Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Original ArticleDiabetes, obesity and metabolism Inhibition of Sodium-Glucose Cotransporter-2 during Serum Deprivation Increases Hepatic Gluconeogenesis via the AMPK/AKT/FOXO Signaling Pathway
 Keypoint
Keypoint
This study aimed to elucidate the effects and mechanisms of SGLT2 inhibition on hepatic glucose metabolism in both serum deprivation and serum supplementation states. SGLT2 inhibitors reduce glucose uptake in hepatocytes. SGLT2 inhibition increases gluconeogenesis in HepG2 during serum deprivation. AMPK, which is increased by SGLT2 inhibition during serum deprivation, upregulates hepatic gluconeogenesis through the AKT-FOXO1 pathway. -
Jinmi Lee1*
 , Seok-Woo Hong1*
, Seok-Woo Hong1* , Min-Jeong Kim1, Yu-Mi Lim1, Sun Joon Moon2, Hyemi Kwon2, Se Eun Park2, Eun-Jung Rhee2
, Min-Jeong Kim1, Yu-Mi Lim1, Sun Joon Moon2, Hyemi Kwon2, Se Eun Park2, Eun-Jung Rhee2 , Won-Young Lee2
, Won-Young Lee2
-
Endocrinology and Metabolism 2024;39(1):98-108.
DOI: https://doi.org/10.3803/EnM.2023.1786
Published online: January 3, 2024
1Institute of Medical Research, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding authors: Won-Young Lee. Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea Tel: +82-2-2001-2579, Fax: +82-2-2001-2049, E-mail: wonyoung2.lee@samsung.com
- Eun-Jung Rhee. Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea Tel: +82-2-2001-2485, Fax: +82-2-2001-2049, E-mail: ejrhee.lee@samsung.com
- *These authors contributed equally to this work.
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,477 Views
- 86 Download
ABSTRACT
-
Background
- Sodium-dependent glucose cotransporter 2 (SGLT2) mediates glucose reabsorption in the renal proximal tubules, and SGLT2 inhibitors are used as therapeutic agents for treating type 2 diabetes mellitus. This study aimed to elucidate the effects and mechanisms of SGLT2 inhibition on hepatic glucose metabolism in both serum deprivation and serum supplementation states.
-
Methods
- Huh7 cells were treated with the SGLT2 inhibitors empagliflozin and dapagliflozin to examine the effect of SGLT2 on hepatic glucose uptake. To examine the modulation of glucose metabolism by SGLT2 inhibition under serum deprivation and serum supplementation conditions, HepG2 cells were transfected with SGLT2 small interfering RNA (siRNA), cultured in serum-free Dulbecco’s modified Eagle’s medium for 16 hours, and then cultured in media supplemented with or without 10% fetal bovine serum for 8 hours.
-
Results
- SGLT2 inhibitors dose-dependently decreased hepatic glucose uptake. Serum deprivation increased the expression levels of the gluconeogenesis genes peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α), glucose 6-phosphatase (G6pase), and phosphoenolpyruvate carboxykinase (PEPCK), and their expression levels during serum deprivation were further increased in cells transfected with SGLT2 siRNA. SGLT2 inhibition by siRNA during serum deprivation induces nuclear localization of the transcription factor forkhead box class O 1 (FOXO1), decreases nuclear phosphorylated-AKT (p-AKT), and p-FOXO1 protein expression, and increases phosphorylated-adenosine monophosphate-activated protein kinase (p-AMPK) protein expression. However, treatment with the AMPK inhibitor, compound C, reversed the reduction in the protein expression levels of nuclear p-AKT and p-FOXO1 and decreased the protein expression levels of p-AMPK and PEPCK in cells transfected with SGLT2 siRNA during serum deprivation.
-
Conclusion
- These data show that SGLT2 mediates glucose uptake in hepatocytes and that SGLT2 inhibition during serum deprivation increases gluconeogenesis via the AMPK/AKT/FOXO1 signaling pathway.
- Two sodium-dependent glucose transporter (SGLT) family members, SGLT1 and sodium-dependent glucose cotransporter 2 (SGLT2), mediate glucose reabsorption and maintain glucose homeostasis. SGLT1 is expressed in the intestine, whereas SGLT2 is mainly expressed in the S1 and S2 segments of the renal proximal tubules [1,2]. SGLT2 inhibitors are used as antidiabetic agents to decrease plasma glucose levels by reducing the reabsorption of filtered glucose in the renal proximal tubule and increasing urinary glucose excretion [3,4]. Recent studies reported the expression of SGLT2 in hepatocytes and in the liver [5,6]. However, the role of SGLT2 in the hepatocytes remains unclear.
- Safety issues associated with newer glucose-lowering medications used in patients with type 2 diabetes mellitus (T2DM) are important. Hypoglycemia is when the body’s blood glucose or sugar levels are lower than normal and can lead to coma or even death [7,8]. Antidiabetic medications such as meglitinides and sulfonylureas cause hypoglycemia by stimulating the release of insulin from pancreatic beta cells [9], whereas SGLT2 inhibitors are a better option for improving glycemic control with a low risk of hypoglycemia, even when co-administered with insulin [10].
- A trial using SGLT2 inhibitors in patients with T2DM reported that dapagliflozin improved insulin sensitivity by lowering plasma glucose and increased gluconeogenesis by increasing the glucagon/insulin ratio [11]. Gluconeogenesis is a metabolic process synthesizing glucose from non-carbohydrate precursors when insufficient or absent dietary intake [12]. During the first 8 hours of fasting, glucose is provided through glycogenolysis, the process of glycogen breakdown in the liver and muscles; when stored glycogen is depleted, glucose is synthesized to maintain homeostasis by increasing gluconeogenesis [13,14]. Gluconeogenesis occurs predominantly in the liver and kidney; during starvation, the liver accounts for 80% to 90% of gluconeogenesis, and the kidney accounts for 10% to 20% [15,16]. Furthermore, gluconeogenesis in the liver is regulated by the activation of key enzymes such as glucose 6-phosphatase (G6pase), fructose 1,6-bisphosphatase (Fbpase), pyruvate carboxylase, and phosphoenolpyruvate carboxykinase (PEPCK).
