Active Surveillance for Low-Risk Papillary Thyroid Carcinoma as an Acceptable Management Option with Additional Benefits: A Comprehensive Systematic Review
Article information
Abstract
Background
Active surveillance (AS) has been introduced as a management strategy for low-risk papillary thyroid carcinoma (PTC) due to its typically indolent nature. Despite this, the widespread adoption of AS has encountered several challenges. The aim of this systematic review was to evaluate the safety of AS related to disease progression and its benefits compared with immediate surgery (IS).
Methods
Studies related to AS in patients with low-risk PTC were searched through the Ovid MEDLINE, Embase, Cochrane Library, and KoreaMed databases. Studies on disease progression, surgical complication, quality of life (QoL), and cost-effectiveness were separately analyzed and narratively synthesized.
Results
In the evaluation of disease progression, the proportions of cases with tumor growth ≥3 mm and a volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Newly detected lymph node metastasis was identified in 0.0%–1.4% of patients. No significant difference was found between IS and delayed surgery in surgical complications, including vocal cord paralysis and postoperative hypoparathyroidism. AS was associated with better QoL than IS. Studies on the cost-effectiveness of AS reported inconsistent data, but AS was more cost-effective when quality-adjusted life years were considered.
Conclusion
AS is an acceptable management option for patients with low-risk PTC based on the low rate of disease progression and the absence of an increased mortality risk. AS has additional benefits, including improved QoL and greater QoL-based cost-effectiveness.
INTRODUCTION
Recent clinical practice guidelines for thyroid cancer have been de-escalated in response to concerns about the overdiagnosis and overtreatment of subclinical thyroid cancer. Although the incidence of thyroid cancer has risen sharply, mortality rates have remained stable [1]. Given the indolent nature of thyroid cancer, particularly papillary thyroid carcinoma (PTC)—the most common type—active surveillance (AS) has emerged as a new management option instead of immediate surgery (IS). This approach was first introduced at Kuma Hospital in Japan in 1993. Initially, markers to differentiate progressive PTC from stable cases were not well-defined, leading to the recommendation of serial follow-up examinations, including ultrasonography (US), for monitoring. Studies on AS for low-risk thyroid cancer have been published in multiple countries, and several prospective cohort studies are currently underway to address the limitations of singlearm studies. Recent guidelines now endorse AS as a suitable treatment option for low-risk thyroid cancer, and in Japan, AS has become an established management strategy [2]. Japanese studies have documented a shift in the proportion of patients with low-risk papillary thyroid microcarcinoma (PTMC) choosing AS, from 42% between 2003 and 2006 to 88% since 2014 at Kuma Hospital [3]. Additionally, a questionnaire survey of 134 institutes revealed that 53.8% of patients with low-risk PTMC were managed with AS [4]. In a retrospective study from 2005 to 2017 in Japan, 7.1% of patients (162 out of 2,288) underwent surgery during AS, with the 5-year cumulative conversion rate dropping from 12.3% in the first period (2005 to 2011) to 4.2% in the second (2011 to 2017) [5]. Unlike the widespread adoption of AS for low-risk prostate cancer, which is based on a similar rationale of indolent biology, AS for low-risk thyroid cancer has been less commonly adopted into actual clinical practice. This has been due to various barriers and slower acceptance in some countries, even though the feasibility of AS is increasingly supported by research and clinical guidelines.
The main challenges in selecting AS for patients with lowrisk PTC include the potential for disease progression from the time of initial diagnosis and the risk of increased surgical complications if surgery is postponed. This study aimed to determine the acceptability and safety of AS as a management option for patients with low-risk PTC by conducting a systematic review that incorporates recently reported long-term follow-up data. To achieve this, we examined disease progression in patients with low-risk PTC and compared surgical complications between those who underwent delayed surgery (DS) following AS and those who underwent IS. We also evaluated other critical factors in the decision-making process for AS, such as quality of life (QoL) and cost-effectiveness.
METHODS
Search strategy
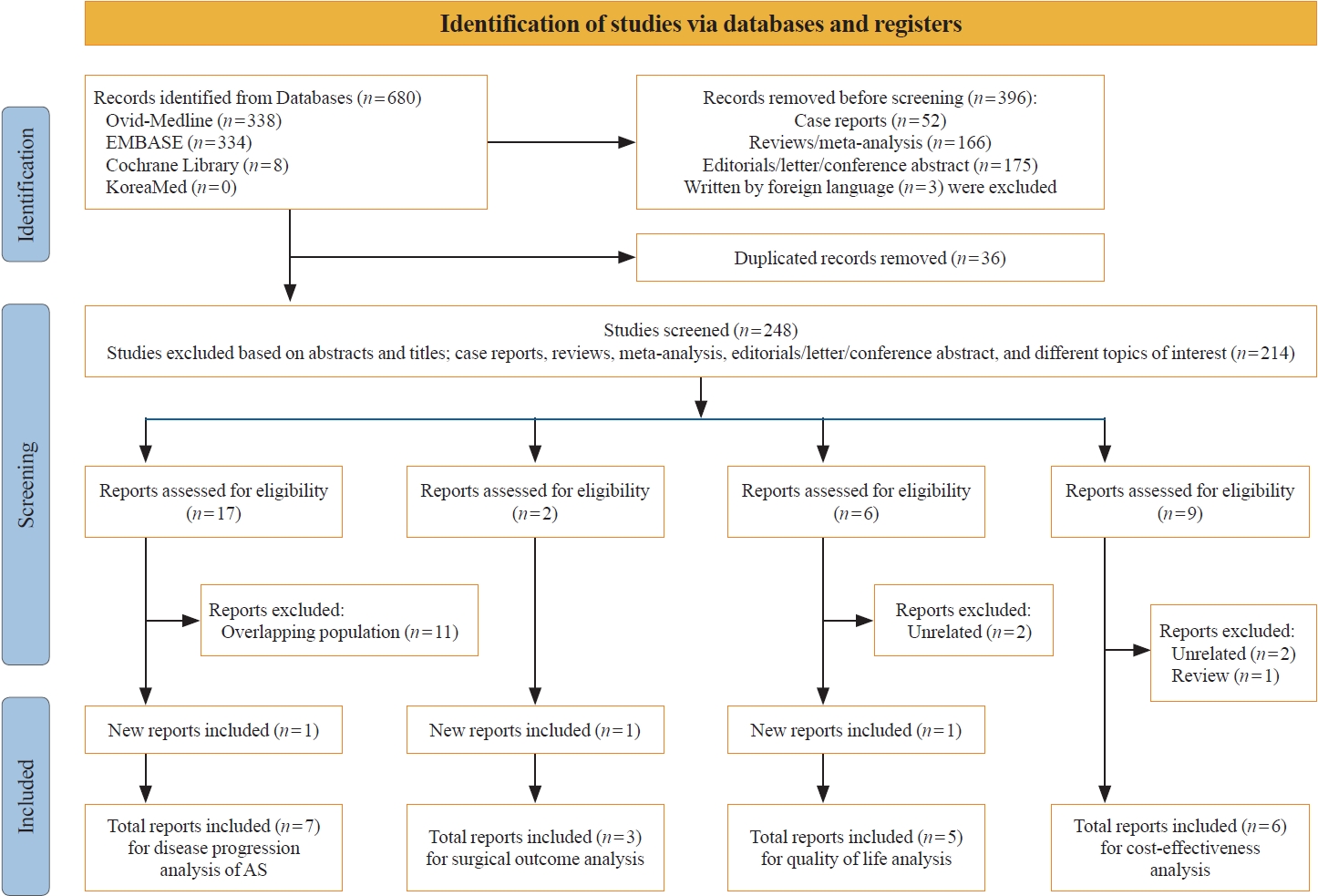
We conducted a literature search of the Cochrane Library, Elsevier Embase, KoreaMed, and Ovid MEDLINE databases up to March 2023. The search terms used were [(active surveillance OR observation)] AND [(papillary thyroid microcarcinoma) OR (thyroid cancer)]. All articles included in this review are written in English. We reported this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1) and registered it at the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022352144) [6]. Prisma checklist was presented in Appendix 1.
Study selection
First, the titles and abstracts were independently evaluated by two reviewers (J.H.Y. and H.K.K.), with articles not meeting the prespecified eligibility criteria being excluded. Any discrepancies between the reviewers were resolved through consultation with a third investigator (H.C.K.). Full-text reviews were conducted based on the following inclusion criteria: (1) studies involving adult patients with PTC; (2) studies including adults who underwent AS, defined as observation with close monitoring for patients with the potential for curative surgery; and (3) studies that reported clinical outcomes, including disease progression, surgical complications between DS and IS, QoL, and cost-effectiveness. Studies that included patients under the age of 18 or were written in a non-English language were excluded. To prevent the inclusion of duplicate participants, only one study from each cohort was selected for the analysis of a specific clinical parameter.
Data extraction and quality assessment
Data on the characteristics of each study were extracted by two independent reviewers (J.H.Y. and H.K.K.), as follows: (1) article characteristics (first author’s name, publication year, country of origin, numbers of included patients); (2) study design (prospective or retrospective); (3) patient characteristics (age and sex); (4) PTC characteristics (tumor size, extra-thyroidal invasion [ETI], lymph node [LN] metastasis, and aggressive variants of PTC); and (5) results (disease progression based on tumor enlargement or newly detected LN metastasis, surgical outcome including aggressiveness [ETI, LN metastasis, and aggressive variants] and complications [the rate of total thyroidectomy and postoperative hypoparathyroidism or vocal cord palsy], QoL, and cost-effectiveness). Quality assessment of the included studies was performed using Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) criteria (Supplemental Figs. S1-S4). Any disagreements were resolved through discussion with a third reviewer (H.C.K.).
Synthesizing the evidence
This review presents synthesized evidence in the format of a narrative review. A meta-analysis could not be performed due to limitations of the included observational studies. There were no randomized trials comparing AS and IS in patients with lowrisk PTC due to ethical problems.
Ethical approval
Ethical approval was waived as this study did not include human participants.
RESULTS
Disease progression in patients with low-risk PTC
Baseline characteristics of the included studies
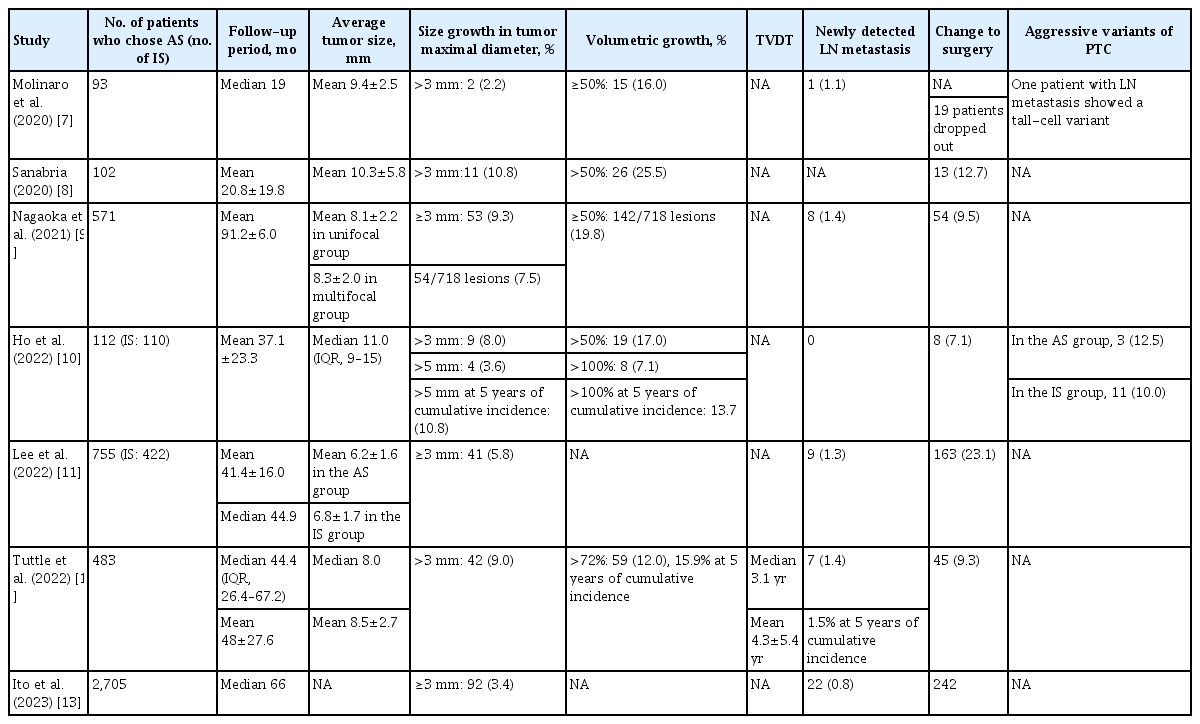
Seven studies [7-13], including only one study from the same cohort to avoid the possibility for duplicated data, were included in the analysis of clinical outcomes under AS in patients with low-risk PTC. Six prospective studies and one retrospective analysis from a prospective cohort were analyzed for disease progression. All the studies were female-dominant, and the mean age of the patients was 40 to 50 years (Supplemental Table S1).
1) Eligibility criteria for AS in patients with low-risk PTC
The inclusion criteria were adult patients with low-risk PTC diagnosed as suspicious of malignancy or malignancy (Bethesda category V or VI) based on fine-needle aspiration or suggestive of malignancy or malignancy by core needle biopsy. However, some studies did not provide detailed information. IS was recommended for patients with high-risk PTC (see Supplemental Table S2). Except for one study from Japan that included patients with low-risk T1b tumors due to patient preference, most studies performed in Japan and Korea mainly included PTMC (T1a). Nevertheless, some studies broadened their inclusion criteria to encompass tumors measuring 2 cm or less (T1b). Two studies included patients with low-risk PTMC [11,13], while five studies involved patients with low-risk PTC, with size thresholds varying by study: less than 1.3 cm in one study [7], less than 1.5 cm in two studies [8,12], and less than 2.0 cm in two studies [9,10].
2) Follow-up under AS
Serial US is required to determine whether disease progression takes place during AS. In most AS studies, comprehensive US assessments were conducted every 6 months in the first 2 years [14]. For patients who show no suspicious signs of disease progression within the first two years, follow-up US is scheduled either annually or biennially, in accordance with study protocols.
Clinical outcomes of AS in patients with low-risk PTC
Disease progression under AS is defined by tumor growth or the emergence of new LN metastasis; however, the threshold for these criteria varies across study protocols. All included studies defined disease progression as an increase in tumor size of at least 3 mm, and five studies [7-10,12] reported increases in tumor volume. Four of these studies [7-10] considered a volume increase of more than 50% compared to baseline, while one study [12] defined volume growth as an increase of more than 72% from baseline. The proportions of cases of tumor growth ≥3 mm and volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Tumor growth equal to or more than 3 mm was observed in the following proportions: 3.4%–9.3% in studies including only PTMCs with 41.4 to 91.2 months of follow-up, 9.0%–10.8% in studies including PTCs measuring ≤1.5 cm with 20.8 to 48.0 months of follow-up, and 8.0% in a study including lesions measuring ≤ 2.0 cm with 37.1 months of follow-up. Molinaro et al. [7] reported a 2.2% incidence of tumor growth of ≥3 mm in low-risk PTCs measuring ≤ 1.3 cm over a 19.0-month follow-up period. The incidence of newly detected LN metastasis ranged from 0.0% to 1.4% (Table 1).
Only one study that included T1b tumors [10] found no significant difference between the T1a and T1b groups in terms of tumor size enlargement (≥3 mm and ≥5 mm) and tumor volume increase (>50% and >100%) over a mean follow-up period of 37.1 months. LN metastasis was not newly observed in either group [10].
Surgical complications between IS and DS
Three selected studies [10,15,16] are summarized in Tables 2, 3. Ho et al. [10] compared eight patients who underwent DS, including four with disease progression, among a total of 112 patients in an AS group and 110 patients in an IS group. No cases of postoperative hypoparathyroidism or laryngeal nerve paralysis were observed in the DS group. Sasaki et al. [15] found no significant differences between the DS and IS groups in the rates of postoperative hypoparathyroidism (26.9% vs. 22.2%) and vocal cord palsy (10.7% vs. 9.5%). Only one patient experienced lateral LN recurrence after DS, whereas nine patients had neck recurrences after IS—six cases of contralateral lobe recurrence and three cases of LN recurrence (0.03% vs. 0.5%, P< 0.001) [15]. Hwang et al. [16] compared 132 patients who underwent DS with 384 patients who underwent IS out of 1,177 enrolled patients with low-risk PTMC. Although the DS group presented with larger tumor sizes, more frequent lymphatic invasion, and multifocality compared to the IS group, the extent of thyroidectomy did not differ significantly between the groups (P=0.283), with total thyroidectomy rates of 33.3% in the DS group and 28.4% in the IS group. Additionally, the rates of postoperative hypoparathyroidism and vocal cord palsy were similar between the two groups. However, a subgroup analysis showed that 39 patients who underwent DS due to disease progression (29.5%) had higher rates of LN metastasis (51.3% vs. 31.0%), tall-cell variant subtype (12.8% vs. 3.4%), and radioiodine (RAI) therapy (30.8% vs. 14.6%) than those who underwent IS.
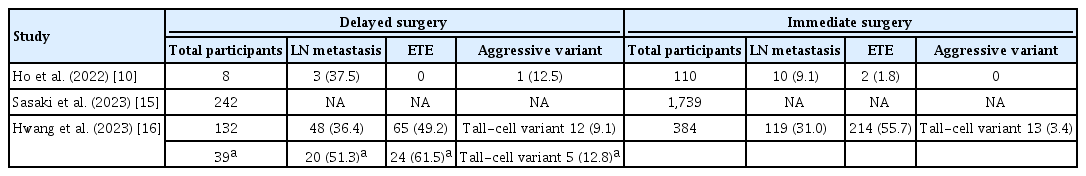
Surgical Outcomes of Delayed Surgery during Active Surveillance Compared with Immediate Surgery: The Aggressiveness of Papillary Thyroid Microcarcinoma between Delayed Surgery and Immediate Surgery
QoL between AS and IS
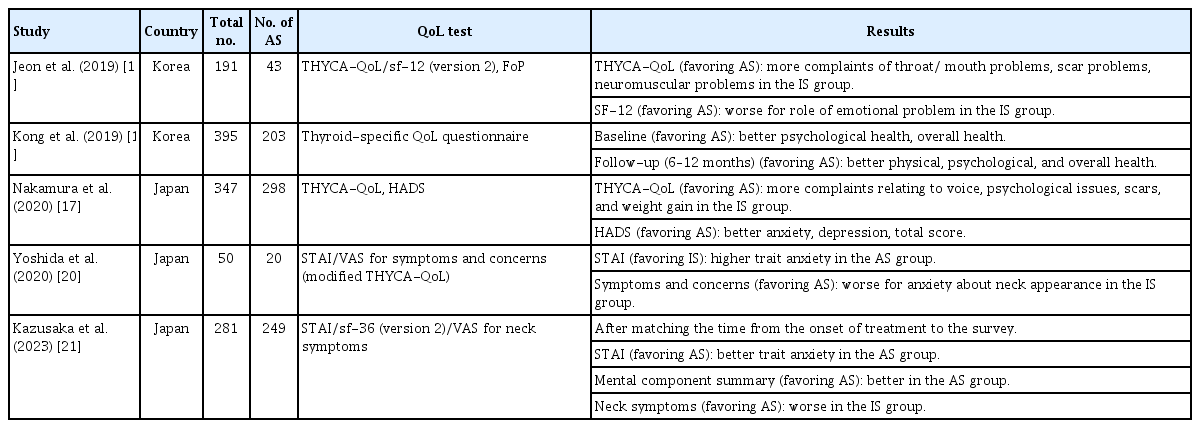
Five selected studies [17-21] that evaluate QoL are summarized in Table 4. A cross-sectional study in Korea using the 12-item Short-Form Health Survey questionnaire reported more frequent role limitations due to emotional problems in the IS group than in the AS group. Furthermore, the IS group had higher scores on the Thyroid Cancer-specific Quality of Life questionnaire (THYCA-QoL) for surgery-related problems, including neuromuscular problems, throat/mouth problems, and scar problems, but there was no statistically significant difference in fear of progression between the two groups [18]. A Korean prospective longitudinal study conducted in Korea compared QoL between the AS and IS groups at baseline and follow-up (mean 8.2±4.6 months after baseline), and the AS group showed better overall and psychological health than the IS group at both time points [19]. However, there were no significant differences between these groups, at either baseline or follow-up, in fear of recurrence/metastasis or the subjective impression of financial burden [19]. In a cross-sectional study from Japan, the IS group reported more issues with voice, psychological distress, scarring, and weight gain than the AS group, as indicated by the THYCA-QoL. The AS group also exhibited lower levels of anxiety and depression than the IS group on the Hospital Anxiety and Depression Scale (HADS) [17]. One Japanese study found higher trait anxiety in the AS group compared to the IS group [20], while another study showed better trait anxiety in the AS group compared with the IS group after matching for the time elapsed since the onset of treatment to the time of the survey [21].
Cost-effectiveness between AS and IS
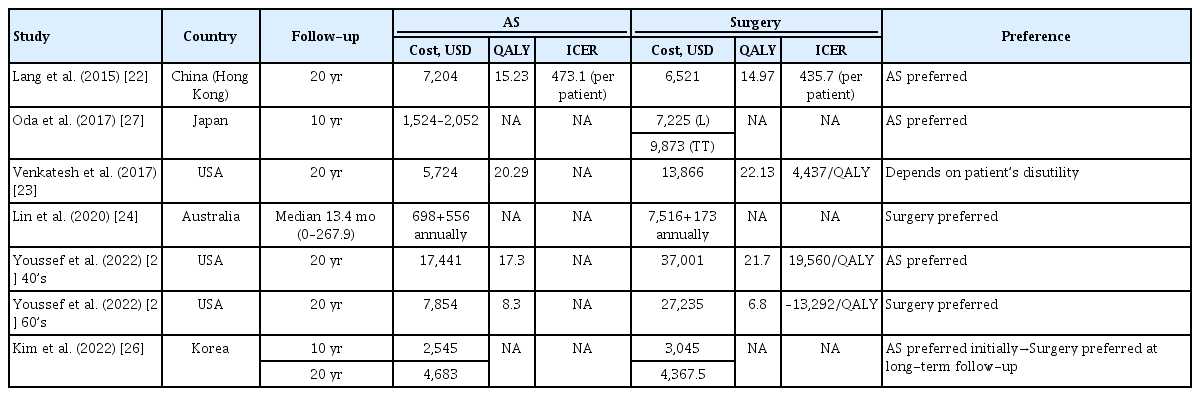
Six selected studies [22-27], including two that provided separate data by age from the same study, are summarized in Table 5. Three of these studies utilized the concept of quality-adjusted life year (QALY), which combines the quantity (i.e., length) of life and QoL [22,23,25]. The QALY calculation involves multiplying the change in utility value resulting from treatment by the effective duration of the treatment. Thus, 1 QALY is equivalent to 1 year of life in perfect health, adjusted by a utility score [28,29]. Venkatesh et al. [23] applied the utility score for prostate cancer, as it is the only cancer for which AS is widely accepted as a management option due to the absence of utility scoring data for thyroid cancer. However, Youssef et al. [25] recently employed a thyroid cancer utility model based on an extensive literature review. Lin et al. [24] found that IS was more cost-effective than AS in patients with PTMC, without evaluating QoL. Youssef et al. [25] demonstrated that total costs were consistently higher for lobectomy compared to AS. However, when QoL was factored in using QALYs, AS proved to be more cost-effective than lobectomy (21.7 vs. 17.3 gained QALYs), aligning with the results of a prior Chinese study (15.23 vs. 14.97 gained QALYs) [22]. In a subgroup analysis by age, lobectomy was more cost-effective than AS for patients aged between 40 and 69 years, but this trend reversed in patients aged 69 years or older [25]. Lang and Wong [22] reported that AS was cost-saving compared to surgery up to 16 years post-diagnosis and remained cost-effective when considering QoL after 17 years. A prospective cohort study in Korea presented similar findings, indicating that cumulative costs between the AS and IS groups were not significantly different at the 10-year follow-up. The cumulative cost curves for AS intersected with those for lobectomy at 22 years without a discount rate and at 17 years with a 3% discount rate [26].
DISCUSSION
Eligibility criteria and follow-up for AS
AS is appropriate for low-risk PTC, while IS should be considered for PTC with high-risk features, including invasion, metastasis, biological aggressiveness, and a risky location. The majority of the studies from other countries included in this review generally shared similar eligibility criteria for low-risk PTC patients. However, the criteria for tumor size varied and there have been only a few studies that included T1b tumors with relatively short follow-up durations [30].
Anderson et al. [31] reported higher rates of lymphovascular invasion (10.2% vs. 3.3%), LN metastasis (35.8% vs. 23.8%), and distant metastasis (0.4% vs. 0.3%) in 51,801 patients with T1b tumors than in 98,111 patients with T1a tumors, among a total of 149,912 patients with differentiated thyroid cancer using data from the National Cancer Data Base (NCDB; 1998–2012) and Surveillance, Epidemiology, and End Results (SEER) program (2004–2012). Therefore, many AS studies included only PTMC (T1a); however, the favorable outcomes of these studies have led to an expansion of the tumor size eligibility for AS to include tumors up to 2 cm (T1b). To date, only two studies have compared clinical outcomes between PTMC (T1a) and T1b PTC. Sakai et al. [32] found no differences in disease progression between the T1a and T1b groups, although low-risk T1b PTC was included only when patients opted for AS after initial surgery was first recommended. Ho et al. [10] also reported no difference in disease progression between T1a and T1b tumors in a prospective AS cohort study that included 59.8% of patients with T1b tumors. However, this review indicates that studies focusing solely on PTMC reported less tumor enlargement than those that included larger tumors, with sizes ranging from 1.5 to 2.0 cm (3.4%–9.3% vs. 8.0%–10.8%). Consequently, the decision to broaden the inclusion criteria for AS beyond PTMC and the criteria used to define tumor growth warrants careful consideration in light of forthcoming large-scale and long-term follow-up data analyses.
Active surveillance (AS) is an approach for managing low-risk cancer to avoid unnecessary surgery; thus, regular US monitoring is necessary to assess the potential for disease progression. Routine chest CT is not recommended, but it could be considered when disease progression is suspected. Patients with low-risk PTC live with the cancer, and currently, there is no consensus on when to conclude sonographic follow-up.
Disease progression and mortality associated with AS
Long-term follow-up results of AS cohort study have recently been reported and this review selected the data with the longest follow-up duration from follow-up studies that evaluated disease progression in the same AS cohort. In Korea, two large prospective multicenter studies are ongoing, and these will be helpful for clarifying the indications for AS in Korean PTMC patients, especially from an ethnic point of view [33,34].
Disease progression during AS is characterized by two main factors: tumor enlargement and the emergence of new LN metastasis. A study conducted in Korea found that a maximal diameter increase of ≥3 mm occurred in 4.4% of cases, while a volume increase of ≥50% was noted in 21.6% of the same cohort [35]. Tumor volume estimations can be inflated when using multiplication equations, which is why increases in maximal diameter are more commonly used as a measure of tumor growth than volumetric increases. Some recent studies have adopted a definition of disease progression that includes either a tumor enlargement ≥5 mm or a volume increase >100%, thereby prolonging AS [10].
The emergence of new LN metastases can increase the extent of surgery required and the likelihood of needing RAI therapy. A Korean retrospective multicenter study reported a 4.5% rate of newly detected LN metastasis during active surveillance in patients with low-risk PTMC [36]. In contrast, prospective studies included in this review indicated a lower LN metastasis rate ranging from 0.0% to 1.4%, suggesting the safety of active surveillance. The simultaneous occurrence of tumor growth and LN metastasis is rare, and LN metastasis has been observed even in patients whose tumor volume has decreased [5]. The pathophysiological mechanisms underlying tumor growth and LN metastasis remain unclear, as they may follow different pathways in disease progression. Therefore, meticulous US is necessary not only for evaluating tumor size but also for detecting LN metastasis before and during AS.
PTMC is indolent, and distant metastases are observed extremely rarely [37]. Mortality due to disease progression during AS has not been reported; however, limitations exist due to the lack of long-term follow-up data, which is particularly relevant considering the indolent natural history of low-risk PTC, especially PTMC, which has an approximately 100% cancer-specific survival rate [38].
Surgical outcomes between AS and IS
This review found no additional surgical complications in the DS group compared to the IS group; however, the extent of thyroidectomy is an important factor in predicting the risk of surgical complications. A meta-analysis of 17 studies reported fewer surgical complications in the hemithyroidectomy group than in the total thyroidectomy group among patients with PTMC, including lower rates of temporary vocal cord palsy (2.0% vs. 4.2%), temporary hypoparathyroidism (2.2% vs. 21.3%), and permanent hypoparathyroidism (0.0% vs. 1.8%) [39].
Two studies [15,16] found no significant differences in the extent of thyroidectomy between the DS and IS groups, which may be associated with the similar rates of surgical complications observed in both groups. In contrast, Ho et al. [10] reported an absence of postoperative hypoparathyroidism and laryngeal nerve paralysis in the DS group, despite a higher frequency of total thyroidectomies compared to the IS group (37.5% vs. 9.1%). It is noteworthy that only eight patients underwent DS, with three of these patients receiving total thyroidectomy due to lymph node metastasis. Meanwhile, most patients in the IS group underwent hemithyroidectomy, adhering to the newly established guidelines [40].
QoL between AS and IS
AS for low-risk PTC was introduced to prevent surgical complications and to improve QoL; thus, QoL is a crucial consideration when choosing between AS and IS. Although the studies were small and utilized various scales, the AS group generally reported better QoL scores than the IS group. This suggests that AS could be a reasonable management option for low-risk PTC in terms of general QoL. AS was associated with improved QoL in terms of physical issues, such as voice changes and neck scarring, compared to the IS group. However, there was a discrepancy in psychological problems, particularly anxiety. Individual differences in premorbid anxiety may influence the preference for surgery due to fears related to cancer. In a Korean longitudinal study examining 2-year QoL outcomes, patients who underwent DS due to disease progression reported better QoL scores than those who underwent DS without disease progression [41]. This finding indicates that high levels of anxiety and persistent worry may remain even after surgery if there is no disease progression. The patient’s anxiety phenotype could persist over time; therefore, individual personality traits should be carefully considered when deciding to pursue AS. Furthermore, state anxiety may decrease as the duration of observation from the start of treatment increases [20], indicating that the time elapsed since the initial diagnosis of low-risk PTC is an important factor for QoL.
Cost-effectiveness between AS and IS
The rapid global increase in the incidence of thyroid cancer has been accompanied by a marked incremental economic burden due to the management of thyroid cancer. However, the cost-effectiveness of AS in patients with thyroid cancer has not been well-established. Universalizing cost-effectiveness is challenging due to the varying medical costs and insurance systems across different countries. Nevertheless, discrepancies in utility scores and statistical modeling—such as Markov and decision tree models—could be addressed by developing a standardized model tailored for thyroid cancer. This review presents conflicting data regarding cost-effectiveness; however, when QoL is considered, AS appears to be the preferred option.
Cost-effectiveness analysis is inherently presumptive and thus has limitations in accurately estimating a range of real clinical situations. Moreover, the interpretation of data is heavily dependent on specific medical cost systems. Currently, there is no established guideline for determining the appropriate endpoint for follow-up during AS, as patients continue to live with cancer. Consequently, the frequency of repeated US scans and the number of significant hospital visits are often correlated with life expectancy. As a result, the age at which thyroid cancer is diagnosed might be one of the main decision points for AS.
In conclusion, AS is a reasonable management strategy for carefully selected patients with low-risk PTCs. Previous studies on AS have confirmed the indolent nature of these tumors and the safety of this watchful waiting approach for low-risk PTC. Additionally, some research has shown no significant difference in surgical complications between DS following AS and IS. The potential for improved QoL and the cost-effectiveness of AS when considering QoL may further justify the selection of AS as a treatment option. Tailored decision-making, which includes proper patient selection and rigorous, compliant follow-up, enhances the effectiveness of AS. The availability of extensive, long-term follow-up data from ongoing prospective studies in various countries will likely support the broader implementation of AS for patients with low-risk PTC.
Supplementary Material
Supplemental Table S1.
Baseline Characteristics of the Included Studies
Supplemental Table S2.
High-Risk Features in Patients with Papillary Thyroid Cancer
Supplemental Fig. S1.
Quality assessment of the included studies for disease progression based on the Risk of Bias for Non-Randomized Studies (RoBANS) tool. (A) Risk of bias graph, (B) risk of bias summary.
Supplemental Fig. S2.
Quality assessment of the included studies for surgical complications based on the Risk of Bias for Non-Randomized Studies (RoBANS) tool. (A) Risk of bias graph, (B) risk of bias summary.
Supplemental Fig. S3.
Quality assessment of the included studies for quality of life based on the Risk of Bias for Non-Randomized Studies (RoBANS) tool. (A) Risk of bias graph, (B) risk of bias summary.
Supplemental Fig. S4.
Quality assessment of the included studies for cost-effectiveness based on the Risk of Bias for Non-Randomized Studies (RoBANS) tool. (A) Risk of bias graph, (B) Risk of bias summary.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: A.R.H., H.K.K., H.C.K. Acquisition, analysis, or interpretation of data: J.H.Y., W.C., J.Y.P., H.K.K. Drafting the work or revising: J.H.Y., H.K.K. Final approval of the manuscript: H.K.K.
Acknowledgements
This study was supported by the Korean Thyroid Association and research funding from the National Cancer Center (grant number 2112570-3).
We acknowledge and thank Miyoung Choi (National Evidence based Healthcare Collaborating Agency, Division of Health Technology Assessment Research) and Chang Hee Cho (Korean Society of Radiology), who contributed to searching and interpreting evidence.
References
Appendix
PRISMA 2020 Checklist
enm-2023-1794-Appendix-1.pdf