Postoperative Thyroid-Stimulating Hormone Levels Did Not Affect Recurrence after Thyroid Lobectomy in Patients with Papillary Thyroid Cancer
Article information
Abstract
Background
Thyroid-stimulating hormone (TSH) suppression is recommended for patients who undergo thyroidectomy for differentiated thyroid cancer (DTC). However, the impact of TSH suppression on clinical outcomes in low-risk DTC remains uncertain. Therefore, we investigated the effects of postoperative TSH levels on recurrence in patients with low-risk DTC after thyroid lobectomy.
Methods
Patients (n=1,528) who underwent thyroid lobectomy for papillary thyroid carcinoma between 2000 and 2012 were included in this study. According to the mean and dominant TSH values during the entire follow-up period or 5 years, patients were divided into four groups (<0.5, 0.5 to 1.9, 2.0 to 4.4, and ≥4.5 mIU/L). Recurrence-free survival was compared among the groups.
Results
During the 5.6 years of follow-up, 21 patients (1.4%) experienced recurrence. Mean TSH levels were within the recommended low-normal range (0.5 to 1.9 mIU/L) during the total follow-up period or 5 years in 38.1% or 36.0% of patients. The mean and dominant TSH values did not affect recurrence-free survival. Adjustment for other risk factors did not alter the results.
Conclusion
Serum TSH levels did not affect short-term recurrence in patients with low-risk DTC after thyroid lobectomy. TSH suppression should be conducted more selectively.
INTRODUCTION
The incidence of thyroid cancer has been increasing, and the detection of small thyroid cancers (<1 cm) contributes to a significant portion of the increase [1]. With an increase in the incidence, the need for thyroid lobectomy in patients has also increased. Moreover, as the tumor size criteria for thyroid lobectomy have been widened from 1 to 4 cm [2], thyroid lobectomy is now being performed more often.
Thyroid-stimulating hormone (TSH) suppression with supraphysiologic doses of levothyroxine is recommended to patients who undergo thyroidectomy for thyroid cancer [2] as TSH suppression can inhibit the proliferation of thyroid cancer cells [34]. Previous studies have reported that TSH suppression improved survival and reduced disease progression and recurrence [5678]. However, effects of TSH suppression were more significant in patients with advanced stage thyroid cancer (stage 3 or 4), and not as significant in patients with early stage thyroid cancer (stage 1 or 2) [56]. In addition, TSH suppression is associated with several morbidities, including cardiovascular and skeletal disease [9] and cardiovascular mortality [10]. TSH suppression therapy is associated with an increased risk of arrhythmias including atrial fibrillation [11] and osteoporosis/bone strength [1121314]. Therefore, TSH suppression should be determined with consideration of these advantages and disadvantages.
Patients who undergo thyroid lobectomy are typically low-risk patients. Thyroid lobectomy is considered in patients with differentiated thyroid cancer (DTC) <4 cm without extrathyroidal extension (ETE) and clinical evidence of any lymph node (LN) metastases [2]. For these patients, American Thyroid Association (ATA) recommends that TSH may be maintained in the mid to lower reference range (0.5 to 2.0 mU/L) after thyroid lobectomy [2]. However, few studies have supported this recommendation, and whether TSH suppression is required in those patients remains controversial. Therefore, we investigated whether serum TSH levels affect the recurrence in patients who underwent thyroid lobectomy for thyroid cancer.
METHODS
Patients
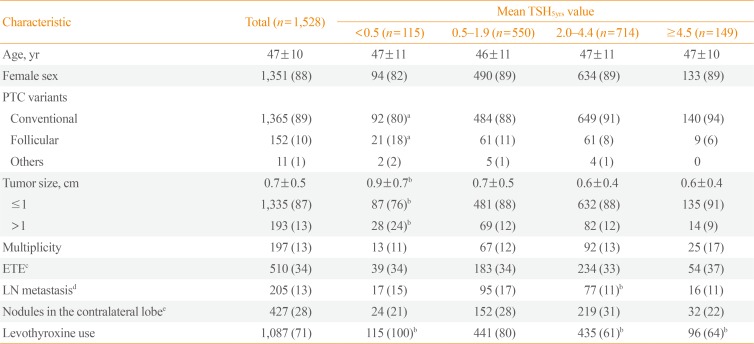
Patients who underwent thyroid lobectomy for papillary thyroid carcinoma (PTC) at the Korea Cancer Center Hospital and Seoul National University Hospital between January 2000 and December 2012 were included in this study. Patients younger than 20 years of age and subtypes of PTC known to have a poor prognosis such as the tall cell variant were excluded. In total, 1,875 patients met these criteria. We then further excluded patients who were followed less than 2 years (n=166) and who had a measured serum TSH concentration less than 5 during the follow-up period (n=181). Finally, 1,528 patients were included in the analysis (mean age 47 years, female 88%, mean duration of follow-up 5.6 years). Overall, 1,260 patients from the Korea Cancer Center Hospital and 268 from Seoul National University Hospital were included. The study protocol was approved by the Institutional Review Board of Korea Cancer Center Hospital (KIRAMS 2018-04-005) and Seoul National University Hospital (H-1707-035-867). Data were collected retrospectively by the review of electronic medical records.
Initial operation and follow-up
Preoperative neck ultrasonography (US) and/or computed tomography was performed in all patients. If there was a nodule (or nodules) found on the contralateral lobe, thyroid lobectomy was performed if the US features of nodules suggested that they were benign or if the results of US-guided fine-needle aspiration (FNA) were benign. Prophylactic central neck dissection was performed depending on the preference of the surgeon.
After the initial operation, serum TSH concentration and neck US were performed at least once per year. During the 5.6 years of follow-up, the median number of TSH measurements was 9 (range, 5 to 40). When cervical LN or a thyroid mass was suspicious for recurrence on neck US, US-guided FNA or core-needle biopsy was performed. Final diagnosis of the recurrence was confirmed by the pathological or cytological results. The time of recurrence was defined as the date of the first pathological or cytological confirmation.
Statistical analyses
Data are expressed as mean±standard deviation. We collected all serum TSH levels measured after surgery. In patients who experienced recurrence only the TSH levels before recurrence were used. After then, mean TSH values were calculated (TSHtotal). Serum TSH levels at each follow-up visit were categorized into four groups (<0.5, 0.5 to 1.9, 2.0 to 4.4, and ≥4.5 mIU/L). The dominant TSH value in each patient was defined as the TSH group comprising most proportion among sequential TSH groups during follow-up period. In the analysis with dominant TSH value, 164 patients who showed multiple peaks of serum TSH levels were excluded because we could not determine the dominant TSH value. In addition, mean and dominant TSH5yrs values were calculated with serum TSH levels for 5 years after surgery. Categorical and continuous variables were analyzed using the chi-square test and Student's t test, respectively. Recurrence-free survival (RFS) was defined as the time interval between the date of thyroid surgery and the date of recurrence. RFS curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard regression was also performed. In multivariate Cox regression analysis, known risk factors such as age, sex, tumor size, multiplicity, ETE, and LN metastasis were included for adjustment. P<0.05 was considered significant. All statistical analyses were performed by SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics
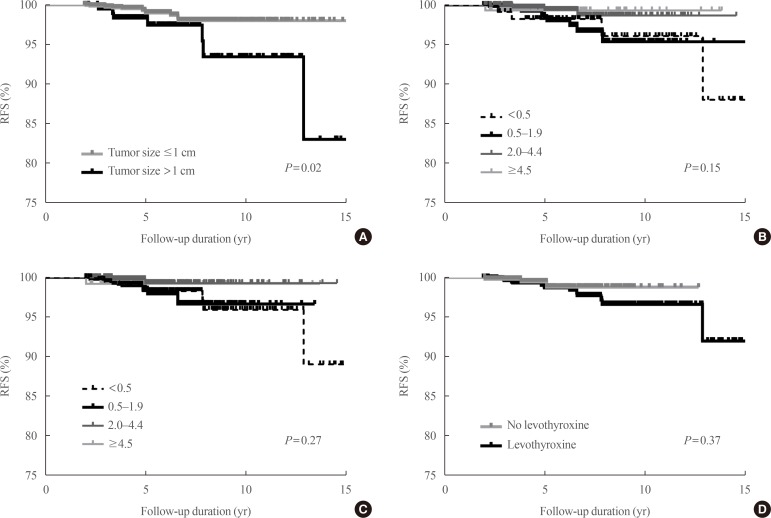
Baseline characteristics of patients are summarized in Table 1. Most patients (89%) had conventional PTC and 87% of the tumors were less than 1 cm. However, 34% and 13% had ETE and central LN metastasis, respectively. During the 5.6 years of follow-up, 21 patients (1.4%) experienced recurrence, and were locoregional; there was no distant metastasis. Among these patients, 14 patients experienced recurrence due to contralateral thyroid nodules and seven patients did due to LN metastasis. All patients underwent completion thyroidectomy and LN dissection. On the final surgical pathology, 16 patients had PTC in the contralateral lobe, and 10 had LN metastasis. The median time to recurrence was 4.9 years (range, 2.1 to 17.0). The 5 and 10-year recurrence rates were 0.8% and 1.4%, respectively. Among the known risk factors for recurrence, only tumor size had a significant impact on the RFS, but the others did not (Fig. 1A, Supplemental Table S1).
Distribution of postoperative TSH levels
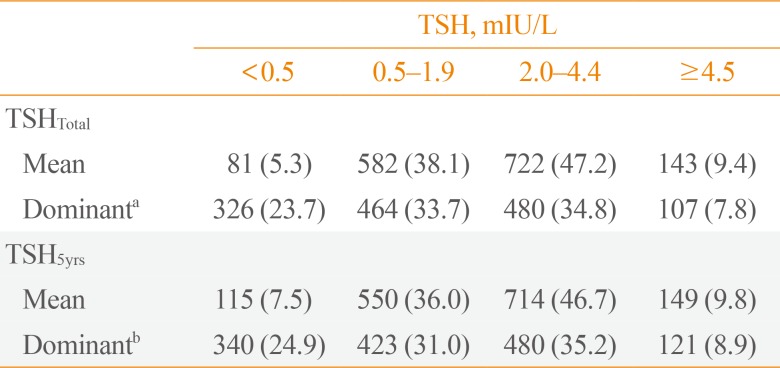
According to the mean and dominant TSHtotal or TSH5yrs, patients were divided into four groups (Table 2). In 36.0% of patients, mean TSH5yrs levels were within the recommended low-normal range (0.5 to 1.9 mIU/L). The mean TSH5yrs level in 46.7% of patients were in the high-normal range (2.0 to 4.4 mIU/L). Overall, 7.5% or 9.8% of patients had a mean TSH5yrs level of <0.5 or ≥4.5 mIU/L, respectively. The distribution of dominant TSH values were similar (Table 2), and patients with dominant TSH5yrs values within the recommended low-normal range (0.5 to 1.9 mIU/L) were 31.0%.
Impact of serum TSH levels on tumor recurrence
We evaluated the effects of serum TSH levels on RFS. Kaplan-Meier RFS curves showed that mean and dominant TSH values did not affect RFS (Fig. 1B, C). Both univariate and multivariate Cox regression analyses with several known risk factors including age, sex, tumor size, multiplicity, ETE, and LN metastasis showed no significant differences in the results (Fig. 2).
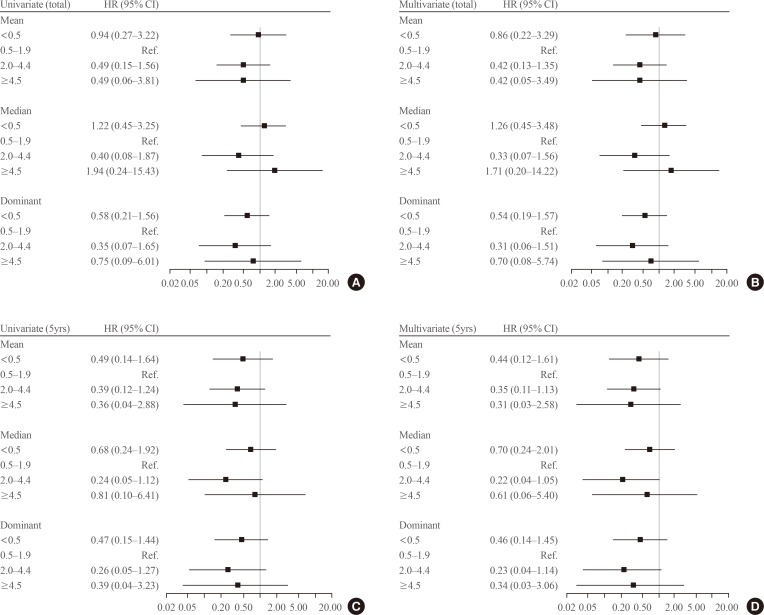
Forest plot for recurrence-free survival according to the mean thyroid-stimulating hormone (TSH) values during the total follow-up period or 5 years. In multivariate Cox regression analysis, known risk factors including age, sex, tumor size, multiplicity, extrathyroidal extension, and lymph node metastasis were adjusted. (A) Univariate Cox regression analysis with mean TSH values were calculated (TSHtotal) values. (B) Multivariate Cox regression analysis with mean TSHtotal values. (C) Univariate Cox regression analysis with mean TSH levels for 5 years after surgery (TSH5yrs) values. (D) Multivariate Cox regression analysis with mean TSH5yrs values. HR, hazard ratio; CI, confidence interval.
There is a possibility that serum TSH levels were suppressed differently based on the characteristics of the patients. Therefore, we compared the baseline characteristics among the groups according to the mean TSH5yrs values (Table 1). Compared to the mean TSH5yrs 0.5 to 1.9 group, the mean TSH5yrs <0.5 group showed a larger tumor size (0.9±0.7 cm vs. 0.7±0.5 cm), and the mean TSH5yrs 2.0 to 4.4 group experienced fewer instances of LN metastasis (11% vs. 17%). Analysis with the dominant TSH values yielded similar results (data not shown). Therefore, we conducted a subgroup analysis according to tumor size and LN metastasis. However, mean TSH5yrs values did not affect RFS in the subgroup analysis (Supplemental Table S2), and dominant TSH values were the same (data not shown).
Impact of levothyroxine use on tumor recurrence
Among all patients, 1,087 (71%) had taken levothyroxine. The mean TSH5yrs levels of these patients was significantly lower than that in patients without levothyroxine (2.46 mIU/L vs. 2.97 mIU/L, P<0.01). The lower TSH5yrs group, levothyroxine was more significantly prescribed (Table 1). Levothyroxine use did not affect RFS (hazard ratio, 1.74; 95% confidence interval, 0.50 to 5.99; P=0.38) (Fig. 1D).
DISCUSSION
In the present study, we demonstrated that postoperative serum TSH levels did not affect recurrence in low-risk PTC patients who underwent thyroid lobectomy. These findings suggest that 20.00there is no evidence on the beneficial effect of TSH suppression on the short-term recurrence.
There is controversy regarding TSH suppression in low-risk DTC patients who undergo thyroid lobectomy because of a lack of evidence. Previous studies have shown that TSH suppression was associated with a decrease in disease recurrence or death [7]; however, those studies included patients at various tumor stages and risks. Therefore, it is difficult to apply the results equally to low-risk DTC patients. The National Thyroid Cancer Treatment Cooperative Study Group (NTCTCS), a large cohort of 11 North American institution, has reported the association between TSH suppression and clinical outcomes [5615]. First, Cooper et al. [5] reported that TSH suppression did not predict disease progression in patients with stage I or II DTC. Next, Jonklaas et al. [6] reported that TSH suppression did not affect disease-specific survival in patients with stage I or II DTC. However, in the most recent study, Carhill et al. [15] reported that modest TSH suppression was associated with improved DFS and overall survival in all stages. However, in a randomized controlled trial in which most subjects (88%) had low-risk, TSH suppression targeted at TSH <0.01 µU/mL did not alter RFS [16]. Among patients with stage I or II DTC, those who undergo thyroid lobectomy typically have a lower risk. Recently, a study with low-risk DTC patients who underwent thyroid lobectomy was reported for the first time, and RFS did not differ according to TSH suppressive therapy or serum TSH levels [17]. To verify the impact of serum TSH levels in low-risk DTC patients who underwent thyroid lobectomy, we conducted the present study with more patients, and the same results were confirmed. TSH suppression in these patients is not necessary and should be reconsidered.
Furthermore, TSH suppression has adverse effects. It can cause arrhythmias, including atrial fibrillation [11] and osteoporosis [1121314], and increase cardiovascular mortality [10]. Considering these adverse effects, TSH suppression should not be performed without a clear evidence of its effectiveness in clinical outcomes.
Thyroid lobectomy may not require life-long thyroid hormone replacement because the contralateral thyroid is left intact. It is one of the major reasons for selecting thyroid lobectomy. However, a recent American study found that 73% of patients at 1 year after thyroid lobectomy had TSH >2 mU/L [18]. This suggests that approximately 73% of patients after thyroid lobectomy will need levothyroxine only for TSH suppression according to the current ATA guideline [2]. If TSH suppression is not necessary in low-risk DTC patients who undergo thyroid lobectomy, many patients may no longer need daily medication. In reality, 29% showed persistent hypothyroidism and only 12% to 13% of patients required levothyroxine replacement after thyroid lobectomy without TSH suppression [1920].
This study has some limitations considering its retrospective design. The recurrence rate was very low because of the nature of low-risk PTC, and previous studies reported that the 10-year recurrence rate was 1.7% to 8.2% [212223]. In this study, the recurrence rate (1.4%) was slightly lower than the previous ones, which can weaken the power of results. Moreover, we cannot determine the effects of serum TSH levels on long-term recurrence because of a short follow-up duration (5.6 years). Thyroid cancers recur steadily even after 5 years [2425]. Therefore, although a significant impact of postoperative TSH levels on the recurrence was not found in this study, TSH suppression can be effective in the long-term clinical outcomes and further studies are warranted in this regard. Lastly, the TSH evaluation period differed individually. TSH5yrs values are estimated using serum TSH levels for up to 5 years after surgery. In our study, the median time to recurrence was 4.9 years, that means about half of the patients had recurrence before 5 years. Therefore, the TSH evaluation period was shorter in patients with recurrence at less than 5 years, compared to the patients without recurrence. This limitation would be avoided by using serum TSH levels until earlier time after surgery, but it was impossible because of the insufficient number of TSH measurements. When using serum TSH levels for up to 2 years after surgery, the proportion of patients who measured serum TSH levels less than five times was 65%.
In the present study, serum TSH levels did not affect the short-term recurrence in patients with low-risk DTC after thyroid lobectomy. Overall, TSH suppression is more useful in terms of patient-specific considerations and is not be necessarily useful for all patients. Therefore, the decision for TSH suppression should be made carefully, particularly in older patients with a short life expectancy or many risk factors for TSH suppression-related adverse events, including arrhythmia or osteoporosis/fractures.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS:
Conception or design: M.J.K., H.S.C., Y.J.P.
Acquisition, analysis, or interpretation of data: M.C.L., M.J.K., H.S.C., S.W.C., G.H.L., Y.J.P., D.J.P.
Drafting the work or revising: M.C.L., M.J.K., H.S.C., Y.J.P.
Final approval of the manuscript: M.J.K., H.S.C.
References
SUPPLEMENTARY MATERIALS
Supplemental Table S1
Cox Regression Analysis with Known Risk Factors for Recurrence-Free Survival
Supplemental Table S2
Cox Regression Analysis for Recurrence-Free Survival According to the Thyroid-Stimulating Hormone Levels