 11.6% (p<0.01), respectively. The serum type I carboxyterminal telopeptide (ICTP), a bone resorption marker, increased progressively until 6 months after the BMT, but thereafter decreased, to the base value after 1 year. Serum osteocalcin, a bone formation marker, decreased progressively, until 3 weeks after the BMT but then increased transiently, and finally returned to the base level at 1 year. The serum IGF-I and FGF-2 also decreased progressively until 3 weeks and 1 week after the BMT, respectively, then increased to the base values at 3 months. The serum M-CSF increased briskly at 1 week post-BMT, then decreased to the base level. There were positive correlations between the percentage changes from the baseline proximal femur BMD and the IGF-I levels 3 weeks and 3 months (r=0.52, p<0.01, r=0.41, p<0.05) post BMT. A Significant correlation was found between the IGF-I and osteocalcin levels pre-BMT, and 3 weeks after the BMT. Another positive correlation was found between the M-CSF and the ICTP levels at 3 weeks post BMT (r=0.54, p<0.05). CONCLUSION: In conclusion, there were significant changes in the serum IGF-I, FGF-2 and M-CSF levels in the immediate post-BMT period, which were related to a decrease in bone formation and loss in the proximal femoral BMD during the year following the BMT

- Effect of Intermittent Etidronate Therapy on the Prevention of Bone Loss after Kidney Transplantation.
- Hye Soo Kim, Jong Min Lee, Sung Kwon Kim, Cheol Whee Park, Chul Woo Yang, Moo Il Kang, Suk Young Kim, Sung Koo Kang, Byung Kee Bang
- J Korean Endocr Soc. 2001;16(4-5):426-437. Published online October 1, 2001
- 1,103 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Osteopenia or osteoporosis is one of the most frequently encountered complications in patients receiving various immunosuppressants after kidney transplantation. The few available preventive strategies for these complications tend to result in various outcomes. In this study, we evaluated the effect of intermittent etidronate therapy for the prevention of bone loss after kidney transplantation. METHODS: Fifty patients who received kidney transplantation for various reasons were recruited and followed for one year. Thirty-eight of these patients commenced etidronate treatment 7 days after operation, the other 12 were followed without etidronate therapy. The treatment consisted of 400mg of etidronate administered orally for 14 days, then repeated four-times every three months. Blood chemistry, iPTH and aluminium levels were tested periodically in all patients. Also checked were bone mineral density of the lumbar spine(L2-4) and femur at baseline, 6 and 12 months after kidney transplantation, as well as D-L spine lateral x-ray at baseline and 12 months. Serum osteocalcin and urine deoxypyridinoline were measured at baseline, 7 days and then every 3 months. RESULTS: Both the etidronate-treated and control groups showed significant decreases in bone mineral densities of the lumbar spine, femur neck and total femur at 6 and 12 months after kidney transplantation(p<0.005). Bone loss was significantly lower in the etidronate-treated group than the control at 12 months after kidney transplantation; lumbar spine(-3.54% vs. -9.51%, p<0.0005), femur neck (-5.41% vs. -8.91%, p<0.0005), total femur (-7.59% vs. -9.07%, p<0.005). Osteocalcin was decreased and deoxypyridinoline increased in both groups. No significant differences in the level or pattern of osteocalcin and deoxypyridinoline were observed in either group. New radiologic compression fractures were found in two patients of the treated group who exhibited severe osteoporosis at baseline during follow-up. CONCLUSIONS: The intermittent administration of etidronate seems to be effective in preventing rapid bone loss after kidney transplantation. Furthermore, this method is safe and convenient for administration and follow-up. Further studies will be required to elucidate the most effective treatment course for the prevention of fractures after kidney transplantation, especially in patients with established severe osteoporosis.

- The Effect of Hematopoietic Stem Cell Transplantation in the Origin and the Osteoblastic Differentiation of the Human Bone Marrow Stromal Cell.
- Moo Il Kang, Seong Won Cho, Eun Sook Oh, Ki Hyun Baik, Won Young Lee, Ki Won Oh, Hye Soo Kim, Je Ho Han, Kun Ho Yoon, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang, Choon Choo Kim
- J Korean Endocr Soc. 2000;15(4-5):571-581. Published online January 1, 2001
- 1,129 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Bone marrow transplantation is the treatment of choice for patients with certain- hematological malignancies, many of whom will survive many years thereafter. Bone disease is a potential longterm complication. But, little is known about the effects of bone marrow transplantation on bone. METHODS: In this study, bone marrow was obtained from healthy donor and transplant recipients. Then mononuclear cells including marrow stromal cells were isolated and cultured. At near confluence, bone marrow stromal cells were subcultured. Thereafter alkaline phosphatase activities of each group were measured by time course of secondary culture. We also analysed the origin of marrow stromal cells by the polymerase chain reaction using YNZ 22 minisatellite probe. RESULTS: l. Cells cultured in our system showed the characteristics of marrow stromal cells differentiated to osteoblasts. They were in fibroblast-like spindle shape and positive to alkaline pbosphatase histochemistry and Von Kossa histochemistry in secondary cultures. 2. The time required for the near confluence in the primary culture was 15 days and 22.9 days on the average in healthy donors and transplant recipients, respectively (p=0.003). 3. In secondary cultures, healthy donors and transplant recipients showed peak alkaline phosphatase activity at 10 days and 17 days, respectively (p=0.031). Alkaline phosphatase activity was lower in BMT recipients than in healthy donors during the whole period of secondary cultures. 4. In polymerase chain reaction analysis using YNZ 22 minisatellite probe, bone marrow stromal cells were of recipient origin. CONCLUSION: Recipient-derived bone marrow stromal cells may be damaged secondary to the effect of chemotherapy, glucocorticoid & total body irradiation which have given before bone marrow transplantation. So it may affect the differentiation of bone marrow stromal cells into the osteoblasts.

- The Nonthyroidal Illness Syndrome: Prognostic Value and Circulating Cytokines after Allogeneic Bone Marrow Transplantation.
- Ki Won Oh, Moo Il Kang, Won Young Lee, Hyun Shik Son, Kun Ho Yoon, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang, Wan Sik Shin, Woo Sung Min, Choon Choo Kim, Byung Young Ahn, Hyung Sun Sohn
- J Korean Endocr Soc. 2000;15(2):214-225. Published online January 1, 2001
- 979 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Alteration of thyroid hormone parameters are frequently observed in sick patients and commonly known as nonthyroidal illness syndrome(NTIS) or euthyroid sick syndrome(ESS). NTIS is seen in starvation, surgery, severe illness, and also bone marrow transplantation(BMT). The degree of reduction in thyroid hormone parameters correlated with the severity of NTIS and might predict the prognosis of underlying illness. Recently, particular attention is focused on the role of cytokines in developing the NTIS. This prospective study was designed to assess the relationship of serum thyroid hormone parameters and serum cytokine levels before and in the short-term follow-up after allogeneic BMT in order to predict patients outcome. METHODS: Included 80 patients that were mainly leukemia and severe aplastic anemia. Serum thyroid hormone parameters and serum cytokine levels were measured before and 7, 14, 21, 28 days and 3, and 6 months after BMT. RESULTS: Near-all patients experienced significant decrease of thyroid hormone levels and also significant increase of cytokine levels after BMT. After post-BMT 3 weeks, the serum cytokine levels were negatively correlated with the serum T3 and T4 levels, but not with the serum TSH levels. The patients treated with high-dose steroid or total-body irradiation tended to show lower levels of TSH and more delayed recovery compared to non-treated patients. The patients died after BMT represented generally lower levels of all thyroid hormone parameters than survival patients during entire follow-up period. CONCLUSION: Development of NTIS is associated with higher probability of fatal outcome after BMT and has prognostic relationship in this group of patients. Increased levels of cytokines, especially IL-6 and TNF-alpha, are often found in post-BMT NTIS patients and correlated with the changes in the levels of thyroid hormone parameters.


 KES
KES

 First
First Prev
Prev