- Forkhead box class O (FOXO) transcription factor family members, including FOXO1, FOXO3a, FOXO4, and FOXO6, play important roles in regulating cellular processes, including cell proliferation, stress resistance, and metabolism [17]. FOXO is phosphorylated by several protein kinases, resulting in its translocation from the nucleus to the cytoplasm, modification of DNA-binding affinity, and alteration of the transcriptional activity of target genes [18,19]. In particular, FOXO1 is closely associated with hepatic gluconeogenesis, and FOXO1 expression is upregulated during fasting and energy starvation [20,21].
- In the present study, we investigated the role and regulatory mechanisms of SGLT2 inhibition in maintaining glucose homeostasis in hepatocytes under serum deprivation and serum supplementation conditions.
INTRODUCTION
- Cell culture
- Huh7 and HepG2 human hepatoma cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) high-glucose (25 mmol/L) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin (Gibco, Thermo Fisher Scientific Inc., Waltham, MA, USA), and incubated in an incubator at 37°C with 5% CO2. All experiments in this study were performed in HepG2 cells, except that the glucose uptake assay was performed in Huh7 cells. HepG2 cells have the characteristic of forming aggregation, so Huh7 cells were used to compare clear differences in the short-term glucose uptake assay.
- Glucose uptake
- Glucose uptake was assessed using a glucose uptake cell-based assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Huh7 cells were cultured in sodium (Na+) buffer, including 140 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgSO4, 1 mM KH2PO4, and 10 mM HEPES, or in sodium-free (Na+-free) buffer, including 140 mM N-methyld-glucamine instead of NaCl, for 1 hour [22]. Cells were treated with 10 nM empagliflozin and dapagliflozin (Cayman Chemical) in glucose-free DMEM low-glucose (5 mmol/L) for 24 hours to determine the change of glucose uptake by SGLT2 inhibitor in hepatocytes. Then, the cell culture medium was replaced by sodium buffer and exposed to 200 μg/mL 2-(N-(7-nitrobenz-2-oxa1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG), a fluorescently labeled deoxyglucose analog, for 1 hour, washed and imaged by fluorescence microscope (IX71, Olympus, Tokyo, Japan). Moreover, cells were lysed with 0.1 M NaOH, neutralized with 0.1 M HCl, and measured using a microplate reader (excitation/emission=485/535 nm).
- Cell viability assay
- HepG2 cell viability was detected using Cell Counting Kit-8 (CCK-8) colorimetric assay (Abcam, Cambridge, UK), according to the manufacturer’s instructions. Briefly, cells were cultured in 96-well plate, then 10 μL of the CCK-8 solution was added to each well and incubated for 2 hours at 37°C. Absorbance was measured using a microplate reader at 450 nm (Tecan, Grödig, Austria).
- Immunofluorescence staining
- HepG2 cells cultured on coverslips were fixed with 4% formaldehyde for 10 minutes, permeabilized with 0.25% Triton X-100, and blocked with a normal goat serum solution for 1 hour. The cells were then incubated overnight with the primary anti-FOXO1 or anti-PEPCK antibody at 4°C and were incubated with an Alexa Fluor 488 secondary antibody or an Alexa Fluor 546 secondary antibody (Invitrogen, Thermo Fisher Scientific, Waltham, CA, USA) for 1 hour at room temperature. After washing with phosphate-buffered saline, the cells were mounted using an aqueous mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (ab104139, Abcam), and images were taken using a fluorescence microscope (BX51, Olympus).
- Quantitative real-time polymerase chain reaction
- Total RNA was extracted from HepG2 cells using TRIzol reagent (Invitrogen) and quantified using spectrophotometry (Nanodrop, Thermo Scientific). cDNA was synthesized using a High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA), and the expression of target genes was measured using a LightCycler 480 SYBR Green (Roche, Lewis, UK). The primer sequences used for polymerase chain reaction (PCR) are listed in Supplemental Table S1. Thermocycling conditions of quantitative real-time PCR were as follows: 45 cycles of denaturation at 95°C for 30 seconds, 55°C for 30 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. β-Actin was used as the reference control to normalize the relative expression levels of the target genes.
- Western blotting
- Total proteins were extracted from cultured cells using radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA, USA) containing Halt Protease and Phosphatase Inhibitor Cocktail (100×) (Thermo Fisher Scientific, Rockford, IL, USA). Nuclear and cytoplasmic protein fractions were isolated from cultured cells using a nuclear extraction kit (Cayman Chemical). Cell lysates containing 20 µg protein were mixed with 4× lithium dodecyl sulfate sample buffer and 10× reducing sample agent (Invitrogen), boiled at 95°C for 10 minutes. Protein samples were loaded onto a 4% to 12% Bis-Tris NuPAGE gel (Thermo Fisher Scientific) and transferred to a polyvinylidene difluoride (PVDF) membrane using an iBlot2 PVDF stack (Invitrogen). The membranes were blocked with 5% bovine serum albumin in tris-buffered saline with Tween-20 for 1 hour at room temperature, following which they were incubated overnight at 4°C with the following specific primary antibodies (Supplemental Table S2). Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Protein bands were visualized using an enhanced chemiluminescence reagent (DAWINBio, Hanam, Korea), and band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
- Transient small interfering RNA transfection
- The cells were transfected with SGLT2 small interfering RNA (siRNA) to determine the modulation of glucose metabolism by SGLT2 inhibition under serum deprivation and serum supplementation conditions. siRNA duplexes against SGLT2 and the negative control were purchased from Bioneer (Daejeon, Korea), and the cells were transfected with SGLT2 siRNA using Lipofectamine RNAiMAX transfection reagent (Invitrogen), according to the manufacturer’s instructions. Cells pre-transfected with SGLT2 siRNA were cultured in serum-free DMEM (25 mmol/L glucose) for 16 hours and then in media supplemented with or without 10% FBS for 8 hours.
