Articles
- Page Path
- HOME > Endocrinol Metab > Volume 30(3); 2015 > Article
-
Review ArticleConnecting Myokines and Metabolism
- Rexford S. Ahima1, Hyeong-Kyu Park2
-
Endocrinology and Metabolism 2015;30(3):235-245.
DOI: https://doi.org/10.3803/EnM.2015.30.3.235
Published online: August 4, 2015
1Division of Endocrinology, Diabetes and Metabolism, and the Institute for Diabetes, Obesity and Metabolism, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
2Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- Corresponding author: Rexford S. Ahima. Division of Endocrinology, Diabetes and Metabolism, Perelman School of Medicine at the University of Pennsylvania, 12-104 Smilow Translational Research Center, 3400 Civic Center Boulevard, Building 421, Philadelphia, PA 19104, USA. Tel: +1-215-573-1872, Fax: +1-215-898-5408, ahima@mail.med.upenn.edu
Copyright © 2015 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Skeletal muscle is the largest organ of the body in non-obese individuals and is now considered to be an endocrine organ. Hormones (myokines) secreted by skeletal muscle mediate communications between muscle and liver, adipose tissue, brain, and other organs. Myokines affect muscle mass and myofiber switching, and have profound effects on glucose and lipid metabolism and inflammation, thus contributing to energy homeostasis and the pathogenesis of obesity, diabetes, and other diseases. In this review, we summarize recent findings on the biology of myokines and provide an assessment of their potential as therapeutic targets.
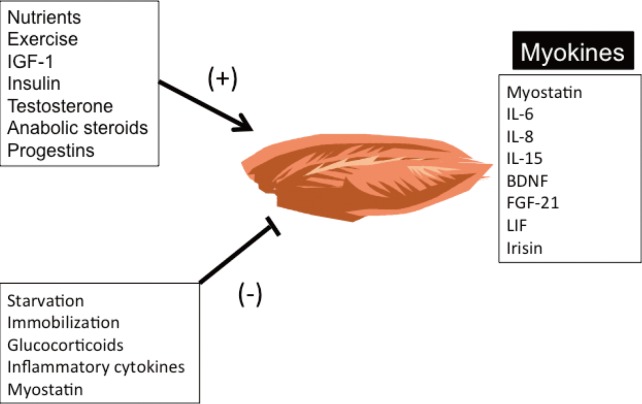
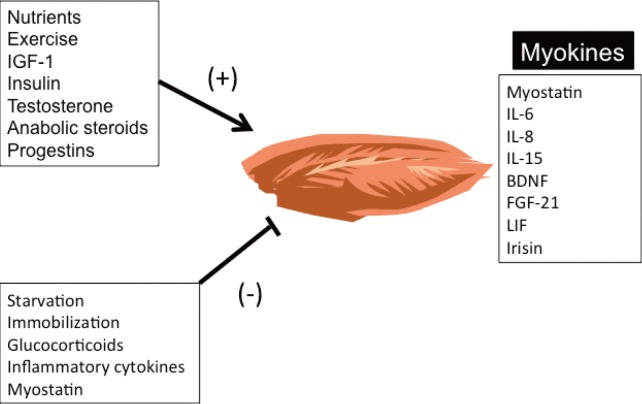
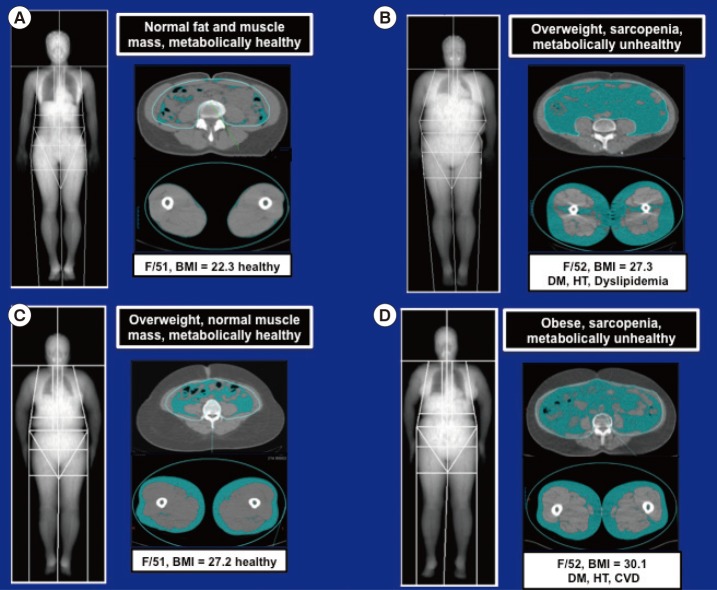
- Lack of exercise and sedentary lifestyle have been linked to obesity, type 2 diabetes, cardiovascular diseases, cancer, osteoporosis, and premature death [1234567]. Skeletal muscle is the most abundant tissue in non-obese adults, accounting for approximately 40% of the body weight [8]. Skeletal muscle adapts to mechanical, neural and humoral stimuli, and plays critical roles in physical activity, energy expenditure, and glucose disposal [910]. Exercise and anabolic hormones, e.g., insulin, insulin-like growth factor 1, growth hormone and testosterone, increase skeletal muscle mass (Fig. 1) [1112]. Conversely, physical inactivity from aging or neuromuscular disorders, and chronic diseases, such as cancer, renal failure, respiratory failure, infection, and some endocrine disorders, e.g., uncontrolled diabetes mellitus, hyperthyroidism and hypercortisolism, cause muscle atrophy or "sarcopenia" (Fig. 1) [1314151617]. Sarcopenia has been linked to obesity, metabolic syndrome, and other diseases in aged populations, particularly in South Asia (Fig. 2) [18192021].
- The concept that skeletal muscle secretes humoral factors that actively communicate with other organs was proposed many years ago [222324]. Henningsen et al. [25] and Pedersen et al. [26] used the term "myokines" to describe cytokines and other peptides expressed and released by muscle cells. This review highlights the biological actions of myostatin and other myokines that regulate skeletal muscle mass and metabolism via autocrine, paracrine, and endocrine mechanisms.
INTRODUCTION
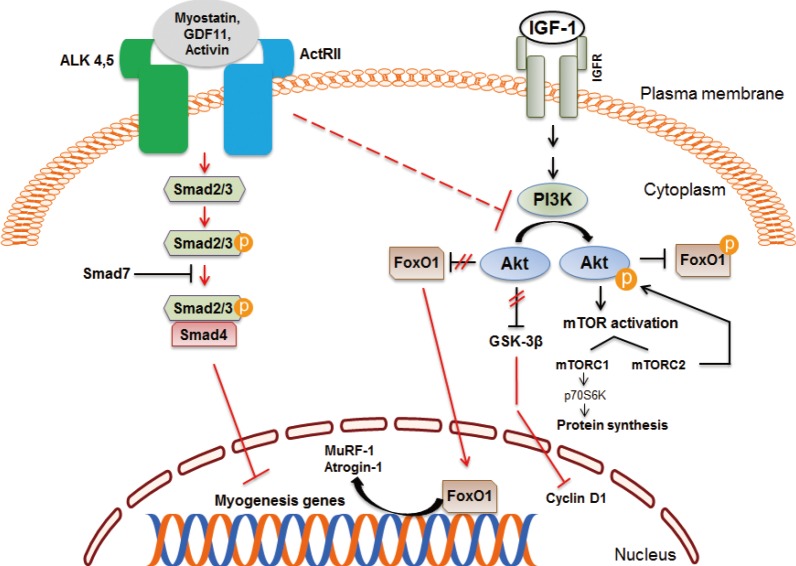
- Much attention has been focused on the biology of the transforming growth factor β (TGF-β) superfamily of proteins since the discovery of myostatin [27]. Myostatin, also known as growth differentiation factor 8, is expressed and secreted predominantly by skeletal muscle and inhibits muscle growth. This function is conserved in many species, as evident by the hypermuscular phenotype resulting from inactivation of myostatin gene in mice, sheep, cattle, and human [2829303132]. During early postnatal development, myostatin inhibits muscle stem cell proliferation, differentiation, and protein synthesis [33]. Normally, the differentiation of skeletal muscle cells requires growth arrest followed by expression of muscle-specific genes. These processes are coordinated by activation of specific cyclins, cyclin-dependent kinases (Cdk), Cdk inhibitors (CdkIs), and muscle regulatory factors. During the proliferation phase, myostatin up-regulates p21 (a CdkI), and decreases the levels of Cdk2 and Cdk4, leading to cell cycle arrest.
- Myostatin inhibits satellite cell activation by down-regulating the transcription factor Pax7, and also controls the myogenic differentiation program through inhibition of myogenic regulatory factors, such as Pax3, MyoD, and Myf5. Studies indicate that myostatin's inhibitory effect on muscle differentiation in the postnatal period is mediated partly by perturbation of Akt/mammalian target of rapamycin complex1 signaling [34353637]. In mature adult muscle fibers, the C-terminal dimer of myostatin binds to activin receptors II (ActRII), mainly ActRIIB and to a lesser degree ActRIIA, which then recruits, phosphorylates and activates activin receptor-like kinase (Alk) 4 and Alk5, leading to phosphorylation and activation of Smad2 and Smad3 [3839]. Phosphorylated Smad2 and Smad3 form a heterodimeric complex with Smad4, which is translocated into the nucleus, and acts as a transcription factor to regulate gene expression. Myostatin signaling also leads to activation of Smad7 which functions as a negative feedback inhibitor [4041]. The activation of myostatin-Smad pathway inhibits the translation initiation complex and protein synthesis. Myostatin suppresses Akt signaling and acts via forkhead box protein O1 transcription factors to promote protein breakdown through activation of the ubiquitin-proteasome system (Fig. 3). Myostatin also inhibits the autophagy-lysosome system [4243].
- Genetic or pharmacologic inhibition of myostatin, ActRIIB, Alk4/Alk5, or Smad2/3 results in skeletal muscle hypertrophy, associated with increased protein synthesis and reduced protein degradation [44]. Myostatin knockout (Mstn-/-) mice have increased skeletal muscle mass as well as reduced body fat [45]. Myostatin-null agouti lethal yellow or leptin deficient mice have drastically reduced body fat and glucose levels raising the possibility that blockade of myostatin signaling may be useful for treating obesity and diabetes [46]. Guo et al. [47] have shown that Mstn-/- mice have increased glucose utilization and insulin sensitivity. To determine whether these effects were due to a lack of myostatin signaling in muscle or adipose tissue, they compared the metabolic phenotypes of mice carrying a dominant negative ActRIIB receptor expressed in adipocytes or skeletal muscle. The absence of myostatin signaling in adipocytes did not affect body composition or glucose homeostasis, whereas inhibition of myostatin signaling in skeletal muscle recapitulated the phenotype of Mstn-/- mice, characterized by hypermuscularity, decreased body fat, and enhanced insulin sensitivity [47].
- We studied the effects of pharmacological blockade of myostatin and related peptides by treating mice on chow and high-fat diets with a soluble activin receptor type IIB (ActRIIB-Fc). ActRIIB-Fc treatment increased lean and skeletal muscle mass, grip strength, and contractile force, decreased body fat, and increased insulin sensitivity [48]. Mice lacking Akt1 or Akt2 have reduced muscle mass, grip strength and contractile force, consistent with a pivotal role of Akt signaling in promoting muscle growth [4950]. Contrary to in vitro studies showing that Akt signaling is necessary for the ability of ActRIIB inhibition to induce muscle hypertrophy, we found that Akt1 and Akt2 deficient mice responded similarly as wild type mice to ActRIIB-Fc in regard to increased muscle size, grip strength and contractile force, indicating these Akt isoforms are not essential for ActRIIB signaling [51].
- ActRIIB-Fc has also been shown to decrease diet-induced obesity and improve glucose and lipid levels in mice [48]. Importantly, ActRIIB-Fc induced the browning of white adipose tissue (WAT), as shown by increased expression of the thermogenic genes uncoupling protein 1 (UCP1) and peroxisomal proliferator-activated receptor-γ coactivator 1α (PGC1α). Thus, the anti-obesity effect of ActRIIB-Fc is partly by increasing skeletal muscle mass as well as inducing thermogenesis in WAT [52]. Other studies have confirmed that deficiency of myostatin signaling in Mstn-/- mice promotes browning of WAT [5354]. WAT of Mstn-/- mice displays features of brown adipose tissue, e.g., increased expression of including UCP1 and PGC1α, as well as expression of beige adipocyte markers, e.g., Tmem26 and CD137. The enhanced browning of adipose tissue appears to be mediated by irisin (fibronectin type III domain-containing 5, Fndc5), a myokine secreted from skeletal muscle in Mstn-/- mice. Myostatin deficiency stimulates AMPK expression and phosphorylation, which then activates PGC1α and irisin and promotes the browning of adipose tissue and thermogenesis [54]. Another study has shown that the reduction of body fat in Mstn-/- mice is due to increased energy expenditure and leptin sensitivity [55]. The cross-talk of myokines and adipokines may provide novel therapeutic tools for treating obesity, diabetes, and diseases associated with muscle atrophy.
- Does myostatin blockade have clinical potential? A double-blind, placebo-controlled study evaluated the safety, pharmacokinetics, and pharmacodynamics of a decoy ActRIIB receptor (ACE-031) in healthy postmenopausal women randomized to receive a single dose of ACE-031 (0.02 to 3 mg/kg subcutaneous) or placebo. ACE-031 treatment had mild adverse events and produced significant increases of lean mass and thigh muscle volume at day 29 in those receiving 3 mg/kg. Moreover, ACE-031 treatment increased adiponectin by 51.3% and decreased leptin by 27.7% demonstrating a favorable metabolic profile [56]. Androgen deprivation therapy for prostate cancer causes sarcopenia and increased body fat. An anti-myostatin peptibody (AMG 745/Mu-S) was evaluated in men undergoing androgen deprivation therapy for non-metastatic prostate cancer [57]. The adverse events in AMG 745 versus placebo treated groups were: diarrhea (13% vs. 9%), fatigue (13% vs. 4%), contusion (10% vs. 0%), and injection site bruising (6% vs. 4%). AMG 745 treatment increased the lean body mass and decreased fat mass. These preliminary results provide support for further investigation into the safety profile and of therapeutic uses of myostatin blockade to reduce sarcopenia and improve metabolism.
