Risk of Subsequent Primary Cancers in Thyroid Cancer Survivors according to the Dose of Levothyroxine: A Nationwide Cohort Study
Article information
Abstract
Background
Current research has not investigated the effect of thyroid-stimulating hormone suppression therapy with levothyroxine on the risk for developing subsequent primary cancers (SPCs). This study aimed to investigate the association between levothyroxine dosage and the risk for SPCs in thyroid cancer patients.
Methods
We conducted a nationwide population-based retrospective cohort study form Korean National Health Insurance database. This cohort included 342,920 thyroid cancer patients between 2004 and 2018. Patients were divided into the non-levothyroxine and the levothyroxine groups, the latter consisting of four dosage subgroups according to quartiles. Cox proportional hazard models were performed to evaluate the risk for SPCs by adjusting for variables including cumulative doses of radioactive iodine (RAI) therapy.
Results
A total of 17,410 SPC cases were observed over a median 7.3 years of follow-up. The high-dose levothyroxine subgroups (Q3 and Q4) had a higher risk for SPC (adjusted hazard ratio [HR], 1.14 and 1.27; 95% confidence interval [CI], 1.05–1.24 and 1.17– 1.37; respectively) compared to the non-levothyroxine group. In particular, the adjusted HR of stomach (1.31), colorectal (1.60), liver and biliary tract (1.95), and pancreatic (2.48) cancers were increased in the Q4 subgroup. We consistently observed a positive association between high levothyroxine dosage per body weight and risk of SPCs, even after adjusting for various confounding variables. Moreover, similar results were identified in the stratified analyses according to thyroidectomy type and RAI therapy, as well as in a subgroup analysis of patients with good adherence.
Conclusion
High-dose levothyroxine use was associated with increased risk of SPCs among thyroid cancer patients regardless of RAI therapy.
INTRODUCTION
Thyroid hormones are key regulators of essential cellular processes, including proliferation, apoptosis, and metabolism. Some studies hypothesized that thyroid hormone deficiency may affect cancer outcomes, showing that hyperthyroidism facilitates tumor growth, while hypothyroidism induces an opposite effect [1]. Many preclinical studies have been conducted in recent decades to determine how thyroid hormones exert their tumor growth-promoting effect [2]. Following the discovery of signal pathways for thyroid hormone action in carcinogenesis, such as phosphoinositide 3-kinase (PI3K) or extracellular signal-regulated kinases (ERK) 1/2 pathway activation, the related mechanisms are now better understood [3]. Some clinical studies support an association between thyroid disorders and cancer risk [4-6]. In a large-sized, prospective study of 26,691 people, low thyroid-stimulating hormone (TSH) levels suggestive of hyperthyroid function were associated with increased cancer risk, especially for lung and prostate cancer, compared with the euthyroid group [5].
Treatment for differentiated thyroid cancer is based on thyroidectomy, and postoperative radioactive iodine (RAI) therapy and TSH suppression with supraphysiologic doses of levothyroxine, which is a synthetic thyroid hormone. Many studies have demonstrated that TSH suppression improves disease-free and disease-specific survival, especially in high-risk patients with differentiated thyroid cancer [7,8]. However, because most patients have a favorable prognosis and a long-life expectancy, there is an increasing concern regarding the long-term health consequences in thyroid cancer patients. In this context, questions have arisen about the possibility that high-dose levothyroxine may induce proliferation of subsequent primary cancers (SPCs) [9]. Patients with differentiated thyroid cancer are at greater risk of developing SPCs than the general population [10]. A meta-analysis confirmed that the risk of developing SPCs was 5% to 31% greater than expected [11]. One of the reasons suggested for this increase, though still controversial, is the potentially carcinogenic effect induced by RAI therapy [12-14]. Recently, Mei et al. [14] conducted a study based on the United States Surveillance, Epidemiology, and End Results database, showing that the risk of experiencing hematologic and breast cancers was greater in patients with thyroid cancer who had received RAI therapy compared to those without RAI therapy. While there have been several prior studies evaluating RAI and risk of SPCs in patients with thyroid cancer, no prior studies have assessed the potential independent influence of levothyroxine use on SPC risks. Therefore, we investigated the relationship between levothyroxine dosage and the risk of SPCs in thyroid cancer patients while adjusting for the effect of RAI therapy, using the Korean National Health Insurance Service (NHIS) database, which covers the entire population of Korea.
METHODS
Data source
This study was approved by the Institutional Review Board of the CHA Bundang Medical Center (no. 2020-01-039). Informed consent was waived by the board. We used the National Health Information Database (NHID) operated by the Korean NHIS, a government-affiliated agency under the Korean Ministry of Health and Welfare that administers and supervises all medical activities in Korea [15]. The NHID contains data on all Korean citizens and registered foreigners, a total of approximately 50,000,000 people, including demographics, medical service use, medications, transaction information, deductions, and claims. When a physician consults with a patient in a medical facility in Korea, the physician must assign a code based on the most appropriate diagnosis. These codes must be based on the Korean Standard Classification of Diseases, which is the same as the World Health Organization’s 10th revision of the International Classification of Diseases (ICD-10). As a result, all such records of medical services performed in the Republic of Korea will be assigned these diagnostic codes and stored in the NHID.
Study design and participant selection
We performed a retrospective cohort study using data between 2002 and 2019. To ensure the sole inclusion of patients who were newly diagnosed with thyroid cancer and had a follow-up period of at least 1 year, we excluded patients diagnosed with thyroid cancer between 2002–2003 and 2019, respectively. Therefore, this study included thyroid cancer patients (ICD-10 code C73) who underwent thyroidectomy (claim codes P4551, P4552, P4553, P4554, and P4561) from January 2004 to December 2018 (n=412,806) (Fig. 1). Patients who met the following criteria were excluded: (1) patients diagnosed with antecedent malignancies within 2 years from the date of thyroid cancer diagnosis (n=49,016); (2) patients with a history of taking levothyroxine before thyroid cancer diagnosis (n=20,115). The maximum follow-up period for participants in the final cohort was until December 2019. Finally, 342,920 patients with thyroid cancer were enrolled in this nationwide study using data from the Korean NHIS. The patients were divided into the levothyroxine group (n=320,325) and the non-levothyroxine group (n=22,595) depending on whether they had taken levothyroxine or not. The levothyroxine group consisted of four dosage subgroups according to quartiles: Q1, Q2, Q3, and Q4. To avoid immortal time bias, the index date was set as the date of the first levothyroxine prescription for the levothyroxine group and the date of thyroidectomy for the non-levothyroxine group.
Exposure (levothyroxine use) and outcome (SPCs)
The levothyroxine group included patients prescribed with levothyroxine after thyroid cancer surgery. Levothyroxine dosage was determined as the mean daily dosage of levothyroxine (µg/day). For participants with SPCs, the average daily dosage of levothyroxine was calculated up to the time of the event. SPCs were defined as cancers meeting the criteria of the same ICD-10 C-code appearing at least three times in outpatient records or at least once in inpatient records. In addition, SPCs excluded thyroid and metastatic cancers (ICD-10 codes C73 and C77–C80) and were defined as cases occurring at least 1 year after the index date. The latency period of 1 year was set to evaluate the effect of levothyroxine on cancer risk, considering the duration required for biological processes to lead to the development and detection of cancer after treatment [12,16,17].
Statistical analyses
Statistical analyses were conducted using the SAS software version 9.4 (SAS Institute, Cary, NC, USA). Cox proportional hazard analysis was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of the incidence of SPC associated with levothyroxine treatment. HR of SPC risk according to levothyroxine dosage per body weight (µg/kg/day) was analyzed once again only in patients (n=260,567) with weight information. Stratified analyses were conducted by the type of thyroidectomy and the use of RAI therapy. Moreover, a subgroup analysis was performed on those who had good adherence, with the medication possession ratio (MPR) of 80% or higher (n=269,859) [18,19].
RESULTS
Table 1 shows participant characteristics according to the dosage of levothyroxine. The mean age at diagnosis of thyroid cancer was 47.8±12.0 years, and females comprised 80.8% of the study population. As for the type of thyroidectomy, most patients in the non-levothyroxine group underwent unilateral thyroidectomy (74.8%), whereas most patients in the levothyroxine group underwent total thyroidectomy (80.4%). No patients received RAI therapy in the non-levothyroxine group, as opposed to 43.6% of patients who did in the levothyroxine group. Especially, increased rates of total thyroidectomy and RAI therapy were observed in the high-dose levothyroxine groups.
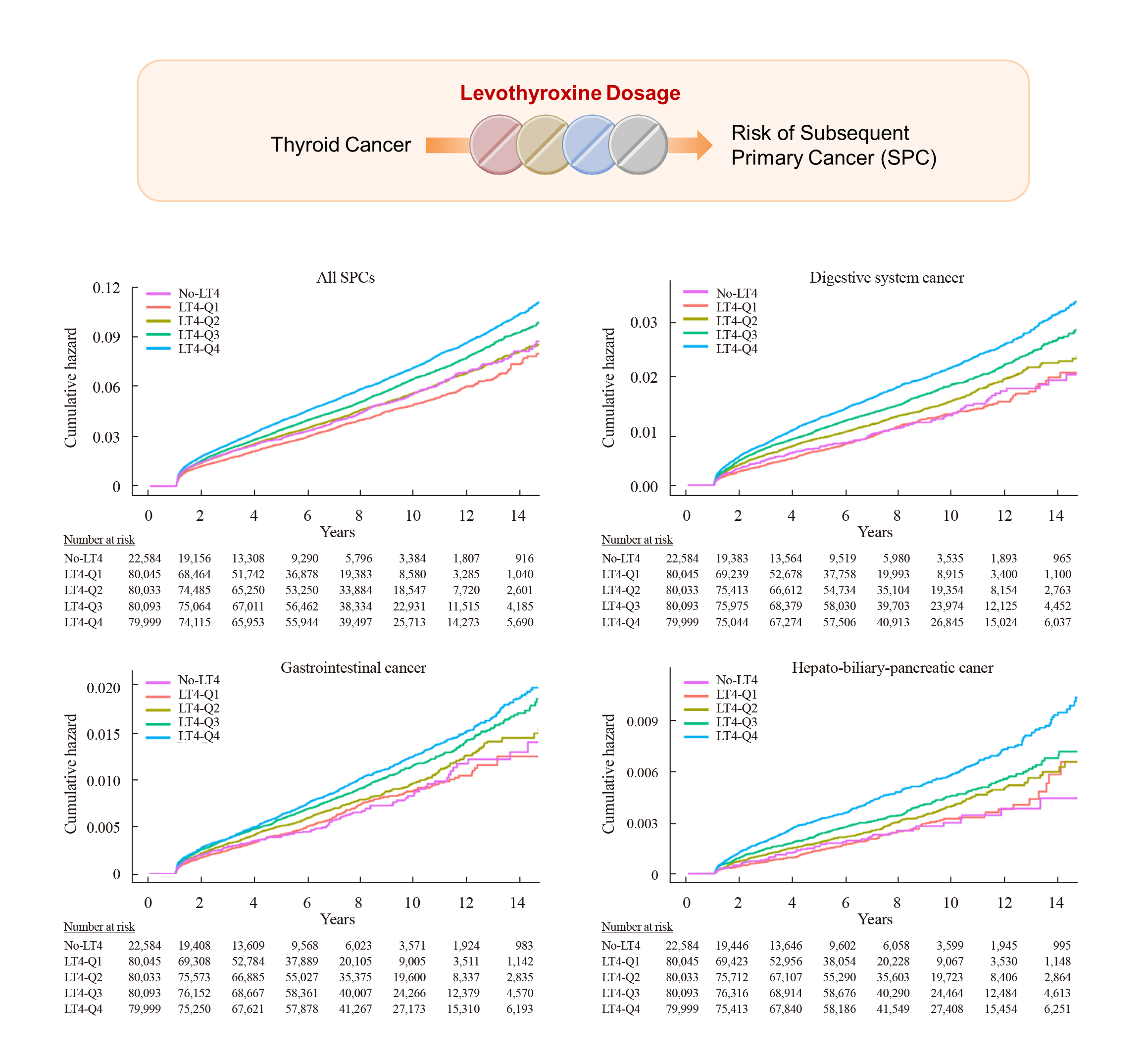
During the median follow-up of 7.3 years, 849 (6.3 per 1,000 person-years) and 16,561 (6.9 per 1,000 person-years) SPCs occurred in the non-levothyroxine and levothyroxine groups, respectively. The risk of SPCs was not different between the two groups after adjusting for age, sex, and cumulative doses of RAI therapy, except that the risks of colorectal cancer and liver and biliary tract cancer were higher in the levothyroxine group (adjusted HR, 1.35 and 1.36; 95% CI, 1.04–1.74 and 1.00–1.86, respectively) (Table 2). However, when we analyzed the levothyroxine group according to its dosage, the incidence rates per 1,000 person-years were 5.8, 6.7, 7.1, and 7.6 in the Q1, Q2, Q3, and Q4 groups, respectively, and the risk of all SPCs increased from Q1 to Q4. Compared with the non-levothyroxine group, the adjusted HRs of all SPCs were 0.91 (95% CI, 0.84 to 0.98), 1.08 (95% CI, 1.00 to 1.17), 1.14 (95% CI, 1.05 to 1.24), and 1.27 (95% CI, 1.17 to 1.37) in the Q1, Q2, Q3, and Q4 groups, respectively (Table 2, Fig. 2A). Especially, the risks of digestive system cancers showed a gradual rise as the levothyroxine dose increased, with significant elevations observed in the high-dose levothyroxine groups. Among them, the risks of stomach, colorectal, and liver and biliary tract cancers were increased in the Q3 (adjusted HR, 1.32, 1.51, and 1.64; 95% CI, 1.01–1.73, 1.14–2.01, and 1.17–2.30, respectively) and Q4 groups (adjusted HR, 1.31, 1.60, and 1.95; 95% CI, 1.01–1.71, 1.20–2.12, and 1.40–2.72, respectively), and the risk of pancreatic cancer was increased in the Q4 group (adjusted HR, 2.48; 95% CI, 1.43 to 4.31) (Fig. 2B-D).

Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer according to Levothyroxine Dosage (n=342,920)

Cumulative hazard of subsequent primary cancers (SPCs) during the follow-up period according to levothyroxine dosage. The Cox proportional hazard model adjusted for age, sex, and cumulative doses of radioactive iodine therapy was used to estimate the risks of all SPCs (A), digestive system (B), gastrointestinal (C), and hepato-biliary-pancreatic cancers (D). LT4, levothyroxine.
These findings remained consistent after further adjustments for comorbidities, as measured by the Charlson comorbidity index (Supplemental Table S1). After additional adjustments for various factors that could influence the occurrence of SPCs, such as obesity, smoking, and alcohol consumption, the levothyroxine group showed a modest increase in the risk of all SPCs compared to the non-levothyroxine group (adjusted HR, 1.12; 95% CI, 1.02 to 1.22). Moreover, this elevated risk was more evident in the high-dose levothyroxine groups (adjusted HR, 1.36 in Q3 and 1.32 in Q4) (Supplemental Table S2). Similar findings were also revealed in the subgroup analysis according to the levothyroxine dosage per body weight, even after adjusting for age, sex, cumulative doses of RAI therapy, Charlson comorbidity index, obesity, smoking, and alcohol consumption (n=260,567) (Table 3, Supplemental Fig. S1).
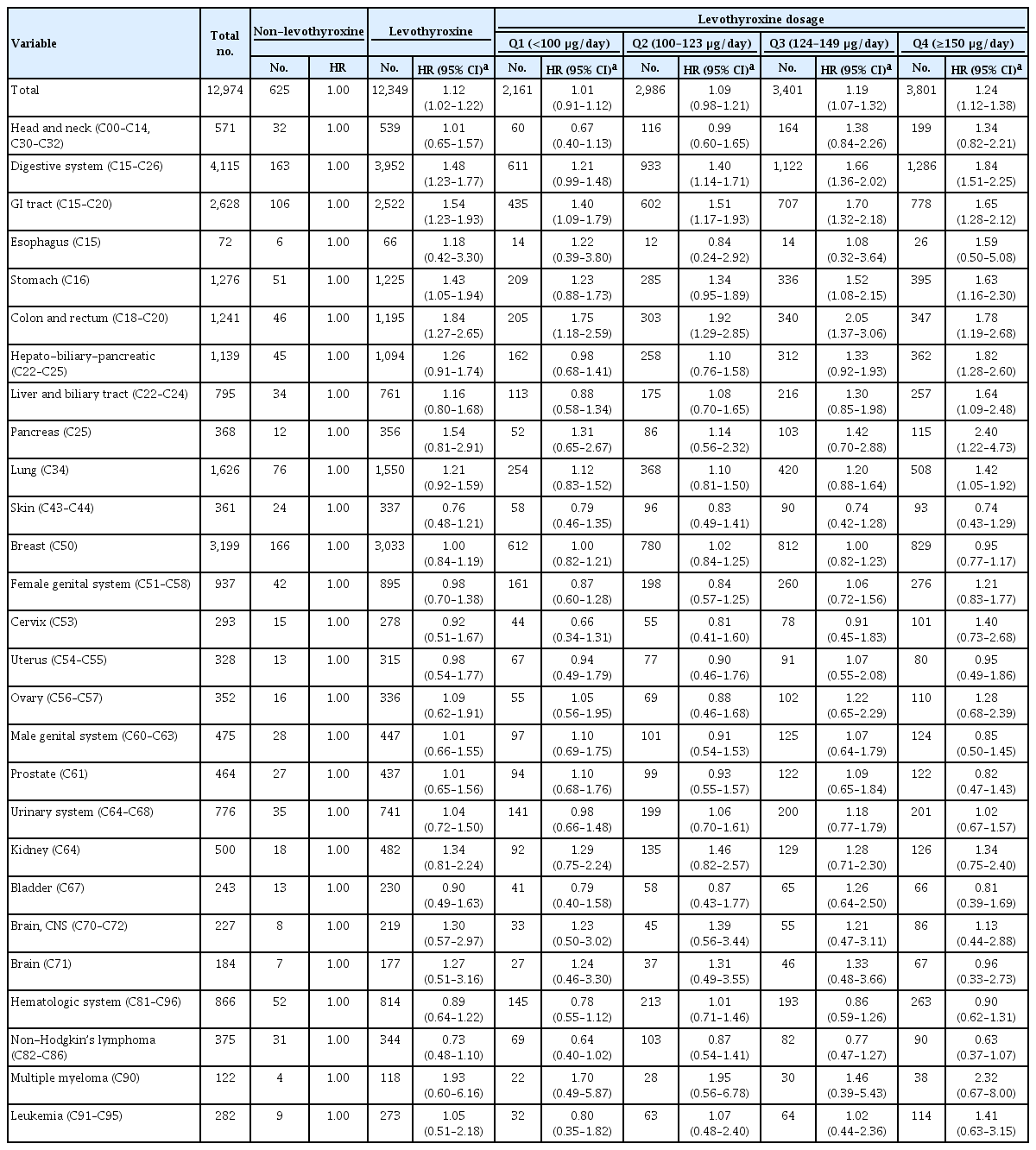
Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer according to Levothyroxine Dosage per Body Weight (n=260,567)
Next, we compared participants in the lowest quartile of levothyroxine use (Q1) with those in higher quartiles of levothyroxine use. In addition to digestive system cancer, the risks of most cancers such as head and neck, lung, breast, female genital system, brain and central nervous system, and hematologic system cancers increased in the high-dose levothyroxine groups (Supplemental Table S3). Comparisons of the high-dose levothyroxine groups to the Q1 group determined by the levothyroxine dosage per body weight also showed consistent results for increased cancer risks (Supplemental Table S4).
In addition, we conducted additional analyses stratified by the type of thyroidectomy (unilateral lobectomy [n=79,733] and completion or total thyroidectomy [n=260,701]) and using RAI therapy (non-RAI [n=203,320] and RAI [n=139,600]). Regardless of the type of thyroidectomy, the risks for all SPCs and digestive system cancer showed gradually increased risks from Q1 to Q4 and were higher in the highest dose levothyroxine group compared to the non-levothyroxine group (Supplemental Tables S5, S6). In thyroid cancer patients not receiving RAI, the risks for all SPCs and digestive system cancer were increased in the Q3 and Q4 groups (Supplemental Table S7). In thyroid cancer patients receiving RAI, there was no patient in the non-levothyroxine group. Thus, when compared to the Q1 group, the high-dose levothyroxine groups showed the elevated risks of more types of cancer such as head and neck, lung, breast, brain and central nervous system, and hematologic system cancers, in addition to digestive system cancer (Supplemental Table S8). Taken together, these findings of the stratified analyses according to the thyroidectomy type and RAI were consistent with those of the overall analysis.
To address the participants’ adherence issue, we conducted further analysis in patients who had good compliance with the MPR of 80% or higher (n=269,859) (Supplemental Table S9). Similar to the overall analysis results, the high-dose levothyroxine was associated with the increased risks of all SPCs and digestive system cancer. Additionally, the Q4 group showed the increased risk of head and neck, breast, uterine, kidney, and brain and central nervous system cancers.
DISCUSSION
In the present study, we observed that patients taking high-dose levothyroxine after thyroid cancer operation had an increased risk of developing SPC compared to patients that were never prescribed with levothyroxine, even after adjustment for the RAI therapy. Since levothyroxine dosage can be based on the patient’s body weight, it may be more accurate to analyze HR according to levothyroxine dosage per kilogram of body weight. The subgroup analysis included patients with known body weight information, who were also most patients included in this study (260,567/342,920 [75.9%]) and revealed a consistently positive association between high levothyroxine dosage per body weight and the risk of SPC. Furthermore, we found similar results in models that adjusted for various confounding variables, in the stratified analyses based on thyroidectomy type and RAI therapy, and in an analysis of a subgroup of patients with strong adherence.
TSH suppression therapy with levothyroxine is a mainstay in the management of thyroid cancer following surgery. However, insufficient evidence of benefit has been documented in lowrisk patients, and aggressive TSH suppression therapy with high-dose levothyroxine can cause side-effects. Therefore, the recent treatment guidelines for patients with differentiated thyroid cancer suggest less aggressive TSH suppression for intermediate-risk and low-risk patients than the previous treatment guidelines [20-22]. Moreover, the 2015 American Thyroid Association guidelines introduced a dynamic risk stratification, which is a restaging system based on the response to initial treatment and recommended tailoring the intensity of therapy over time for individual patients [20]. The most frequently documented side-effects of TSH suppression therapy include exacerbation of ischemic heart disease [23], increased risk of cardiac arrhythmias [24], and deterioration of osteoporosis [25]. This study showed that thyroid cancer patients taking high-dose levothyroxine were at a higher risk of developing gastrointestinal tract, and hepato-biliary-pancreatic cancers in a dose-dependent manner. Thus, TSH suppression therapy and optimal dosage of levothyroxine should be decided after weighing the potential risks and benefits.
Previous studies have shown that thyroid hormones play a significant role in the carcinogenesis process via complex mechanisms [26]. Thyroid hormones are known to influence a myriad of oncological events via genomic and non-genomic pathways, controlling transcription factors, altering angiogenesis, and promoting invasiveness, and act in a cell type-specific manner [27,28]. Moreover, the expression of thyroid hormone receptors and iodothyronine deiodinases, enzymes which are involved in the activation and deactivation of thyroid hormones, varies across organs and is also affected by thyroid hormone status [29]. However, the extent to which thyroid hormones influence cancer development in each organ has yet to be determined. This study is noteworthy in this aspect because it identified the effects of levothyroxine on cancer development across organs according to dosage.
In our study, the risk of several cancers was found to be the least in the group of patients receiving the lowest dose of levothyroxine (Q1 group) compared to the non-levothyroxine group. As a result, when compared to the Q1 group, participants in the high-dose levothyroxine groups had a higher risk of more types of cancer when compared to participants in the Q1 group. Based on the results of previous Korean cohort studies that presented TSH levels according to levothyroxine administration after thyroid lobectomy [30,31], the Q1 group may have a lower TSH level than the non-levothyroxine group. Some studies reported that TSH has biological functions in human cells other than thyroid cells [32-35], and it may especially promote angiogenesis via directly stimulating endothelial cells [34]. Thus, the lowest dose of levothyroxine therapy might have a protective effect on the development of certain types of cancers by blocking the cancer-promoting effects of TSH. However, further studies are necessary to confirm this effect.
Levothyroxine is primarily absorbed in the upper gastrointestinal tract ranging from 40% to 80%. Consequently, 20% to 60% of levothyroxine is excreted in the stool, resulting in a supra-physiological exposure of the colonic epithelium to thyroid hormone [36]. The thyroid hormone status affects the growth and homeostasis of gastrointestinal organs through binding to thyroid hormone receptors in the gastrointestinal epithelium [37]. Therefore, our results might show that thyroid cancer patients taking high-dose levothyroxine are at a higher risk of developing gastrointestinal tract cancers. However, the effect of levothyroxine on carcinogenesis remains controversial as there is little or no evidence regarding its association with this type of cancer [38-43]. In addition, this study showed that thyroid cancer patients taking high-dose levothyroxine had a higher risk of developing hepato-biliary-pancreatic cancers in a dose-dependent manner. The thyroid hormone status might influence the liver tumorigenesis, but the effects are complex. Some studies suggest that this may depend on the thyroid hormone receptor expression status, cancer stage, or other co-effectors present in the tumor microenvironment [26,44].
The main strength of this study is its novelty. To the best of our knowledge, this is the first study that evaluated the risk of SPC in thyroid cancer patients according to the dose of levothyroxine received. Another strength is the assurance of consistent results through meticulous control of potential influencing factors. This involved employing methods such as models that adjusted for multiple confounding variables and various subgroup analyses. In addition, the study benefits from the availability of comprehensive medical records for each participant without any recall bias. Patients’ medical records were extracted from the NHID; hence all recorded data were not distorted by individual and subjective memories, and no patient was overlooked during the study period. Finally, the results of our study are nationally representative findings of the entire Korean population.
However, our study had several limitations. First, we used health insurance claims data, which could not assess the actual patients’ compliance with levothyroxine. However, we applied the MPR, which is the most commonly used method for calculating adherence [18], and validated the results by conducting a subgroup analysis on patients who had good adherence with the MPR of 80 or higher [19]. Moreover, medical data are even more accurate compared to survey or subjective data which are susceptible to recall bias. Second, our claims data do not include results of thyroid function tests, including thyroid hormone or TSH levels. We considered the high-dose levothyroxine group to be on relatively aggressive TSH suppression therapy, and thyroid hormone or TSH levels may be associated with the risk of SPCs than levothyroxine itself. However, the dosage of levothyroxine can be affected by several factors, such as age, sex, body weight, and the type of thyroidectomy. Thus, we cannot exclude the possibility of bias in determining the degree of TSH suppression with levothyroxine doses. Nevertheless, we evaluated the risk of SPCs according to levothyroxine dosage per body weight and performed a stratified analysis of unilateral lobectomy and completion or total thyroidectomy. The findings of these analyses were consistent to the results of the overall analysis. Finally, as the limitations of health insurance claim data, we did not include information such as tumor stage, which could lead to residual confounding. Nevertheless, we adjusted for cumulative doses of RAI therapy, the most well-known factor influencing SPC risks in thyroid cancer patients. Furthermore, we demonstrated comparable positive associations between levothyroxine dosage and the risk of SPCs in the stratified analyses according to the thyroidectomy type and RAI therapy.
In conclusion, this nationwide population-based study identified a link between high-dose levothyroxine use and an increase in the incidence of SPC among thyroid cancer patients. Given the relationship, physicians should carefully tailor levothyroxine dosage to patients before and during TSH suppression therapy.
Supplementary Material
Supplemental Table S1.
Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer according to Levothyroxine Dosage (n=342,920)
Supplemental Table S2.
Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer according to Levothyroxine Dosage (n=342,920)
Supplemental Table S3.
Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer, Comparing the Lowest Quartile with the Higher Quartiles of Levothyroxine Use (n=342,920)
Supplemental Table S4.
Hazard Ratios of Subsequent Primary Cancer Risk in Patients with Thyroid Cancer, Comparing the Lowest Quartile with the Higher Quartiles of Levothyroxine Dosage per Body Weight (n=260,567)
Supplemental Table S5.
Hazard Ratios of Subsequent Primary Cancer Risk in Thyroid Cancer Patients Undergoing Unilateral Lobectomy according to Levothyroxine Dosage (n=79,733)
Supplemental Table S6.
Hazard Ratios of Subsequent Primary Cancer Risk in Thyroid Cancer Patients Undergoing Completion/Total Thyroidectomy according to Levothyroxine Dosage (n=260,701)
Supplemental Table S7.
Hazard Ratios of Subsequent Primary Cancer Risk in Thyroid Cancer Patients Not Receiving Radioactive Iodine according to Levothyroxine Dosage (n=203,320)
Supplemental Table S8.
Hazard Ratios of Subsequent Primary Cancer Risk in Thyroid Cancer Patients Receiving Radioactive Iodine according to Levothyroxine Dosage (n=139,600)
Supplemental Table S9.
Hazard Ratios of Subsequent Primary Cancer Risk in Thyroid Cancer Patients with Medication Possession Ratio ≥80%, according to Levothyroxine Dosage (n=269,859)
Supplemental Fig. S1.
Cumulative hazard of subsequent primary cancers (SPCs) during the follow-up period according to levothyroxine dosage per body weight in the subgroup analysis (n=260,567). The Cox proportional hazard model adjusted for age, sex, cumulative doses of radioactive iodine therapy, Charlson comorbidity index, obesity, smoking, and alcohol consumption was used to estimate the risks of all SPCs (A), head and neck (B), gastrointestinal (C), and hepato-biliary-pancreatic cancers (D). LT4, levothyroxine.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.S.K., Y.S.S. Acquisition, analysis, or interpretation of data: J.W.L., M.K.H., Y.S.S. Drafting the work or revising: M.S.K., Y.S.S. Final approval of the manuscript: M.S.K., J.W.L., M.K.H., Y.S.S.
Acknowledgements
This study was funded by grants from the Korean Thyroid Association (2020) and the National Research Foundation (NRF) of Korea (no. NRF-2020R1C1C1003924).