- Statistical analysis
- All experiments were performed in triplicate, and data are presented as mean±standard error values. Statistical comparisons between groups were conducted using the SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). The data were analyzed using a one-way analysis of variance, followed by Bonferroni post hoc pairwise comparisons to calculate significant differences between the mean values obtained from the different experimental groups. P<0.05 was considered statistically significant.
METHODS
- SGLT2 inhibitors reduce glucose uptake in hepatocytes
- SGLT2, mainly present in the renal proximal tubule, maintains glucose homeostasis by regulating glucose reabsorption in a sodium-dependent manner [3]. Huh7 cells were treated with various concentrations of the SGLT2 inhibitors, empagliflozin and dapagliflozin, to determine the direct role of SGLT2 in regulating hepatic glucose metabolism. Empagliflozin and dapagliflozin decreased the glucose uptake dose-dependently (Fig. 1). Glucose uptake increased in cells treated with sodium buffer compared to those treated with sodium-free buffer. Interestingly, however, the expression of glucose transporter 2 (GLUT2), another glucose transporter in the liver, was increased in the presence of glucose but was not regulated by SGLT2 inhibitors (Supplemental Fig. S1). These results indicate that SGLT2 plays a role in hepatic glucose metabolism and that SGLT2 inhibitors may play a role in directly inhibiting glucose uptake in hepatocytes without affecting GLUT2.
- SGLT2 inhibition increases gluconeogenesis in HepG2 during serum deprivation
- Glucose deprivation induces cell death. Cells pre-transfected with SGLT2 siRNA were cultured under serum deprivation (serum-free media for 24 hours) and serum supplementation (serum-free media for 16 hours+serum-supplemented media for 8 hours) conditions to determine the effects of SGLT2 inhibition on serum starved cells. SGLT2 inhibition by siRNA in HepG2 cells did not lead to a compensatory increase in GLUT2 gene expression, but increased the expression of sirtuin 1 (SIRT1), whose expression is increased during starvation, and gluconeogenesis-associated genes, including peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α), PEPCK, and G6pase under serum deprivation conditions (Fig. 2). In addition, SGLT2 inhibition during serum deprivation decreased the expression of extracellular signal-regulated kinase (ERK), the c-Jun N-terminal kinases (JNK), and proliferating cell nuclear antigen (PCNA), which are involved in cell proliferation (Supplemental Fig. S2A). Nevertheless, the expression of cleaved caspase-3 protein did not change between the groups, and serum deprivation for 24 hours in cells pre-transfected with SGLT2 siRNA did not induce cell death (Supplemental Fig. S2A, B). These results indicate that SGLT2 inhibition under serum deprivation conditions decreases cell proliferation and increases gluconeogenesis for cell survival.
- SGLT2 inhibition increases the expression of p-AMPK and decreases the expression of nuclear p-AKT and p-FOXO1
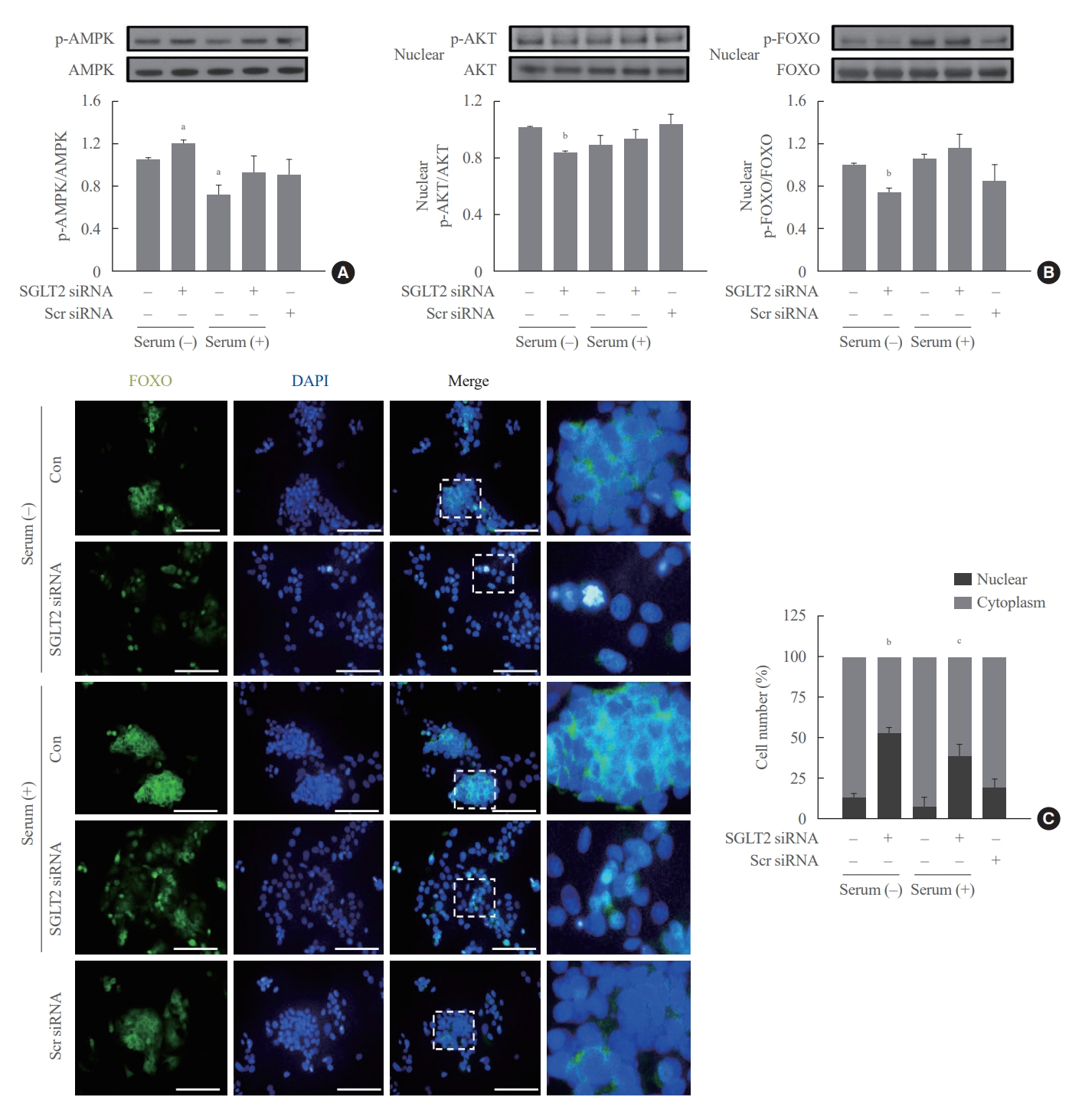
- We examined whether SGLT2 inhibition during serum deprivation and serum supplementation regulates the expression of FOXO1, a transcription factor involved in hepatic gluconeogenesis. AKT in the nucleus phosphorylates FOXO1, translocates FOXO1 to the cytoplasm, and inhibits its transcription activities [23,24]. SGLT2 inhibition by siRNA during serum deprivation, but not during serum supplementation, increased the expression of phosphorylated-adenosine monophosphate-activated protein kinase (p-AMPK) and decreased the expression of nuclear p-AKT and p-FOXO1 (Fig. 3A, B). In addition, SGLT2 inhibition by siRNA increased the nuclear localization of FOXO1, demonstrated by immunofluorescence staining (Fig. 3C). These results in cells transfected with SGLT2 siRNA were also consistently seen in cells treated with SGLT2 inhibitors, empagliflozin and dapagliflozin (Supplemental Fig. S3).
- AMPK inhibition in HepG2 cells transfected with SGLT2 siRNA under serum deprivation increases the expression of nuclear p-AKT and p-FOXO1 and reduces the expression of PEPCK
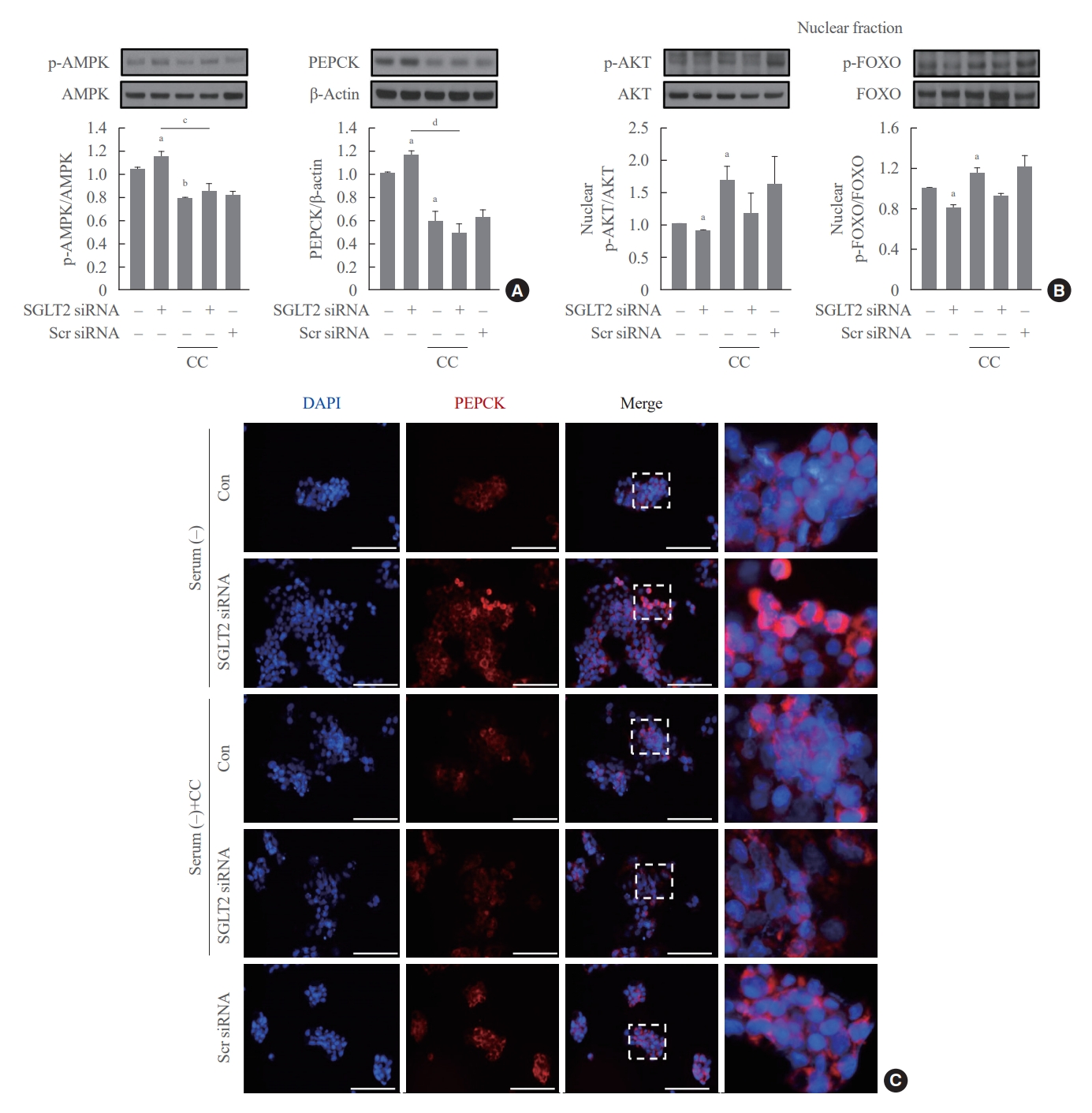
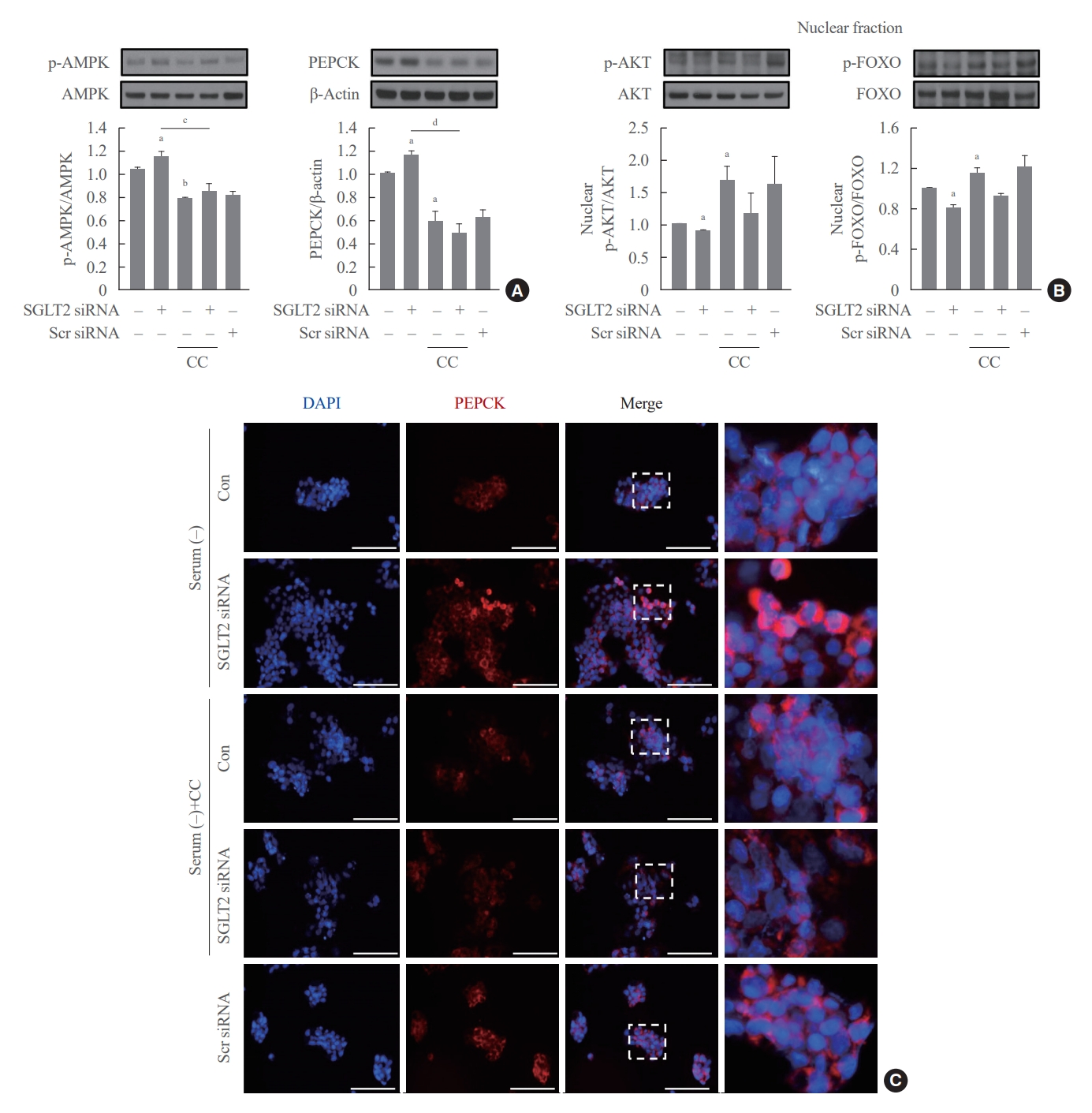
- To determine whether AMPK increased by SGLT2 inhibition modulates AKT-FOXO1 signaling, cells pre-transfected with SGTL2 siRNA were treated with the AMPK inhibitor compound C. Inhibition of AMPK by compound C treatment decreased the expression of p-AMPK and PEPCK proteins and increased the expression of nuclear p-AKT and p-FOXO1 proteins (Fig. 4A, B). In addition, the increase in p-AMPK and PEPCK protein expression and decrease in nuclear p-AKT and p-FOXO1 protein expression in cells transfected with SGLT2 siRNA were reversed by treatment with compound C. Consistently, immunofluorescence staining for PEPCK demonstrated that the increased expression of PEPCK in cells transfected with SGLT2 siRNA was reduced by compound C (Fig. 4C). These data show that AMPK, increased by SGLT2 inhibition during serum deprivation, increases hepatic gluconeogenesis through the AKT-FOXO1 pathway.
RESULTS
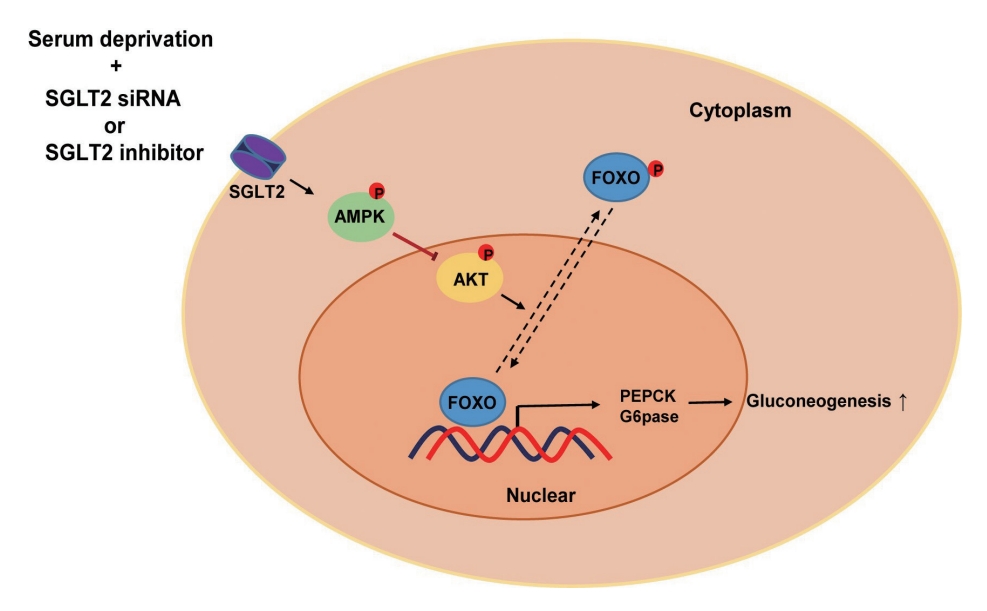
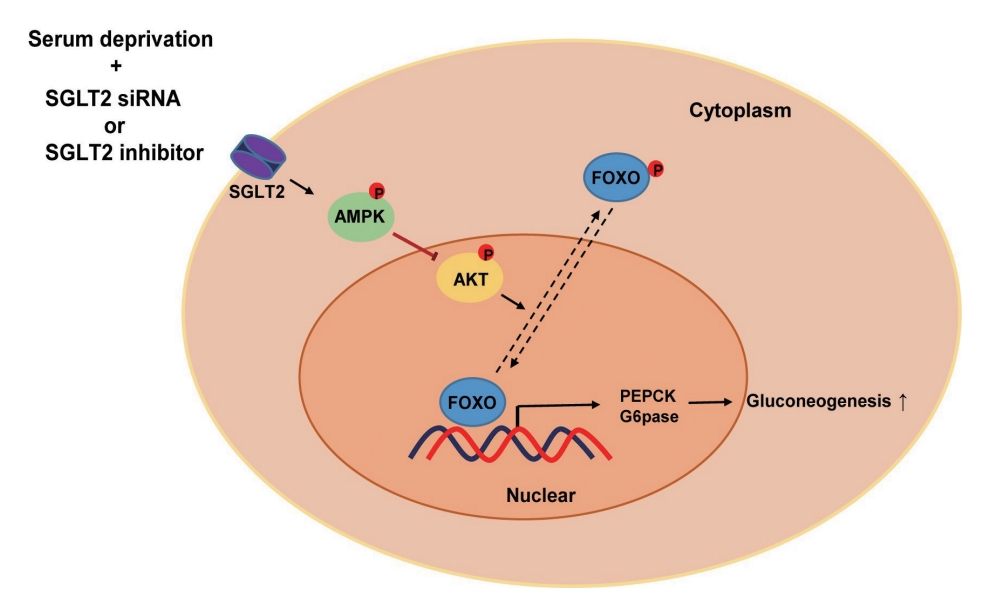
- In this study, we determined that SGLT2 inhibition during serum deprivation, but not during serum supplementation, increased hepatic gluconeogenesis via the AMPK/AKT/FOXO1 signaling pathway to protect against hypoglycemia-induced apoptosis (Fig. 5). The SGLT2 inhibitors, empagliflozin and dapagliflozin, decreased the sodium-dependent glucose uptake in Huh7 cells. SGLT2 inhibition under serum deprivation conditions increased the expression levels of gluconeogenesis-associated genes and AMPK protein and reduced the expression of nuclear p-AKT and p-FOXO1 proteins. In addition, nuclear localization of FOXO1, a major transcription factor for hepatic gluconeogenesis, was observed in cells transfected with SGLT2 siRNA during serum deprivation. However, in cells transfected with SGLT2 siRNA during serum deprivation, treatment with the AMPK inhibitor compound C reversed the decrease in the expression of nuclear p-AKT and p-FOXO1 and decreased the expression of PEPCK.
- The liver plays a vital role in the regulation of glucose homeostasis. Excessive glucose in the liver is used as a substrate for fatty acid synthesis, causing fatty liver disease, whereas glucose deprivation in hepatocytes induces growth arrest and cell death [1,25]. Gluconeogenesis is stimulated by glucagon, growth hormone, epinephrine, and cortisol, which regulate transcription factors for gluconeogenesis such as cAMP response element binding protein (CREB) and FOXO and transcriptional co-activator such as CREB binding protein (CBP)/p300, CREB regulated transcription co-activator 2 (CRTC2), and PGC-1α [26,27]. During hypoglycemia, glucagon, a hormone secreted by pancreatic α cells, releases glucose from the liver into the blood by activating glycogenolysis and gluconeogenesis [28]. In patients with T2DM, the SGLT2 inhibitor dapagliflozin improves insulin sensitivity by reducing plasma glucose concentrations via glycosuria and increases endogenous glucose production by increasing the glucagon/insulin ratio [11]. Bonner et al. [29] reported that dapagliflozin promotes glucagon secretion from pancreatic alpha cells and hepatic gluconeogenesis. In addition, even when combined with insulin, SGLT2 inhibitors improve glycosylated hemoglobin A1c levels, fasting plasma glucose levels, and body weight without increasing the risk of hypoglycemia [30]. In this study, we observed that despite the absence of glucagon, SGLT2 inhibition in hepatocytes increased the expression of gluconeogenesis-associated genes during serum deprivation. These results indicate that serum starvation would aggravate energy deficiency in cells transfected with SGLT2 siRNA, which blocks glucose uptake, and that SGLT2 inhibition directly prevents hypoglycemia by increasing hepatic gluconeogenesis during serum deprivation.
- FOXO proteins are transcription factors that play significant roles in autophagy, cell death, stress resistance, cell viability, metabolism, and differentiation [31]. Aoyama et al. [32] reported that feeding promotes the phosphorylation and nuclear exclusion of Foxo1 and reduces the expression levels of gluconeogenesis-associated genes such as G6pase, PEPCK, and PGC-1 in C57BL/6 mice. Whereas FOXO1 translocates to the nucleus during fasting and promotes the transcription of G6pase and PEPCK genes in the liver [26,33]. Phosphorylation of FOXO by AKT induces its translocation to the cytosol, thereby suppressing the transcriptional function of FOXO [23,34]. Under glucose deprivation, AMPK plays an opposite role to that of AKT in protecting cells from metabolic stress, and the AMPK agonists 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and phenformin dephosphorylate AKT [35]. In this study, AMPK expression was increased in cells transfected with SGLT2 siRNA during serum deprivation. AMPK inhibitor treatment reversed the decreased expression of nuclear p-AKT and p-FOXO1 and decreased the expression of cytoplasmic PEPCK in cells transfected with SGLT2 siRNA during serum deprivation. Thus, these data suggest that AMPK increased by SGLT2 inhibition during serum deprivation stimulates hepatic gluconeogenesis through the AKT-FOXO1 signaling pathway to protect cells from energy deprivation-induced stress.
- The regulatory effect of AMPK on FOXO-mediated gluconeogenesis is controversial. Guo et al. [36] reported that metformin, as an AMPK agonist, decrease FOXO1 S273 by inhibiting protein kinase A (PKA), blocks FOXO nuclear translocation, suppressing gluconeogenesis, in cells treated with glucagon. However, FOXO has multiple phosphorylation sites, and the nuclear-cytoplasmic translocation of FOXO depends on the activation of each phosphorylation site. In this study, we observed that AMPK activated by SGLT2 inhibition during serum deprivation, decreased phospho-FOXO1 S256 by inhibiting AKT and increased gluconeogenesis. FOXO1 is phosphorylated at T24, S256, and S319 by AKT that is followed by nuclear exclusion [37]. Thus, the regulation of gluconeogenesis by AMPK through FOXO depends on the phosphorylation site of FOXO and the subsequent localization of FOXO. Therefore, the conflicting results in the two studies may be due to differences in the mechanism activating FOXO as a transcription factor and the experimental conditions (glucagon treatment vs. serum deprivation condition).
- Two families of glucose transporters regulate glucose uptake: GLUTs and SGLTs. The GLUT family contains 14 members and is expressed in a tissue-specific manner. GLUT2 is the main glucose-facilitating transporter isoform in the liver that controls glucose uptake depending on the circulating glucose concentration [38,39]. The role of SGLT2 in mediating the active transport of glucose is sodium-dependent. In this study, glucose uptake was reduced in a sodium-free medium compared to that in a sodium medium, indicating that GLUT2 regulates sodium-independent glucose uptake. Interestingly, no compensatory increase in GLUT2 expression was observed in cells treated with SGLT2 inhibitors or in cells transfected with SGLT2 siRNA compared to control cells. In addition, the inhibition of SGLT2 during serum deprivation further reduced the expression of cell proliferation-related factors, such as EKR, JNK, and PCNA, compared to serum supplementation. The mitogen-activated protein (MAP) kinase pathway, mediated by ERK, JNK, and p38 protein kinases, regulates proliferation, differentiation, development, transformation, and apoptosis [40]. PCNA, a cell proliferation marker, is a downstream factor of ERK and PCNA expression is down-regulated by MAPK inhibitor or siRNA [41]. Thus, these results suggest that GLUT2 cannot replace SGLT2, and SGLT2 inhibition during serum deprivation, compared to serum supplementation, seems to minimize energy expenditure by inhibiting cell proliferation.
- In conclusion, the present study showed that SGLT2 plays a role in regulating glucose uptake in hepatocytes and that inhibition of SGLT2 during serum deprivation protects against starvation-induced cell damage by increasing hepatic gluconeogenesis. Therefore, SGLT2 inhibition may be a safe approach for lowering blood glucose levels in patients with diabetes and simultaneously improving liver disease associated with high blood glucose levels.
DISCUSSION
Supplementary Material
Supplemental Table S1.
Supplemental Table S2.
Supplemental Fig. S1.
Supplemental Fig. S2.
Supplemental Fig. S3.
-
CONFLICTS OF INTEREST
Won-Young Lee is an editor-in-chief and Eun-Jung Rhee is a deputy editor of the journal. But they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.L., S.W.H., S.J.M., E.J.R., W.Y.L. Acquisition, analysis, or interpretation of data: J.L., S.W.H., M.J.K., Y.M.L., S.J.M., H.K., S.E.P., E.J.R., W.Y.L. Drafting the work or revising: J.L., S.W.H., M.J.K., Y.M.L., S.J.M., H.K., S.E.P., E.J.R., W.Y.L. Final approval of the manuscript: J.L., S.W.H., E.J.R., W.Y.L.
Article information
-
Acknowledgements
- This study was supported by the National Research Foundation (NRF) funded by the Korean government (NRF-2022R1F1A 1072882) (http://www.nrf.re.kr). The funders played no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.


- 1. Adeva-Andany MM, Perez-Felpete N, Fernandez-Fernandez C, Donapetry-Garcia C, Pazos-Garcia C. Liver glucose metabolism in humans. Biosci Rep 2016;36:e00416.ArticlePubMedPMCPDF
- 2. Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig 2020;11:770–82.ArticlePubMedPMCPDF
- 3. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011;22:104–12.ArticlePubMedPMC
- 4. Kim JH, Ko HY, Wang HJ, Lee H, Yun M, Kang ES. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes Metab 2020;22:373–82.ArticlePubMedPDF
- 5. Obara K, Shirakami Y, Maruta A, Ideta T, Miyazaki T, Kochi T, et al. Preventive effects of the sodium glucose cotransporter 2 inhibitor tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic mice. Oncotarget 2017;8:58353–63.ArticlePubMedPMC
- 6. Li L, Li Q, Huang W, Han Y, Tan H, An M, et al. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol 2021;12:589273.ArticlePubMedPMC
- 7. Akirov A, Grossman A, Shochat T, Shimon I. Mortality among hospitalized patients with hypoglycemia: insulin related and noninsulin related. J Clin Endocrinol Metab 2017;102:416–24.ArticlePubMed
- 8. Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: an update on pathophysiology, treatment, and prevention. World J Diabetes 2021;12:2036–49.ArticlePubMedPMC
- 9. Anderson M, Powell J, Campbell KM, Taylor JR. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab Syndr Obes 2014;7:85–94.PubMedPMC
- 10. Filippas-Ntekouan S, Filippatos TD, Elisaf MS. SGLT2 inhibitors: are they safe? Postgrad Med 2018;130:72–82.ArticlePubMed
- 11. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–14.ArticlePubMedPMC
- 12. Zhang X, Yang S, Chen J, Su Z. Unraveling the regulation of hepatic gluconeogenesis. Front Endocrinol (Lausanne) 2019;9:802.ArticlePubMedPMC
- 13. Geisler CE, Hepler C, Higgins MR, Renquist BJ. Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr Metab (Lond) 2016;13:62.ArticlePubMedPMCPDF
- 14. Rui L. Energy metabolism in the liver. Compr Physiol 2014;4:177–97.ArticlePubMedPMCPDF
- 15. Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care 2002;6:317–21.ArticlePubMedPMC
- 16. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010;27:136–42.ArticlePubMedPMC
- 17. Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal 2005;7:752–60.ArticlePubMed
- 18. Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int 2014;2014:925350.ArticlePubMedPMCPDF
- 19. Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol 2013;28 Suppl 1:125–31.ArticlePubMedPDF
- 20. Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, et al. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A 2019;116:13533–42.ArticlePubMedPMC
- 21. Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 2012;50:437–43.ArticlePubMedPMC
- 22. Lu YT, Ma XL, Xu YH, Hu J, Wang F, Qin WY, et al. A fluorescent glucose transport assay for screening SGLT2 inhibitors in endogenous SGLT2-expressing HK-2 cells. Nat Prod Bioprospect 2019;9:13–21.ArticlePubMedPMCPDF
- 23. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 2011;1813:1978–86.ArticlePubMed
- 24. Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 2011;1813:1938–45.ArticlePubMed
- 25. Zhou Y, Yu H, Cheng S, Chen Y, He L, Ren J, et al. Glutamate dehydrogenase 1 mediated glutaminolysis sustains HCC cells survival under glucose deprivation. J Cancer 2022;13:1061–72.ArticlePubMedPMC
- 26. Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep 2013;46:567–74.ArticlePubMedPMC
- 27. Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 2003;285:E685–92.ArticlePubMed
- 28. Moon JS, Won KC. Pancreatic α-cell dysfunction in type 2 diabetes: old kids on the block. Diabetes Metab J 2015;39:1–9.ArticlePubMedPMC
- 29. Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–7.ArticlePubMedPDF
- 30. Yang Y, Chen S, Pan H, Zou Y, Wang B, Wang G, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6944.PubMedPMC
- 31. Link W, Fernandez-Marcos PJ. FOXO transcription factors at the interface of metabolism and cancer. Int J Cancer 2017;141:2379–91.ArticlePubMedPDF
- 32. Aoyama H, Daitoku H, Fukamizu A. Nutrient control of phosphorylation and translocation of Foxo1 in C57BL/6 and db/db mice. Int J Mol Med 2006;18:433–9.ArticlePubMed
- 33. Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003;423:550–5.ArticlePubMedPDF
- 34. He L, Li Y, Zeng N, Stiles BL. Regulation of basal expression of hepatic PEPCK and G6Pase by AKT2. Biochem J 2020;477:1021–31.ArticlePubMedPMCPDF
- 35. Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer 2017;16:79.ArticlePubMedPMCPDF
- 36. Guo X, Li X, Yang W, Liao W, Shen JZ, Ai W, et al. Metformin targets Foxo1 to control glucose homeostasis. Biomolecules 2021;11:873.ArticlePubMedPMC
- 37. Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 2004;378(Pt 3):839–49.ArticlePubMedPMCPDF
- 38. Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol 2012;18:6771–81.ArticlePubMedPMC
- 39. Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch 2020;472:1273–98.ArticlePubMedPMCPDF
- 40. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002;12:9–18.ArticlePubMedPDF
- 41. Lu R, Wang X, Chen ZF, Sun DF, Tian XQ, Fang JY. Inhibition of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway decreases DNA methylation in colon cancer cells. J Biol Chem 2007;282:12249–59.ArticlePubMed
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite