Frequency of TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients
Article information
Abstract
Background
Telomerase reverse transcriptase (TERT) promoter mutations are associated with increased recurrence and mortality in patients with thyroid carcinoma. Previous studies on TERT promoter mutations were retrospectively conducted on a limited number of patients.
Methods
We prospectively collected data on all consecutive patients who underwent thyroid carcinoma surgery between January 2019 and December 2020 at the Samsung Medical Center, Seoul, Korea. We included 2,092 patients with thyroid carcinoma.
Results
Of 2,092 patients, 72 patients (3.4%) had TERT promoter mutations. However, the frequency of TERT promoter mutations was 0.5% in papillary thyroid microcarcinoma (PTMC) ≤1 cm and it was 5.8% in papillary thyroid carcinoma (PTC) >1 cm. The frequency of TERT promoter mutations was significantly associated with older age at diagnosis (odds ratio [OR], 1.12; P<0.001), larger primary tumor size (OR, 2.02; P<0.001), and aggressive histological type (OR, 7.78 in follicular thyroid carcinoma; OR, 10.33 in poorly differentiated thyroid carcinoma; OR, 45.92 in anaplastic thyroid carcinoma; P<0.001). Advanced T stage, advanced N stage, and distant metastasis at diagnosis were highly prevalent in mutated thyroid cancers. However, initial distant metastasis was not present in patients with TERT promoter mutations in PTMC. Although the C228T mutation was more highly detected than the C250T mutation (64 cases vs. 7 cases), there were no significant clinicopathological differences.
Conclusion
This study is the first attempt to investigate the frequency of TERT promoter mutations in a real-world setting. The frequency of TERT promoter mutations in PTC was lower than expected, and in PTMC, young patients, and female patients, the frequency was very low.
INTRODUCTION
The incidence of thyroid carcinoma has increased with time, but there has been no increase in mortality rate [1-3]. Most patients with thyroid carcinoma have a long survival. Therefore, it is important to distinguish patients who need aggressive treatment from those who do not. Conventional prognostic factors cannot predict the outcome of each patient completely [4-6]. Therefore, more precise factors for estimating the oncologic outcome are required to select a proper therapeutic strategy for each patient.
Telomerase reverse transcriptase (TERT) promoter mutations are the main genetic alteration in the risk stratification of thyroid carcinoma. TERT promoter mutations increase telomerase activity, which protects telomeres from shortening and allows carcinoma cells to undergo immortalization. The TERT promoter mutations have been reported to be highly prevalent in tumors with aggressive or dedifferentiated histology [7-9], and it is associated with increased recurrence and mortality in patients with thyroid carcinoma [7,10-14]. Our team reported that TERT promoter mutations can be a molecular prognostic marker, and may improve the prediction of a prognosis when merged with the conventional staging system in differentiated thyroid carcinoma (DTC) [15-17].
Although it is well known that TERT promoter mutations are significant factors in oncologic outcomes, the real-world frequency of TERT promoter mutations is unclear. Previous studies on TERT promoter mutations were retrospectively conducted on limited subjects and did not reflect the actual situation in the clinical setting. The studies may overestimate the frequency of TERT promoter mutations because of potential selection bias. In this study, we prospectively performed TERT promoter mutation tests in all consecutive patients who underwent surgery for thyroid carcinoma in our institution. Therefore, this study can provide real-world data on TERT promoter mutation frequency.
METHODS
Patients and clinicopathological data
We collected data from 2,143 consecutive patients with thyroid tumors who underwent thyroidectomy or neck dissection between January 2019 and December 2020 at the Samsung Medical Center, Seoul, Korea. The TERT promoter mutation test was performed immediately after surgery regardless of individual clinical risks. Among the 2,143 patients, nine patients were pathologically diagnosed with medullary thyroid carcinoma and 42 patients who were suspected of malignancy in preoperative fine-needle aspiration or core-needle biopsy were diagnosed with benign disease (follicular adenoma, Hurthle cell adenoma, well-differentiated tumor of uncertain malignant potential, and follicular tumor of uncertain malignant potential) after surgery were excluded. Finally, 2,092 thyroid carcinoma patients were included in the study. We defined papillary thyroid microcarcinoma (PTMC) as a primary tumor size ≤1 cm.
The clinical information of patients was obtained from electronic medical records. Cancer staging was conducted by using the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. The Institutional Review Board at the Samsung Medical Center approved this study (IRB 2022-01-072). Patient consent was waived by the committee because of the retrospective chart review study design and the use of only deidentified clinicopathologic information.
Detection of TERT promoter mutations
Genomic DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue using a Qiagen DNA FFPE Tissue Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) sequencing was carried out to identify TERT promoter mutations using an iTERT mutation detection kit (Geninus Inc., Seoul, Korea). The PCR reactions were assembled on ice and preincubated at 94°C for 15 minutes, followed by 40 cycles at 94°C for 20 seconds, 58°C for 40 seconds, 72°C for 1 minute, and a final extension at 72°C for 5 minutes using a C1000 Touch Thermal Cycler Kit (Bio-Rad, Hercules, CA, USA). Bidirectional sequencing was performed using a BigDye Terminator v.3.1 Kit (Applied Biosystems, Foster City, CA, USA) on an ABI 3130xL Genetic Analyzer. The sample was considered mutation-positive if mutations were detected in both the forward and reverse DNA strands.
Statistical analysis
Continuous variables are presented as mean±standard deviation (SD), and categorical variables are presented as numbers and percentages. Student’s t test was performed for the comparison of continuous variables, and the chi-square test or Fisher’s exact test was performed for the comparison of categorical variables as appropriate. P values were two-sided, and P values less than 0.05 were considered statistically significant. Linear by linear association was used to analyze P for trend, age, and primary tumor size. Univariate logistic regression was used to evaluate the association between TERT promoter mutations and clinicopathological variables. Statistical analysis was performed using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
The detailed clinicopathological data of 2,092 patients with thyroid carcinoma are presented in Table 1. The mean±SD age was 45.6±13.0 years, and the group included 1,539 (73.6%) females. The mean±SD tumor size was 1.33±1.14 cm. Most patients (96.6%) had papillary thyroid carcinoma (PTC), 38 (1.8%) patients had follicular thyroid carcinoma (FTC), 14 (0.7%) patients had Hurthle cell thyroid carcinoma (HTC), 13 (0.6%) patients had poorly differentiated thyroid carcinoma (PDTC), and seven (0.3%) patients had anaplastic thyroid carcinoma (ATC). According to the 8th edition of the AJCC staging system, 1,671 (79.9%) patients had T1 stage, 204 (9.8%) had T2 stage, 175 (8.4%) had T3 stage, and 42 (2.0%) had T4 stage. The proportion of N0 was 771 (36.9%), N1a was 669 (32.0%), and N1b was 292 (14.0%). Distant metastasis at the time of diagnosis was found in 32 (1.5%) patients. Out of 72 patients with TERT promoter mutations, 64 had a TERT promoter mutation at hotspot C228T (chr5: 1,295,228C>T), seven patients had the C250T (chr5: 1,295,250C>T) mutation, and one patient had the C228A (chr5: 1,295,228C>A) mutation.
Comparison of clinicopathological characteristics of patients with TERT promoter mutations to patients with wild-type
The clinicopathological comparison of patients with TERT promoter mutations to those with wild-type are shown in Table 2. The mean±SD age of the 72 patients with TERT promoter mutations was 62.5±12.9 years. The frequency of TERT promoter mutations increased significantly with age (odds ratio [OR], 1.13; P<0.001), and it was significantly higher in males compared with females (OR, 2.22; P=0.003). This significance was consistent when patients were classified according to the age at diagnosis. TERT promoter mutations were not found in patients under the age of 19, but it was detected in 0.5% of patients 20 to 29 years old, 0.6% of patients 30 to 39 years old, 1.2% of patients 40 to 49 years old, 3.3% of patients 50 to 59 years old, 9.2% of patients 60 to 69 years old, 25.4% of patients 70 to 79 years old, and 62.5% of patients 80 years and older (P for trend <0.001) (Fig. 1). In particular, the proportion of TERT promoter mutations reached 0.9% in female patients aged under 49 years.

Association of TERT Promoter Mutation Status with Clinicopathological Variables in 2,092 Thyroid Carcinoma Patients

The frequency of telomerase reverse transcriptase (TERT) promoter mutations according to age in 2,092 thyroid carcinoma patients.
The primary tumor size in the patients with TERT promoter mutations was significantly larger than in those with wild-type (OR, 2.02; P<0.001). This significance was consistent when patients were classified according to the primary tumor size. The frequency of TERT promoter mutations increased significantly as the tumor size increased (P for trend <0.001) (Fig. 2). TERT promoter mutations were found in 0.5% of tumors less than 1 cm in size, 3.5% of tumors 1.1 to 2.0 cm, 8.0% of tumors 2.1 to 3.0 cm, 12.0% of tumors 3.1 to 4.0 cm, 18.4% of tumors 4.1 to 5.0 cm, and 31.1% of tumors more than 5.1 cm. The increase in mutations as the tumor size increased was more prominent in men than in women.
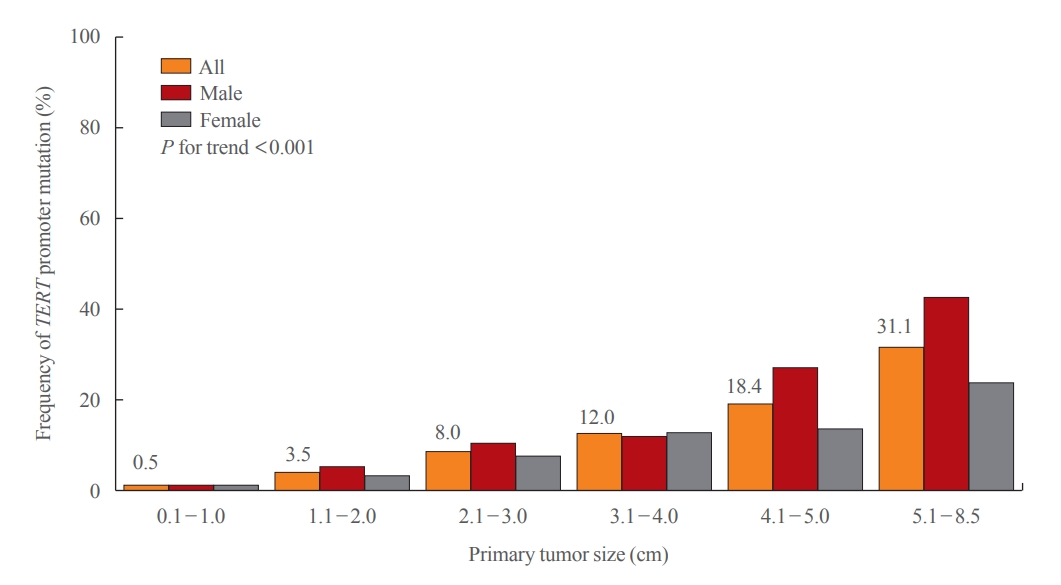
The frequency of telomerase reverse transcriptase (TERT) promoter mutations according to tumor size in 2,092 thyroid carcinoma patients.
The frequency of TERT promoter mutations significantly differed among the histological type. FTC, PDTC, and ATC had significantly higher TERT promoter mutations than PTC ([OR, 7.78; P<0.001], [OR, 10.33; P<0.001], and [OR, 45.92; P<0.001], respectively). In PTC, TERT promoter mutations were detected in 2.5% of classic PTC, 7.6% of tall cell variant PTC or classic with tall cell feature PTC, and 33.3% of solid variant PTC. TERT promoter mutations were not detected in cribriform-morular variant PTC, diffuse sclerosing variant PTC, Hobnail variant PTC, oncocytic variant PTC, and Warthin-like variant PTC (Supplemental Table S1). In FTC, TERT promoter mutations were detected in 9.7% of minimally invasive FTC, 40% of encapsulated angioinvasive FTC, and 100% of widely invasive FTC (Supplemental Table S2).
Advanced T stage and N stage were also significantly associated with TERT promoter mutations. The frequency of distant metastasis at the time of diagnosis was significantly higher in patients with TERT promoter mutations. The significance of the association between the TERT promoter mutational status and conventional clinicopathological variables were consistent when analyzed only in PTC patients (Supplemental Table S3, Supplemental Figs. S1, S2).
Association of TERT promoter mutation status according to primary tumor size and age at diagnosis in PTC
The patients with PTC, the largest subgroup, were categorized into two groups based on primary tumor size. The frequency of TERT promoter mutations in PTMC was 0.52%, and 5.8% in PTC >1 cm (Table 3). In the PTMC group, age (categorical), sex, and presence of initial distant metastasis were not significantly different. In six patients with TERT promoter mutations, one patient had recurrent laryngeal nerve invasion (T4) and three patients had lymph node metastasis; one patient was N1a, and two patients were N1b. Hence, the clinicopathological characteristics of PTC patients were similar in the entire cohort.

Comparison of Clinicopathological Characteristics according to Primary Tumor Size and TERT Promoter Mutation Status in 2,020 PTC Patients
We also compared clinicopathological characteristics according to age at diagnosis in PTC (Table 4). The frequency of TERT promoter mutations in age <55 years was 0.80%, and 9.8% in age ≥55 years. Age at diagnosis was significantly older in patients with TERT promoter mutations in both groups.
Comparison of clinicopathological characteristics between C228T and C250T mutations
Out of 72 patients with TERT promoter mutation, 64 patients (88.9%) had TERT promoter mutation at hotspot C228T (chr5: 1,295,228C>T), seven patients (9.7%) had the C250T (chr5: 1,295,250C>T) mutation, and one patient (1.4%) had the C228A (chr5: 1,295,228C>A) mutation. Clinicopathological characteristics were not significantly different between C228T and C250T mutations (Table 5).
DISCUSSION
This study reviewed the prospectively collected data from 2,092 consecutive patients with thyroid carcinoma for the last two years in a real-world setting. Thus, we demonstrated the real-world situation of TERT promoter mutations in a large number of patients. The frequency of TERT promoter mutations was 3.4% in all types of thyroid carcinoma and 2.8% in PTC. In addition, the frequency of TERT promoter mutations was only 0.5% in PTMC but it was 5.8% in PTC with primary tumor size larger than 1 cm. Overall, TERT promoter mutational status was significantly associated with old age, male sex, large tumor size, advanced histological type, advanced T and N stage, and presence of distant metastasis.
In Korea, the incidence of thyroid carcinoma abruptly increased after the introduction of high-resolution ultrasonography and peaked in 2012, and then decreased [18]. Since the introduction of the Revised Korean Thyroid Association management guidelines, most thyroid tumors of 1 cm or less do not undergo cytologic examination, and active surveillance is selected as an optional method of treatment [19]. However, if the tumor grows or progresses during observation, or if the patient strongly desires surgery, surgery is performed [20-22]. In Korea, approximately half of thyroid carcinoma patients undergo surgery for small tumors of less than 1 cm in diameter despite the effort to avoid overtreatment. As a consequence, more than half of the subjects in this study are diagnosed with PTMC (n=1,143). Therefore, we could provide real-world data for PTMC.
In a meta-analysis, TERT promoter mutations were observed in 10% (4.5% to 25.5%) of patients with PTC, 17% (13.8% to 36.4%) of those with FTC, 40% (21.4% to 51.7%) of those with PDTC, and 40% (12.6% to 60.0%) of those with ATC [7-10,13]. de Biase et al. [23] reported the frequency of PTMC as 4.7%. Since, previous studies reported TERT promoter mutations in PTC were associated with clinicopathological features such as old age, and larger tumor size [10,11,23-26], we compare patients’ baseline characteristics between this study and previous studies (Supplemental Table S4). The mean age at diagnosis of wild-type TERT patients was similar to previous reports, and it was similar to or older than previous reports in patients with TERT promoter mutations. Also, the proportion of female patients in each group was similar.
Despite the baseline characteristics being similar to previous reports, we found that the frequency of TERT promoter mutations is much lower in real-world data. The frequency is still lower than previous reports when analyzed in PTC with primary tumor size >1 cm. In addition, initial distant metastasis was not presented in any of the six patients with TERT promoter mutations in PTMC. The clinical significance of TERT promoter mutations might be lower when the primary tumor size is smaller, and this result is consistent with a previous report [23]. In PTMC, active surveillance is one of the treatment options [27]. Previous studies have tried to find biological markers to predict PTMC growth [28,29]. TERT promoter mutations are associated with poor prognosis; thus, Yabuta et al. [29] assessed 26 of 1,252 patients with molecular analysis during active surveillance. However, none of them had TERT promoter mutations. This result is understandable considering the low frequency of TERT promoter mutations in real-world data. The clinical significance of TERT promoter mutations might be lower when the primary tumor size is smaller, and this result is consistent with a previous report [23].
TERT promoter mutations are rarely found in childhood thyroid carcinoma, whereas they are detected more commonly in the elderly [7,9,30-32]. During proliferation in the cells of older individuals, telomere dysfunction or further telomere crisis occurs because of shorter telomeres and deficient telomerase. This result triggers genomic instability and induces the development of TERT promoter mutations [26]. In the present study, the frequency of TERT promoter mutations increased significantly with age (OR, 1.13; P<0.001). Furthermore, the significance was present consistently in categorical analysis (P for trend <0.001). This result was more predominant in women. No female patient aged younger than 29 years had TERT promoter mutations, 0.3% of the females aged between 30 and 39 years had mutations, and 0.9% in those aged between 40 and 49 years, whereas the frequency of TERT promoter mutations was up to 3.0% in male patients aged under 49 years. Although some studies have provided different results, the majority of previous studies have reported that TERT promoter mutations were found more frequently in men than in women [33]. In addition, the frequency of TERT promoter mutations increased significantly as the tumor size increased (OR, 2.02; P<0.001). In patients categorized according to primary tumor size, ORs were significantly increased as the primary tumor size increased. This result is consistent with previous reports showing that the frequency of TERT promoter mutations was higher in large tumors [7,9].
Two mutations in the TERT promoter (chr5: 1295228C>T, termed C228T, and chr5: 1295250C>T, termed C250T) were found in follicular cell-derived thyroid carcinoma [8,10,26,34-36]. C228T was reported to be far more dominant than C250T, and these two mutations occur in a mutually exclusive manner. Their mutual exclusivity suggests that each of them may play a specific role in thyroid tumorigenesis [8,9,37]. In this study, C228T mutations were found more frequently than C250T mutations, which is consistent with a previous report. One patient with tall cell variant PTC had C228A mutations. There were no significant differences in clinicopathological characteristics between C228T and C250T. However, these results need to be validated in larger studies because the number of C250T patients was too small.
TERT promoter mutations have been reported to be highly prevalent in tumors with aggressive or dedifferentiated histology [7-9]. In this study, similar results were found. The frequencies of TERT promoter mutations were 18.4% (7 of 38) in FTC, 7.1% (1 of 14) in HTC, 23.0% (3 of 13) in PDTC, and 57.1% (4 of 7) in ATC, whereas the frequency was 2.8% (57 of 2020) in PTC. In PTC, TERT promoter mutations were found more frequently in the tall cell variants than in classic or follicular variants (7.6% vs. 2.5% vs. 2.4%), which was consistent with previous reports. TERT promoter mutations have been reported to be more prevalent in the tall cell variants than classic or follicular variants [8,9,38]. On the other hand, the frequency of TERT promoter mutations in HTC was lower than that in previous reports [34,39]. Chindris et al. [39] assayed TERT promoter sequence variation in 61 of 173 patients with HTC treated at Mayo Clinic over 11 years. The authors reported that the frequency was 13.1% (widely invasive 6/48 vs. minimally invasive 2/13) [39]. Landa et al. [34] reported that the frequency in HTC was 16.0%, but TERT promoter mutations were restricted to widely invasive HTC and did not occur in minimally invasive HTC (widely invasive 4/17 vs. minimally invasive 0/8). The incidence of HTC in Korea is very low and accounts for less than 1% of all thyroid carcinomas. In this study, the incidence of HTC was only 0.7% of all thyroid carcinomas, and only 14 patients with HTC were included. Therefore, it is difficult to compare the results of this study with other results because of the large differences in subject numbers. Nevertheless, one of the 14 (7.1%) HTC patients had TERT promoter mutations, and the histological subtype was encapsulated angioinvasive type. According to a previous study, a high expression level of TERT was seen in two structural rearrangements in widely invasive FTC [40]. Thus, high TERT alteration frequency can have TERT mutations such as fusion as well as TERT promoter mutations. Since we performed targeted sequencing methods that could not detected structural rearrangement, there might be more tumors with TERT rearrangements.
Previous reports showed that the TERT promoter mutations are highly prevalent in advanced stages and distant metastasis [9-11,13,33,34,37], and the results of this study were consistent with this finding. In addition, the frequency of TERT promoter mutations was significantly higher in men than in women (OR, 2.05; P=0.003). Although some studies have obtained different results, the majority of previous studies have reported that TERT promoter mutations are found more frequently in men than in women [33].
Given the low mortality rate despite the increased incidence of thyroid carcinoma, aggressive treatment is necessary only for a small number of patients with thyroid carcinoma, and therefore it is very important to find a prognostic factor to discriminate these patients in advance. Among the many proposed prognostic factors, TERT promoter mutations are reported to be a powerful marker of poor prognosis, independent of tumor histological type [41]. However, previous studies on TERT promoter mutations were retrospectively conducted on a limited number of subjects. In the present study, we prospectively tested TERT promoter mutations in a large number of patients with thyroid carcinoma. Therefore, this study is the first attempt to investigate the real-world frequency of TERT promoter mutations in thyroid carcinoma patients. The real-world frequency of TERT promoter mutations seems lower than expected. In particular, the frequency of TERT promoter mutations in PTMC, young age, and female patients was very low. However, we could not obtain long-term outcomes because of the short follow-up duration. Thus, further studies would be needed to provide long-term outcomes.
In conclusion, the frequency of TERT promoter mutations was 3.4% in all types of thyroid carcinoma, and it was 2.8% in PTC. However, the frequency of TERT promoter mutations was only 0.5% in PTMC. The frequency of TERT promoter mutations was significantly higher in old age, large tumors, and aggressive histology. There were no clinicopathological differences between C228T and C250T. This study provides basic data for further investigations on TERT promoter mutations.
Acknowledgements
This research has been presented as poster presentation at the 2022 Korean Endocrine Society Annual Congress, which was held in Seoul, Korea 7th to 9th April, 2022. The poster was awarded a prize during the congress.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION
Conception or design: J.H.C.
Acquisition, analysis, or interpretation of data: H.Y., H.P., H.J.R., J.H., J.S.K., Y.L.O., J.H.C., J.H.K., J.S.K., H.W.J., T.H.K., S.W.K.
Drafting the work or revising: H.Y., H.P.
Final approval of the manuscript: J.H.C.
Supplementary Information
Histological Subtypes of 2,020 Papillary Thyroid Carcinoma Patients
Histological Subtypes of Follicular Thyroid Carcinoma Patients, and Hurthle cell Thyroid Carcinoma Patients
Association of TERT Promoter Mutations Status with Clinicopathological Variables in 2,020 Papillary Thyroid Carcinoma Patients
Summary of Clinicopathological Characteristics of Papillary Thyroid Carcinoma according to TERT Promoter Mutational Status in Previous Studies and This Study
The frequency of telomerase reverse transcriptase (TERT) promoter mutations according to age in 2,020 papillary thyroid carcinoma patients.
The frequency of telomerase reverse transcriptase (TERT) promoter mutations according to tumor size in 2,020 papillary thyroid carcinoma patients.