- As discussed earlier, myostatin deficiency or blockade of ActRIIB receptor potently decreases body fat and improves metabolic outcomes in obese mice [535455]. Human obesity is associated with increased myostatin expression and plasma myostatin levels. The secretion of myostatin from myotubes derived from muscle biopsies is increased in obese compared with lean women [5859]. The biological significance of these findings, and whether myostatin and other TGF-β peptide superfamily can be targeted specifically for treatment of obesity and metabolic disorders requires further studies.
MYOSTATIN
- The cytokine interleukin 6 (IL-6) was named a myokine because its levels increased in response to exercise and muscle contraction [606162]. Evidence supporting the notion that is the source of IL-6 is based on transcriptional analysis of IL-6 mRNA levels during exercise, in situ hybridization and immunohistochemistry of IL-6, microdialysis of contracting skeletal muscle, and measurement of arteriovenous IL-6 concentrations and blood flow across an exercising leg [63]. Skeletal muscle adapts to exercise by altering glycogen content, increasing β-oxidation of fatty acids, increasing intramyocellular triglyceride hydrolysis, and enhancing epinephrine-induced lipolysis [64]. Thus, the trained skeletal muscle uses fat as a substrate and is less dependent on glucose and muscle glycogen during exercise.
- Epidemiological studies have found an inverse correlation of the amount of physical activity and plasma IL-6 concentration. The basal plasma levels of IL-6 are strongly associated with physical inactivity, obesity and metabolic syndrome [656667]. Chronic exercise decreases the basal levels of IL-6, and the increases in plasma IL-6 and muscle IL-6 mRNA content during acute exercise are also blunted in response to endurance training [68]. IL-6 receptor (IL-6R) α is regulated opposite to IL-6, and the basal IL-6Rα mRNA content in muscle is increased during endurance training, perhaps counteracting the reduction in IL-6 [69].
- What are the biological roles of IL-6? Treatment of rat L6 myocytes with IL-6 increases basal glucose uptake via glucose transporter 4 translocation, as well as insulin-stimulated glucose uptake [70]. The in vitro effect of IL-6 on glucose uptake is mediated, at least partly, through AMP-activated protein kinase (AMPK) activation. Studies have also suggested that IL-6 may stimulate fatty acid oxidation via AMPK [717273]. In resting humans, acute administration of IL-6 infused to achieve physiological concentrations had no effect on either endogenous glucose production or glucose disposal [74]. In contrast, when IL-6 was infused to mimic the plasma of IL-6 observed during high-intensity exercise, the endogenous glucose production was markedly increased, suggesting that a muscle-liver crosstalk mediated via IL-6 may have a role in regulating plasma glucose levels through endogenous glucose production during exercise [70]. In addition to its effects on glucose metabolism, infusion of IL-6 in healthy volunteers stimulates lipolysis in skeletal muscle without affecting adipose tissue [7175]. IL-6 inhibits endotoxin-induced tumor necrosis factor (TNF) production in human monocytes, and infusion of IL-6 during exercise attenuates the ability of endotoxin to increase TNF levels in healthy individuals. These anti-inflammatory properties of IL-6 are associated with induction of anti-inflammatory cytokines, e.g., IL-1 receptor agonist and IL-10 [76].
INTERLEUKIN 6
- IL-15 was classified as an interleukin based on its 4-α-helical secondary structure and its ability to mimic the functions of IL-2 [77]. The plasma membrane receptor for IL-15 was shown to be composed of IL-2 receptor β (IL-2Rβ), the common gamma chain (γc), and a specific IL-15 receptor α (IL-15Rα) chain [7879]. Transcripts for IL-15 and IL-15Rα are widely expressed, and skeletal muscle expresses IL15 and IL15RA mRNAs [7778]. In vitro experiments in myogenic cells suggested that IL-15 was an anabolic factor for skeletal muscle; however, increasing IL-15 levels in vivo did not induce muscle hypertrophy [80818283]. Nonetheless, studies have revealed different locomotor phenotypes of mice lacking IL-15Rα or IL-15 or IL-2Rβ [8485]. Furthermore, single nucleotide polymorphisms (SNPs) in the human IL15 and IL15RA genes have been associated with different muscle phenotypes, responses to resistance training, and obesity [8687888990].
- We hypothesized that IL-15Rα has a role in determining the muscle phenotype in mice. We found that loss of IL-15Rα leads to a remodeling of fast skeletal muscles to a more oxidative phenotype associated with increased spontaneous locomotor activity and exercise capacity, and resistance to fatigue [91]. The molecular signature of oxidative muscle phenotype from IL-15Rα knockout mice included altered mitochondrial biogenesis and calcium homeostasis. Consistent with our observations in mice, we found a significant association between a SNP in exon 3 of the IL15RA gene and endurance in human athletes [91].
- A recent paper by O'Connell and Pistilli [92] has shown mechanisms by which IL-15Rα induced an oxidative skeletal muscle phenotype. Muscle-specific deletion of IL-15Rα resulted in a greater mitochondrial density and reduced twitch:tetanus ratio in extensor digitorum longus and soleus muscles indicating a oxidative shift in muscle phenotype. However, the spontaneous activity was not different in muscle IL-15Rα deficient mice, unlike the whole body IL-15Rα knockout mouse [93]. Thus, muscle IL-15Rα has a role in altering contractile properties and fatigue characteristics of skeletal muscles, but the locomotor behavior is likely to be controlled by IL-15Rα targets in brain [84]. Further studies are needed to evaluate whether IL-15Rα can be targeted specifically for obesity treatment by increasing energy expenditure and fatty acid oxidation.
INTERLEUKIN 15
- Chronic exercise increases skeletal muscle mitochondrial biogenesis, which is regulated by PGC1α [949596]. Bostrom et al. [97] demonstrated that the inguinal subcutaneous WAT had increased levels of UCP1 and Cidea in muscle-specific PGC1α transgenic mice compared to wild-type mice. To address whether the browning of the subcutaneous WAT was due to a myokine, they cultured primary murine subcutaneous adipocytes with conditioned media from PGC1α overexpressing myocytes, and found that the conditioned media increased expression of brown-fat specific genes in adipocytes. Using gene array and bioinformatic methods, Bostrom et al. [97] identified FNDC5 as a gene target of PGC1α, and showed that FNDC5 expression was increased in muscle obtained from exercise-trained mice and humans. Primary subcutaneous adipocytes treated with recombinant-FNDC5 displayed an increased expression of brown adipose genes, i.e., UCP1, Elovl3, Cox7a, and Otop1. Moreover, UCP1-positive cells treated with FNDC5 developed multi-loculated lipid droplets, increased mitochondrial content and oxygen consumption, consistent with a thermogenic phenotype. Based on these results, the authors surmised that FNDC5 induced a beige phenotype of WAT in mice, and this effect was attenuated by a peroxisome proliferator-activated receptor α antagonist treatment [97]. Further experiments revealed that the full-length FNDC5 was a transmembrane protein, and the extracellular N-terminal portion of FNDC5 was secreted and was highly homologous between mouse and humans. This myokine was named "irisin" after the Greek messenger goddess Iris. Plasma irisin levels were shown to be increased in mice and humans after short-term exercise. Adenoviral expression of FNDC5 in liver increased plasma irisin levels which led to the browning of subcutaneous WAT, increased energy expenditure, and protection against obesity and insulin resistance [97].
- However, questions have been raised about the biology of FNDC5 expression and plasma irisin levels [9899]. It is unclear whether irisin is truly a myokine since human WAT is capable of expressing FNDC5 and secreting irisin [100]. Some studies indicate that neither acute nor chronic exercise consistently increases expression FNDC5 and/or irisin in humans [101102]. However, others have shown associations of plasma irisin and aging, obesity, physical activity, and metabolic outcomes [103104]. These controversies surrounding the role of irisin may arise from different exercise regimens and assays for measuring irisin, suboptimal storage of tissue samples, as well as differences in the function of irisin in mouse versus human [101104105].
IRISIN
- Myokines are proposed to play important roles in mediating the beneficial effects of skeletal muscle mass and exercise on health. Myokines have been implicated in the pathogenesis of obesity, substrate oxidation, lipid partitioning, insulin sensitivity, and inflammation. While the list of putative myokines keeps growing, the specific physiological and pathological effects of these molecules are poorly understood. Important questions that need to be answered for a presumed myokine include whether skeletal muscle is the main or only source, how the local and systemic concentrations of the myokine are regulated, whether there are biological differences among species, and what specific signaling mechanisms mediate the biological effects of the myokine in various organs. A better understanding of the actions of myokines may identify novel therapies for obesity, diabetes, cardiovascular diseases, cancer, and other diseases known to be improved by exercise.
CONCLUSIONS
-
Acknowledgements
- We thank Dr. Lim Soo of the Seoul National University College of Medicine for providing MRI scans (Fig. 2). RSA is supported by American Diabetes Association grant #7-13-BS-004, and National Institutes of Health grants R01-NS084965 and P01-DK049210.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003;289:323–330. ArticlePubMed
- 2. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc 2008;40:1863–1872. ArticlePubMedPMC
- 3. Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One 2011;6:e19657ArticlePubMedPMC
- 4. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350. ArticlePubMed
- 5. Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–658. ArticlePubMed
- 6. Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil 2008;15:239–246. ArticlePubMed
- 7. Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology 2007;18:137–157. ArticlePubMed
- 8. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. ArticlePubMed
- 9. Friedrichsen M, Mortensen B, Pehmoller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol 2013;366:204–214. ArticlePubMed
- 10. Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol 2014;220:T61–T79. ArticlePubMed
- 11. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 2013;280:4294–4314. ArticlePubMed
- 12. Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr 2014;99:237S–242S. ArticlePubMedPDF
- 13. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014;49:59–68. ArticlePubMed
- 14. Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010;91:1128S–1132S. ArticlePubMedPDF
- 15. Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013;45:2163–2172. ArticlePubMed
- 16. Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 2013;45:2200–2208. ArticlePubMed
- 17. Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol 2013;45:2215–2229. ArticlePubMed
- 18. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One 2010;5:e10805ArticlePubMedPMC
- 19. Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract 2013;7:e301–e307. ArticlePubMed
- 20. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J 2014;61:61–70. ArticlePubMed
- 21. Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008-2010. PLoS One 2014;9:e86902ArticlePubMedPMC
- 22. Goldstein MS. Humoral nature of the hypoglycemic factor of muscular work. Diabetes 1961;10:232–234. ArticlePubMed
- 23. Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 2003;24:113–119. ArticlePubMed
- 24. Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S. Computational reconstruction of the human skeletal muscle secretome. Proteins 2006;62:776–792. ArticlePubMed
- 25. Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics 2010;9:2482–2496. ArticlePubMedPMC
- 26. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–465. ArticlePubMedPDF
- 27. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997;387:83–90. ArticlePubMedPDF
- 28. Szabo G, Dallmann G, Muller G, Patthy L, Soller M, Varga L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome 1998;9:671–672. ArticlePubMed
- 29. Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 2006;38:813–818. ArticlePubMedPDF
- 30. Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 1997;17:71–74. ArticlePubMedPDF
- 31. Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 1997;7:910–916. ArticlePubMed
- 32. Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 2004;350:2682–2688. ArticlePubMed
- 33. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 2008;29:513–534. ArticlePubMedPMCPDF
- 34. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484. ArticlePubMed
- 35. Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem 2007;282:3799–3808. ArticlePubMed
- 36. Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009;150:286–294. ArticlePubMedPDF
- 37. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 2009;296:C1258–C1270. ArticlePubMed
- 38. Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell 1998;95:737–740. ArticlePubMed
- 39. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A 2001;98:9306–9311. ArticlePubMedPMC
- 40. Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 2004;26:262–272. ArticlePubMed
- 41. Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol 2006;206:264–272. ArticlePubMed
- 42. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399–412. ArticlePubMedPMC
- 43. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 2004;14:395–403. ArticlePubMed
- 44. Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci 2014;71:4361–4371. ArticlePubMedPDF
- 45. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun 2002;291:701–706. ArticlePubMed
- 46. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 2002;109:595–601. ArticlePubMedPMC
- 47. Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 2009;4:e4937ArticlePubMedPMC
- 48. Akpan I, Goncalves MD, Dhir R, Yin X, Pistilli EE, Bogdanovich S, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond) 2009;33:1265–1273. ArticlePubMedPMCPDF
- 49. Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 2001;15:2203–2208. ArticlePubMedPMC
- 50. Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 2003;112:197–208. ArticlePubMedPMC
- 51. Goncalves MD, Pistilli EE, Balduzzi A, Birnbaum MJ, Lachey J, Khurana TS, et al. Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS One 2010;5:e12707ArticlePubMedPMC
- 52. Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, et al. A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology 2012;153:3133–3146. ArticlePubMedPMCPDF
- 53. Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 2012;55:183–193. ArticlePubMedPDF
- 54. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J 2013;27:1981–1989. ArticlePubMedPMC
- 55. Choi SJ, Yablonka-Reuveni Z, Kaiyala KJ, Ogimoto K, Schwartz MW, Wisse BE. Increased energy expenditure and leptin sensitivity account for low fat mass in myostatin-deficient mice. Am J Physiol Endocrinol Metab 2011;300:E1031–E1037. ArticlePubMedPMC
- 56. Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve 2013;47:416–423. ArticlePubMed
- 57. Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2014;99:E1967–E1975. ArticlePubMed
- 58. Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009;58:30–38. ArticlePubMedPMC
- 59. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 2011;43:1828–1835. ArticlePubMedPMC
- 60. Bartoccioni E, Michaelis D, Hohlfeld R. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. Immunol Lett 1994;42:135–138. ArticlePubMed
- 61. Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 1998;508(Pt 3):949–953. ArticlePubMedPMC
- 62. Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 2001;15:2748–2750. ArticlePubMed
- 63. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008;88:1379–1406. ArticlePubMed
- 64. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17:162–184. ArticlePubMed
- 65. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004;52:1098–1104. ArticlePubMed
- 66. Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia 2006;49:2078–2085. ArticlePubMedPDF
- 67. Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh-Manoux A, et al. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation 2012;126:928–933. ArticlePubMedPMC
- 68. Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2004;287:E1189–E1194. ArticlePubMed
- 69. Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol (1985) 2005;99:2075–2079. ArticlePubMed
- 70. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006;55:2688–2697. ArticlePubMed
- 71. van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 2003;88:3005–3010. ArticlePubMed
- 72. Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 2004;287:E616–E621. ArticlePubMed
- 73. Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 2006;20:3364–3375. ArticlePubMedPDF
- 74. Steensberg A, Fischer CP, Sacchetti M, Keller C, Osada T, Schjerling P, et al. Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J Physiol 2003;548(Pt 2):631–638. ArticlePubMedPMC
- 75. Wolsk E, Mygind H, Grondahl TS, Pedersen BK, van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;299:E832–E840. ArticlePubMed
- 76. Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003;285:E433–E437. ArticlePubMed
- 77. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994;264:965–968. ArticlePubMed
- 78. Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J 1995;14:3654–3663. ArticlePubMedPMC
- 79. Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J 1994;13:2822–2830. ArticlePubMedPMC
- 80. Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology 1995;136:3669–3672. ArticlePubMedPDF
- 81. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 2002;280:55–63. ArticlePubMed
- 82. Furmanczyk PS, Quinn LS. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol Int 2003;27:845–851. ArticlePubMed
- 83. Pistilli EE, Alway SE. Systemic elevation of interleukin-15 in vivo promotes apoptosis in skeletal muscles of young adult and aged rats. Biochem Biophys Res Commun 2008;373:20–24. ArticlePubMedPMC
- 84. He Y, Wu X, Khan RS, Kastin AJ, Cornelissen-Guillaume GG, Hsuchou H, et al. IL-15 receptor deletion results in circadian changes of locomotor and metabolic activity. J Mol Neurosci 2010;41:315–321. ArticlePubMedPDF
- 85. Wu X, He Y, Hsuchou H, Kastin AJ, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun 2010;24:1340–1346. ArticlePubMedPMC
- 86. Pistilli EE, Devaney JM, Gordish-Dressman H, Bradbury MK, Seip RL, Thompson PD, et al. Interleukin-15 and interleukin-15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine 2008;43:45–53. ArticlePubMedPMC
- 87. Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol (1985) 2004;97:2214–2219. ArticlePubMed
- 88. Di Renzo L, Bigioni M, Bottini FG, Del Gobbo V, Premrov MG, Cianci R, et al. Normal Weight Obese syndrome: role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C-->T genes in the relationship between body composition and resting metabolic rate. Eur Rev Med Pharmacol Sci 2006;10:235–245. PubMed
- 89. Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab 2008;93:4486–4493. ArticlePubMedPDF
- 90. Di Renzo L, Gloria-Bottini F, Saccucci P, Bigioni M, Abenavoli L, Gasbarrini G, et al. Role of interleukin-15 receptor alpha polymorphisms in normal weight obese syndrome. Int J Immunopathol Pharmacol 2009;22:105–113. ArticlePubMed
- 91. Pistilli EE, Bogdanovich S, Garton F, Yang N, Gulbin JP, Conner JD, et al. Loss of IL-15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest 2011;121:3120–3132. ArticlePubMedPMC
- 92. O'Connell GC, Pistilli EE. Interleukin-15 directly stimulates pro-oxidative gene expression in skeletal muscle in-vitro via a mechanism that requires interleukin-15 receptor alpha. Biochem Biophys Res Commun 2015;458:614–619. ArticlePubMed
- 93. O'Connell G, Guo G, Stricker J, Quinn LS, Ma A, Pistilli EE. Muscle-specific deletion of exons 2 and 3 of the IL-15RA gene in mice: effects on contractile properties of fast and slow muscles. J Appl Physiol (1985) 2015;118:437–448. ArticlePubMed
- 94. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002;16:1879–1886. ArticlePubMed
- 95. Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 2004;18:357–368. ArticlePubMed
- 96. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–735. ArticlePubMedPDF
- 97. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–468. ArticlePubMedPMCPDF
- 98. Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, et al. Evidence against a beneficial effect of irisin in humans. PLoS One 2013;8:e73680ArticlePubMedPMC
- 99. Erickson HP. Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte 2013;2:289–293. ArticlePubMedPMC
- 100. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013;98:E769–E778. ArticlePubMedPDF
- 101. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, et al. Irisin and exercise training in humans: results from a randomized controlled training trial. BMC Med 2013;11:235ArticlePubMedPMCPDF
- 102. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 2014;281:739–749. ArticlePubMed
- 103. Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012;61:1725–1738. ArticlePubMedPMC
- 104. Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab 2015;100:E453–E457. ArticlePubMed
- 105. Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, et al. Irisin: a myth rather than an exercise-inducible myokine. Sci Rep 2015;5:8889ArticlePubMedPMCPDF
References
Positive and negative regulators of skeletal muscle mass. Myokines are produced and secreted by skeletal muscle and act via autocrine, paracrine and endocrine mechanisms to regulate skeletal muscle mass and metabolism. IGF-1, insulin-like growth factor 1; IL, interleukin; BDNF, brain-derived neurotrophic factor; FGF-21, fibroblast growth factor 21; LIF, leukemia inhibitory factor.
Magnetic resonance imaging scans comparing the distributions of abdominal and thigh fat and muscle in (A) lean, (B, C) overweight, and (D) obese women. As in (B) and (D), increased visceral adiposity and sarcopenia are associated with diabetes mellitus (DM), hypertension (HT), dyslipidemia, and cardiovascular disease (CVD). BMI, body mass index.
Myostatin and insulin-like growth factor 1 (IGF-1) signaling pathways in skeletal muscle. Myostatin and other transforming growth factor β family members signal via activin receptor II (ActRII), Smad2, and Smad3, which blocks muscle differentiation and leads to muscle atrophy. Inhibition of regulatory-associated protein of mammalian target of rapamycin (mTOR) and mTOR complex 1 (mTORC1) has an additive effect on myostatin signaling. The IGF-1/Akt pathway induces skeletal muscle hypertrophy. ALK, activin receptor-like kinase; GDF11, growth differentiation factor 11; IGFR, IGF receptor; FoxO1, forkhead box protein O1; PI3K, phosphatidylinositol 3-kinase; GSK-3β, glycogen synthase kinase 3β; MuRF, muscle ring finger; p70S6K, p70 S6 kinase.
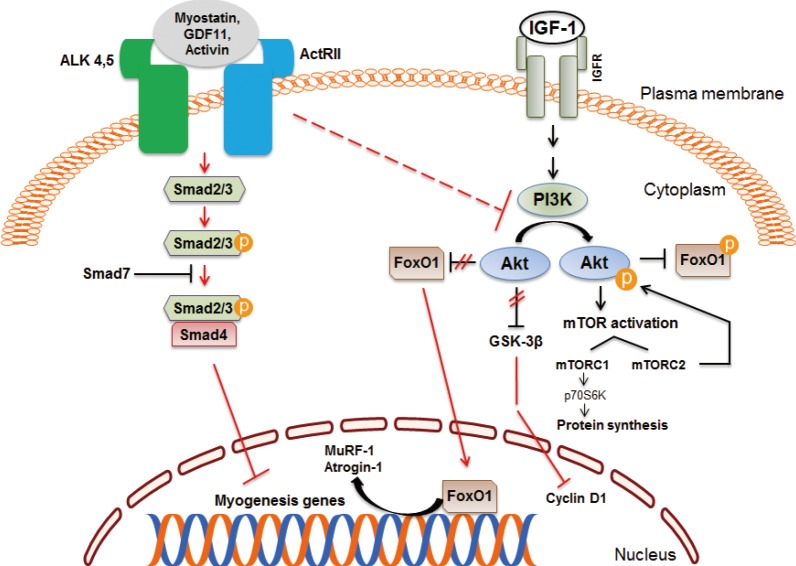
Figure & Data
References
Citations

- Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases
Helena Trevisan Schroeder, Carlos Henrique De Lemos Muller, Thiago Gomes Heck, Mauricio Krause, Paulo Ivo Homem de Bittencourt
Cell Stress and Chaperones.2024; 29(1): 116. CrossRef - Exercise affects high-fat diet-stimulated breast cancer metastasis through irisin secretion by altering cancer stem cell properties
YuJin Lee, SoDam Park, SeungHwa Park, Hye Ji Kwon, Sang-Ho Lee, Yuri Kim, Jung-Hyun Kim
Biochemistry and Biophysics Reports.2024; 38: 101684. CrossRef - Myokines: A central point in managing redox homeostasis and quality of life
Richa Rathor, Geetha Suryakumar
BioFactors.2024;[Epub] CrossRef - Correlations between serum testosterone and irisin levels in a sample of Egyptian men with metabolic syndrome; (case-control study)
Inass Hassan Ahmad, Eman Roshdy Mohamed Mostafa, Shymaa Abdelhafeez Mohammed, Walaa Shipl, Amany Ahmed Soliman, Marwa Said
Archives of Physiology and Biochemistry.2023; 129(1): 180. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef - Myokines: Crosstalk and Consequences on Liver Physiopathology
Aurore Dumond Bourie, Jean-Baptiste Potier, Michel Pinget, Karim Bouzakri
Nutrients.2023; 15(7): 1729. CrossRef - Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia
Archana Arjunan, Juhyun Song
Biomedicine & Pharmacotherapy.2023; 163: 114847. CrossRef - Muscle quality: the assessment, prognosis, and intervention
翔 畑中, 洋祐 大須賀
Nippon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics.2023; 60(2): 103. CrossRef - Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors
Hamed Alizadeh Pahlavani
Frontiers in Endocrinology.2022;[Epub] CrossRef - Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs
Alessandra Renzini, Marco D’Onghia, Dario Coletti, Viviana Moresi
Frontiers in Physiology.2022;[Epub] CrossRef - Irisin response to acute moderate intensity exercise and high intensity interval training in youth of different obesity statuses: A randomized crossover trial
Benjamin H. Colpitts, Brittany V. Rioux, Ashley L. Eadie, Keith R. Brunt, Martin Sénéchal
Physiological Reports.2022;[Epub] CrossRef - The effects of whole‐body vibration amplitude on glucose metabolism, inflammation, and skeletal muscle oxygenation
Adeola A. Sanni, Anson M. Blanks, Cassandra C. Derella, Chase Horsager, Reva H. Crandall, Jacob Looney, Savanna Sanchez, Kimberly Norland, Bingwei Ye, Jeffrey Thomas, Xiaoling Wang, Ryan A. Harris
Physiological Reports.2022;[Epub] CrossRef - Preoperative Thoracic Muscle Mass Predicts Bone Density Change After Parathyroidectomy in Primary Hyperparathyroidism
Seung Won Burm, Namki Hong, Seunghyun Lee, Gi Jeong Kim, Sang Hyun Hwang, Jongju Jeong, Yumie Rhee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(6): e2474. CrossRef - Single skeletal muscle fiber mechanical properties: a muscle quality biomarker of human aging
Jae-Young Lim, Walter R. Frontera
European Journal of Applied Physiology.2022; 122(6): 1383. CrossRef - Hybrid HIIT/isometrics strength training programs: a paradigm shift for physical exercise
Luis Wyche, Guillermo Rojo-Gil, María Marín-Peiró, José Antonio Pérez-Turpin, Jaime Enrique Gómez-Paternina, Carlos Elvira, Duncan Ayers
Scientific Journal of Sport and Performance.2022; 1(1): 37. CrossRef - The Effect of Irisin on Proliferation, Apoptosis, and Expression of Metastasis Markers in Prostate Cancer Cell Lines
Atiye Saeedi Sadr, Hassan Ehteram, Elahe Seyed Hosseini, Marziyeh Alizadeh Zarei, Hassan Hassani Bafrani, Hamed Haddad Kashani
Oncology and Therapy.2022; 10(2): 377. CrossRef - Molecular mechanisms of adaptive and therapeutic effects of physical activity in patients with cardiovascular diseases
V.E. Vladimirsky, E.V. Vladimirsky, A.N. Lunina, A.D. Fesyun, A.P. Rachin, O.D. Lebedeva, M.Yu. Yakovlev, M.A. Tubekova
Voprosy kurortologii, fizioterapii i lechebnoi fizicheskoi kul'tury.2022; 99(2): 69. CrossRef - Studies in Rats of Combined Muscle and Liver Perfusion and of Muscle Extract Indicate That Contractions Release a Muscle Hormone Directly Enhancing Hepatic Glycogenolysis
Xiao X. Han, Jens J. Holst, Henrik Galbo
Journal of Personalized Medicine.2022; 12(5): 837. CrossRef - Local and systemic transcriptomic responses from acute exercise induced muscle damage of the human knee extensors
Eric A. Kirk, Christina A. Castellani, Timothy J. Doherty, Charles L. Rice, Shiva M. Singh
Physiological Genomics.2022; 54(8): 305. CrossRef - Upregulation of IL‐8, osteonectin, and myonectin mRNAs by intermittent hypoxia via OCT1‐ and NRF2‐mediated mechanisms in skeletal muscle cells
Shin Takasawa, Ryogo Shobatake, Asako Itaya‐Hironaka, Mai Makino, Tomoko Uchiyama, Sumiyo Sakuramoto‐Tsuchida, Yoshinori Takeda, Hiroyo Ota, Akiyo Yamauchi
Journal of Cellular and Molecular Medicine.2022; 26(24): 6019. CrossRef - Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview
Amritpal Dhaliwal, Jonathan I. Quinlan, Kellie Overthrow, Carolyn Greig, Janet M. Lord, Matthew J. Armstrong, Sheldon C. Cooper
Nutrients.2021; 13(2): 656. CrossRef - Role of Interleukin-6 in Vascular Health and Disease
Paulina Villar-Fincheira, Fernanda Sanhueza-Olivares, Ignacio Norambuena-Soto, Nicole Cancino-Arenas, Felipe Hernandez-Vargas, Rodrigo Troncoso, Luigi Gabrielli, Mario Chiong
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Computed Tomography-Derived Myosteatosis and Metabolic Disorders
Iva Miljkovic, Chantal A. Vella, Matthew Allison
Diabetes & Metabolism Journal.2021; 45(4): 482. CrossRef - Skeletal muscle energy metabolism in obesity
Abel M. Mengeste, Arild C. Rustan, Jenny Lund
Obesity.2021; 29(10): 1582. CrossRef - Myokines and adipomyokines: inflammatory mediators or unique molecules of targeted therapy for obesity?
O. V. Vasyukova, Yu. V. Kasyanova, P. L. Okorokov, O. B. Bezlepkina
Problems of Endocrinology.2021; 67(4): 36. CrossRef - Associations Between Lipoprotein Subfractions and Area and Density of Abdominal Muscle and Intermuscular Adipose Tissue: The Multi-Ethnic Study of Atherosclerosis
Megan M. Marron, Matthew Allison, Alka M. Kanaya, Britta Larsen, Alexis C. Wood, David Herrington, Philip Greenland, Iva Miljkovic
Frontiers in Physiology.2021;[Epub] CrossRef - Musclin Is Related to Insulin Resistance and Body Composition, but Not to Body Mass Index or Cardiorespiratory Capacity in Adults
Yeliana L. Sánchez, Manuela Yepes-Calderón, Luis Valbuena, Andrés F. Milán, María C. Trillos-Almanza, Sergio Granados, Miguel Peña, Mauricio Estrada-Castrillón, Juan C. Aristizábal, Raúl Narvez-Sanchez, Jaime Gallo-Villegas, Juan C. Calderón
Endocrinology and Metabolism.2021; 36(5): 1055. CrossRef - Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial
Hélio José Coelho-Júnior, Ivan de Oliveira Gonçalves, Ricardo Aurélio Carvalho Sampaio, Priscila Yukari Sewo Sampaio, Eduardo Lusa Cadore, Riccardo Calvani, Anna Picca, Mikel Izquierdo, Emanuele Marzetti, Marco Carlos Uchida
International Journal of Environmental Research and Public Health.2020; 17(10): 3435. CrossRef - Prostate tumor–derived GDF11 accelerates androgen deprivation therapy–induced sarcopenia
Chunliu Pan, Neha Jaiswal Agrawal, Yanni Zulia, Shalini Singh, Kai Sha, James L. Mohler, Kevin H. Eng, Joe V. Chakkalakal, John J. Krolewski, Kent L. Nastiuk
JCI Insight.2020;[Epub] CrossRef - Muscle–Organ Crosstalk: The Emerging Roles of Myokines
Mai Charlotte Krogh Severinsen, Bente Klarlund Pedersen
Endocrine Reviews.2020; 41(4): 594. CrossRef - Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets?
Ai Guo, Kai Li, Qian Xiao
Experimental Gerontology.2020; 139: 111022. CrossRef - Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging
Rosaly Correa-de-Araujo, Odessa Addison, Iva Miljkovic, Bret H. Goodpaster, Bryan C. Bergman, Richard V. Clark, Joanne W. Elena, Karyn A. Esser, Luigi Ferrucci, Michael O. Harris-Love, Steve B. Kritchevsky, Amanda Lorbergs, John A. Shepherd, Gerald I. Shu
Frontiers in Physiology.2020;[Epub] CrossRef - Muscle-Organ Crosstalk: Focus on Immunometabolism
Marie Lund Bay, Bente Klarlund Pedersen
Frontiers in Physiology.2020;[Epub] CrossRef - Impact of diets rich in olive oil, palm oil or lard on myokine expression in rats
Chantal Gauze-Gnagne, Fabrice Raynaud, Youzan Ferdinand Djohan, Céline Lauret, Christine Feillet-Coudray, Charles Coudray, Absalome Monde, Gervais Koffi, Marion Morena, Massara Camara-Cisse, Jean Paul Cristol, Eric Badia
Food & Function.2020;[Epub] CrossRef - Long-term treatment with insulin and retinoic acid increased glucose utilization in L6 muscle cells via glycogenesis
Matthew Goff, Guoxun Chen
Biochemistry and Cell Biology.2020; 98(6): 683. CrossRef - Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review
Vahid Maleki, Hamed Jafari-Vayghan, Sevda Saleh-Ghadimi, Mahsa Adibian, Sorayya Kheirouri, Mohammad Alizadeh
Complementary Therapies in Medicine.2019; 43: 20. CrossRef - Effect of a Very-Low-Calorie Ketogenic Diet on Circulating Myokine Levels Compared with the Effect of Bariatric Surgery or a Low-Calorie Diet in Patients with Obesity
Sajoux, Lorenzo, Gomez-Arbelaez, Zulet, Abete, Castro, Baltar, Portillo, Tinahones, Martinez, Crujeiras, Casanueva
Nutrients.2019; 11(10): 2368. CrossRef - Extracellular Vesicles: Delivery Vehicles of Myokines
Eleonora Trovato, Valentina Di Felice, Rosario Barone
Frontiers in Physiology.2019;[Epub] CrossRef - Effects of Exercise to Improve Cardiovascular Health
Kelsey Pinckard, Kedryn K. Baskin, Kristin I. Stanford
Frontiers in Cardiovascular Medicine.2019;[Epub] CrossRef - Does exercise-induced apelin affect sarcopenia? A systematic review and meta-analysis
Jun Hyun Bae, Seong Eun Kwak, Ji Hyun Lee, Zhang Yangjie, Wook Song
Hormones.2019; 18(4): 383. CrossRef - Skeletal muscle denervation investigations: selecting an experimental control wisely
Haiming Liu, LaDora V. Thompson
American Journal of Physiology-Cell Physiology.2019; 316(3): C456. CrossRef - Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease
Yung-Shuen Lin, Fang-Yu Lin, Ya-Hsin Hsiao
Molecular Neurobiology.2019; 56(3): 1984. CrossRef - Skeletal muscle as a protagonist in the pregnancy metabolic syndrome
Raul Narvaez-Sanchez, Juan C. Calderón, Gloria Vega, Maria Camila Trillos, Sara Ospina
Medical Hypotheses.2019; 126: 26. CrossRef - Oncostatin M, a muscle-secreted myokine, recovers high-glucose-induced impairment of Akt phosphorylation by Fos induction in hippocampal neuron cells
William Won Seok Hyung, Sung Gon Lee, Keun Tae Kim, Hyeon Soo Kim
NeuroReport.2019; 30(11): 765. CrossRef - If my muscle could talk: Myokines as a biomarker of frailty
Hélio J. Coelho-Junior, Anna Picca, Riccardo Calvani, Marco C. Uchida, Emanuele Marzetti
Experimental Gerontology.2019; 127: 110715. CrossRef - Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer
Hiroshi Fukushima, Kosuke Takemura, Hiroaki Suzuki, Fumitaka Koga
International Journal of Molecular Sciences.2018; 19(10): 2999. CrossRef - Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes
Hye Soo Chung, Soon Young Hwang, Ju Hee Choi, Hyun Jung Lee, Nam Hoon Kim, Hye Jin Yoo, Ji-A Seo, Sin Gon Kim, Nan Hee Kim, Sei Hyun Baik, Kyung Mook Choi
Diabetes Research and Clinical Practice.2018; 136: 100. CrossRef - Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non‐massaged hindlimb
Benjamin F. Miller, Karyn L. Hamilton, Zana R. Majeed, Sarah M. Abshire, Amy L. Confides, Amanda M. Hayek, Emily R. Hunt, Patrick Shipman, Frederick F. Peelor, Timothy A. Butterfield, Esther E. Dupont‐Versteegden
The Journal of Physiology.2018; 596(1): 83. CrossRef - La funzione endocrina del muscolo scheletrico
Francesco Marampon, Clara Crescioli
L'Endocrinologo.2018; 19(1): 10. CrossRef - RESPUESTA INFLAMATORIA Y ANTIINFLAMATORIA TRAS EL ESFUERZO AGUDO EN NATACIÓN // INFLAMMATORY AND ANTINFLAMMATOY RESPONSE AFTER SWIMMING ACUTE EFFORT
G.A. Pussieldi, C.E. Veneroso, J.A. de Paz
Revista Internacional de Medicina y Ciencias de la Actividad Física y del Deporte.2018; 18(71): 413. CrossRef - Alpha-linolenic acid and linoleic acid differentially regulate the skeletal muscle secretome of obese Zucker rats
Alex Rajna, Heather Gibling, Ousseynou Sarr, Sarthak Matravadia, Graham P. Holloway, David M. Mutch
Physiological Genomics.2018; 50(8): 580. CrossRef - Serum irisin associates with breast cancer to spinal metastasis
Zheng-ping Zhang, Xue-fang Zhang, Hui Li, Tuan-jiang Liu, Qin-peng Zhao, Lin-hong Huang, Zi-jun Cao, Li-min He, Ding-jun Hao
Medicine.2018; 97(17): e0524. CrossRef - Causes and solutions to “globesity”: The new fa(s)t alarming global epidemic
Liliya V. Vasileva, Andrey S. Marchev, Milen I. Georgiev
Food and Chemical Toxicology.2018; 121: 173. CrossRef - Chronic inflammation and sarcopenia: A regenerative cell therapy perspective
Jagadish K. Chhetri, Philipe de Souto Barreto, Bertrand Fougère, Yves Rolland, Bruno Vellas, Matteo Cesari
Experimental Gerontology.2018; 103: 115. CrossRef - Level of Interleukins IL-6 and IL-15 in Blood Plasma of Mice after Forced Swimming Test
L. V. Kapilevich, T. A. Kironenko, A. N. Zakharova, A. V. Kabachkova, S. N. Orlov
Bulletin of Experimental Biology and Medicine.2017; 163(1): 10. CrossRef - Exercise Inhibits the Effects of Smoke-Induced COPD Involving Modulation of STAT3
Maysa Alves Rodrigues Brandao-Rangel, Andre Luis Lacerda Bachi, Manoel Carneiro Oliveira-Junior, Asghar Abbasi, Adriano Silva-Renno, Auriléia Aparecida de Brito, Ana Paula Ligeiro de Oliveira, Alessandra Choqueta Toledo-Arruda, Maria Gabriela Belvisi, Rod
Oxidative Medicine and Cellular Longevity.2017; 2017: 1. CrossRef - The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report
Rosaly Correa-de-Araujo, Michael O. Harris-Love, Iva Miljkovic, Maren S. Fragala, Brian W. Anthony, Todd M. Manini
Frontiers in Physiology.2017;[Epub] CrossRef - Myostatin and carbohydrate disturbances
Yavor S. Assyov, Tsvetelina V. Velikova, Zdravko A. Kamenov
Endocrine Research.2017; 42(2): 102. CrossRef -
OPA
1 deficiency promotes secretion of
FGF
21 from muscle that prevents obesity and insulin resistance
Renata Oliveira Pereira, Satya M Tadinada, Frederick M Zasadny, Karen Jesus Oliveira, Karla Maria Pereira Pires, Angela Olvera, Jennifer Jeffers, Rhonda Souvenir, Rose Mcglauflin, Alec Seei, Trevor Funari, Hiromi Sesaki, Matthew J Potthoff, Christopher M
The EMBO Journal.2017; 36(14): 2126. CrossRef - Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort
R Burrows, P Correa-Burrows, M Reyes, E Blanco, C Albala, S Gahagan
Pediatric Diabetes.2017; 18(8): 895. CrossRef - Impact of sarcopenia in the management of urological cancer patients
Hiroshi Fukushima, Fumitaka Koga
Expert Review of Anticancer Therapy.2017; 17(5): 455. CrossRef - Prognostic value of preoperative total psoas muscle area on long-term outcome in surgically treated oesophageal cancer patients
Seong Yong Park, Joon-Kee Yoon, Su Jin Lee, Seokjin Haam, Joonho Jung
Interactive CardioVascular and Thoracic Surgery.2017; 24(1): 13. CrossRef - Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide
F. Scaldaferri, M. Pizzoferrato, L. R. Lopetuso, T. Musca, F. Ingravalle, L. L. Sicignano, M. Mentella, G. Miggiano, M. C. Mele, E. Gaetani, C. Graziani, V. Petito, G. Cammarota, E. Marzetti, A. Martone, F. Landi, A. Gasbarrini
Gastroenterology Research and Practice.2017; 2017: 1. CrossRef - Circulating Irisin Is Reduced in Male Patients with Type 1 and Type 2 Myotonic Dystrophies
Elena Dozio, Elena Passeri, Rosanna Cardani, Stefano Benedini, Carmen Aresta, Rea Valaperta, Massimiliano Corsi Romanelli, Giovanni Meola, Valeria Sansone, Sabrina Corbetta
Frontiers in Endocrinology.2017;[Epub] CrossRef - Implication of hepatokines in metabolic disorders and cardiovascular diseases
Tae Woo Jung, Hye Jin Yoo, Kyung Mook Choi
BBA Clinical.2016; 5: 108. CrossRef - Development of a high‐throughput method for real‐time assessment of cellular metabolism in intact long skeletal muscle fibre bundles
Rui Li, Frederik J. Steyn, Michael B. Stout, Kevin Lee, Tanya R. Cully, Juan C. Calderón, Shyuan T. Ngo
The Journal of Physiology.2016; 594(24): 7197. CrossRef - Integrated data mining of transcriptomic and proteomic datasets to predict the secretome of adipose tissue and muscle in ruminants
M. Bonnet, J. Tournayre, I. Cassar-Malek
Molecular BioSystems.2016; 12(9): 2722. CrossRef - CHI3L1 - a novel myokine
H. Kainulainen
Acta Physiologica.2016; 216(3): 260. CrossRef - Elevated circulating irisin is associated with lower risk of insulin resistance: association and path analyses of obese Chinese adults
Xiulin Shi, Mingzhu Lin, Changqin Liu, Fangsen Xiao, Yongwen Liu, Peiying Huang, Xin Zeng, Bing Yan, Suhuan Liu, Xiaoying Li, Shuyu Yang, Xuejun Li, Zhibin Li
BMC Endocrine Disorders.2016;[Epub] CrossRef - Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia
Kyoung Min Kim, Hak Chul Jang, Soo Lim
The Korean Journal of Internal Medicine.2016; 31(4): 643. CrossRef - Relation of serum irisin level with metabolic and antropometric parameters in obese children
Gönül Çatlı, Tuncay Küme, Hale Ünver Tuhan, Ahmet Anık, Özlem Gürsoy Çalan, Ece Böber, Ayhan Abacı
Journal of Diabetes and its Complications.2016; 30(8): 1560. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite