Prognostic Roles of Inflammatory Biomarkers in Radioiodine-Refractory Thyroid Cancer Treated with Lenvatinib
Article information
Abstract
Background
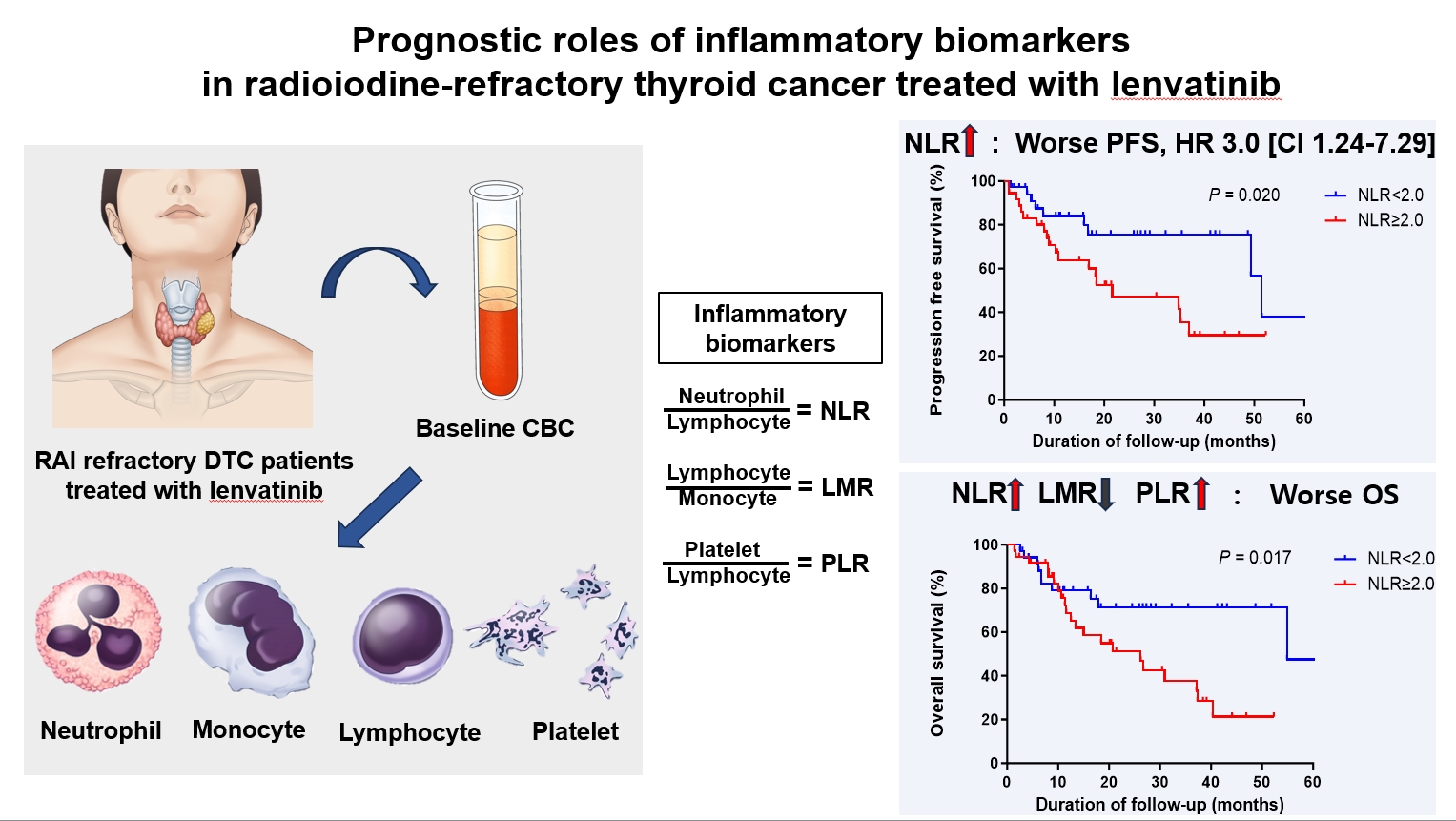
Inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR), serve as valuable prognostic indicators in various cancers. This multicenter, retrospective cohort study assessed the treatment outcomes of lenvatinib in 71 patients with radioactive iodine (RAI)-refractory thyroid cancer, considering the baseline inflammatory biomarkers.
Methods
This study retrospectively included patients from five tertiary hospitals in Korea whose complete blood counts were available before lenvatinib treatment. Progression-free survival (PFS) and overall survival (OS) were evaluated based on the median value of inflammatory biomarkers.
Results
No significant differences in baseline characteristics were observed among patients grouped according to the inflammatory biomarkers, except for older patients with a higher-than-median NLR (≥2) compared to their counterparts with a lower NLR (P= 0.01). Patients with a higher-than-median NLR had significantly shorter PFS (P=0.02) and OS (P=0.017) than those with a lower NLR. In multivariate analysis, a higher-than-median NLR was significantly associated with poor OS (hazard ratio, 3.0; 95% confidence interval, 1.24 to 7.29; P=0.015). However, neither the LMR nor the PLR was associated with PFS. A higher-than-median LMR (≥3.9) was significantly associated with prolonged OS compared to a lower LMR (P=0.036). In contrast, a higher-than-median PLR (≥142.1) was associated with shorter OS compared to a lower PLR (P=0.039).
Conclusion
Baseline inflammatory biomarkers can serve as predictive indicators of PFS and OS in patients with RAI-refractory thyroid cancer treated with lenvatinib.
INTRODUCTION
Inflammatory and immune cells in the tumor microenvironment can affect the development and suppression of malignancies [1]. The thyroid is an immune-related organ, and inflammation within its tumor microenvironment is marked by the presence of infiltrating leukocytes [2]. Among these leukocytes, tumor-infiltrating lymphocytes (TILs) interfere with carcinogenesis, and their presence is linked to improved overall survival (OS) in patients with papillary thyroid carcinoma (PTC) [3,4]. Conversely, tumor-associated macrophages (TAMs), which originate from circulating monocytes, are the most abundant leukocytes found in tumors and are implicated in promoting tumor growth [5,6]. Furthermore, neutrophils, which serve as a first-line defense mechanism of host immunity, act as pro-tumorigenic cells, and their density is correlated with tumor progression [7,8].
Assessing a prognostic biomarker that measures the severity of inflammation associated with cancer can play an important role in determining the extent of malignancy. Systemic changes in inflammatory responses can affect peripheral blood leukocytes; therefore, a complete blood count (CBC) can be used to evaluate the severity of inflammation. The peripheral lymphocyte-to-monocyte ratio (LMR) may be indicative of TILs. Several studies have reported that a low LMR may be a poor prognostic marker in thyroid cancers [9,10]. Previous studies have also reported an elevated peripheral neutrophil-to-lymphocyte ratio (NLR) as a poor prognostic factor in several malignancies, including differentiated thyroid cancer and anaplastic thyroid cancer (ATC) [11-14]. Moreover, the platelet-to-lymphocyte ratio (PLR) has been proposed as a biomarker in thyroid cancer [15,16].
We previously reported that the LMR was associated with poor clinical outcomes in patients with ATC and progressive radioactive iodine (RAI)-refractory thyroid cancers who were treated with sorafenib [10,17]. Recently, a post hoc analysis of the Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT) revealed that a low NLR was associated with improved progression-free survival (PFS) and OS in patients with progressive RAI-refractory thyroid cancers treated with lenvatinib [18]. Lenvatinib is known for its ability to block angiogenesis and inhibit multiple kinase activities, thereby affecting immune cells—including TAMs, cytotoxic T cells, and regulatory T cells—and modulating the tumor microenvironment [19,20]. However, the prognostic value of NLR, LMR, and PLR for patients requiring tyrosine kinase inhibitor (TKI) treatment for progressive RAI-refractory thyroid cancer remains uncertain. Similarly, it is unclear how to use these biomarkers most effectively in clinical settings. In this study, we investigated the treatment outcomes of lenvatinib according to the baseline levels of the NLR, LMR, and PLR in patients with RAI-refractory thyroid cancer. We also proposed a predictive model for the prognosis of these patients using these inflammatory biomarkers.
METHODS
Subjects
This multicenter, retrospective cohort study assessed 71 patients with progressive RAI-refractory thyroid cancer who received lenvatinib treatment across five tertiary hospitals in Korea: Asan Medical Center, Pusan National University Hospital, Severance Hospital, Seoul St. Mary’s Hospital, and Chonnam National University Hospital, from 2016 to 2021. These patients had previously been part of multicenter cohort studies that examined the real-world efficacy of lenvatinib treatment [21,22]. Patients whose CBCs were available within 3 months before lenvatinib treatment were included in this study. Baseline CBC data were collected when the patients were medically stable and lacked factors that could affect the CBC, such as active infection, hematologic disease, or history of steroid use. The study protocol was reviewed and approved by the Institutional Review Board of Asan Medical Center (IRB no: 2021-1066) and each participating institution. Written informed consent by the patients was waived due to a retrospective nature of our study. All experiments were performed in accordance with relevant guidelines and regulations.
Treatment and outcomes
Lenvatinib was self-administered, with a median initial dose of 20 mg per day. Tumor response and disease progression were evaluated every 2 to 3 months. PFS was calculated as the duration from the start of lenvatinib administration to the date of disease progression defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 classification [23]. We also evaluated the disease control rate, which included complete response, partial response, and stable disease for 6 months or more. Efficacy outcomes were determined using repeated axial images obtained from computed tomography or magnetic resonance imaging as previously described [22]. OS was calculated as the duration from the start of lenvatinib administration to the date of death from any cause. Disease-related symptoms were defined as symptoms associated with neck mass or metastatic lesions from the lung or bone, such as neck swelling, dyspnea, hemoptysis, and back pain.
Inflammatory biomarkers
Inflammatory biomarkers were calculated from the baseline CBC data. The NLR was calculated as the ratio of neutrophil percentage to lymphocyte percentage. The LMR was calculated as the ratio of lymphocyte percentage to monocyte percentage. The PLR was calculated as the ratio of the absolute platelet count to the absolute lymphocyte count. The cutoff level of each inflammatory biomarker was determined based on the median value obtained in the present study, and patients were then divided into subgroups based on the cutoff levels as previously described [18]. PFS and OS were evaluated based on inflammatory biomarkers.
Statistical analysis
Statistical analysis was conducted using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Continuous variables are presented as medians with interquartile ranges (IQRs) and were compared using the Wilcoxon rank sum test. Categorical variables are presented as numbers (percentages) and were compared using the Pearson chi-square test. Survival curves were plotted with the Kaplan-Meier method using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA; http://www.graphpad.com). The log-rank test was used to determine significance. A Cox proportional hazards model was utilized to evaluate prognostic values, based on hazard ratios (HRs) and 95% confidence intervals (CIs). All P values are two-sided, and a P<0.05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics of patients
Table 1 presents the baseline characteristics of patients who received lenvatinib treatment. The median age at diagnosis was 58.0 years (IQR, 60.6 to 71.3), with men comprising 42% of the cohort. PTC was the most prevalent pathology, affecting 71.8% of patients, followed by poorly differentiated carcinoma (15.5%) and follicular carcinoma (11.3%). The median duration from thyroid cancer diagnosis to the initiation of lenvatinib was 5.9 years (IQR, 2.5 to 13.6), and the median age at the commencement of lenvatinib therapy was 67.4 years (IQR, 60.6 to 71.3). The median follow-up time from the start of lenvatinib to the end of the observation period was 1.5 years (IQR, 0.7 to 5.5). Prior to lenvatinib treatment, all patients had undergone total thyroidectomy and RAI therapy, with a median cumulative RAI dose of 230 mCi (IQR, 150 to 400). At the start of lenvatinib, 30 patients (42.3%) were symptomatic. Among the 71 patients, 68 presented with distant metastases, while only three had locally advanced disease. The median serum thyroglobulin (Tg) level was 128.0 ng/mL (IQR, 15.7 to 1,140.0), and the median serum anti-Tg antibody level was 22.8 U/mL (IQR, 10.1 to 43.1). The lung was the most common site of distant metastatic lesions (83.1%) and target lesions (57.7%). Regarding metastatic sites, 25 patients had metastasis at one site, whereas 46 had metastases at two or more sites, with up to five sites observed. The baseline number of metastatic organs analyzed based on inflammatory markers is shown in Supplemental Fig. S1. A low baseline LMR and high PLR were associated with the presence of two or more metastatic organs (P=0.0098 and P=0.0055, respectively). The median initial and maintenance doses of lenvatinib dose were 20 and 10 mg, respectively. The disease control rate was 70.4%, and 47 patients (66.2%) had a disease control duration of at least 6 months. The median baseline absolute white blood cell count was 5.9 (IQR, 5.1 to 5.9) ×106/μL, and neutrophils and lymphocytes accounted for 55.4% and 29.0%, respectively. The median baseline NLR, LMR, and PLR were 2.0, 3.9, and 142.1 respectively.
Clinical parameters according to inflammatory biomarkers
Table 2 shows the clinical parameters according to each inflammatory biomarker. The levels of inflammatory biomarkers showed no significant associations with sex, pathology, cumulative RAI dose, time from diagnosis, longest diameter of the target lesion, cancer-related symptoms, lung-only metastasis, serum Tg level, and the number of metastatic sites. Patients with a high NLR (NLR ≥2) were significantly older than those with a low NLR (P=0.01). Other baseline characteristics were not significantly different between the NLR and PLR groups.
PFS and OS according to the inflammatory biomarkers
Fig. 1 presents the PFS of patients according to each inflammatory biomarker. The median PFS was 49.3 months (95% CI, 31.6 to 67.0). Patients in the high NLR group (NLR ≥2) had significantly shorter PFS (median PFS, 21.6 months; 95% CI, 0.5 to 42.7 months) than those in the low NLR group (median PFS, 51.5 months; 95% CI, 47.1 to 55.9 months) (HR, 2.51; 95% CI, 1.12 to 5.61; P=0.02) (Fig. 1A). There was no significant difference in PFS according to the LMR and PLR groups, despite a trend for shorter PFS in the low LMR (LMR <3.9) and high PLR (PLR ≥142.1) groups (Fig. 1B, C).
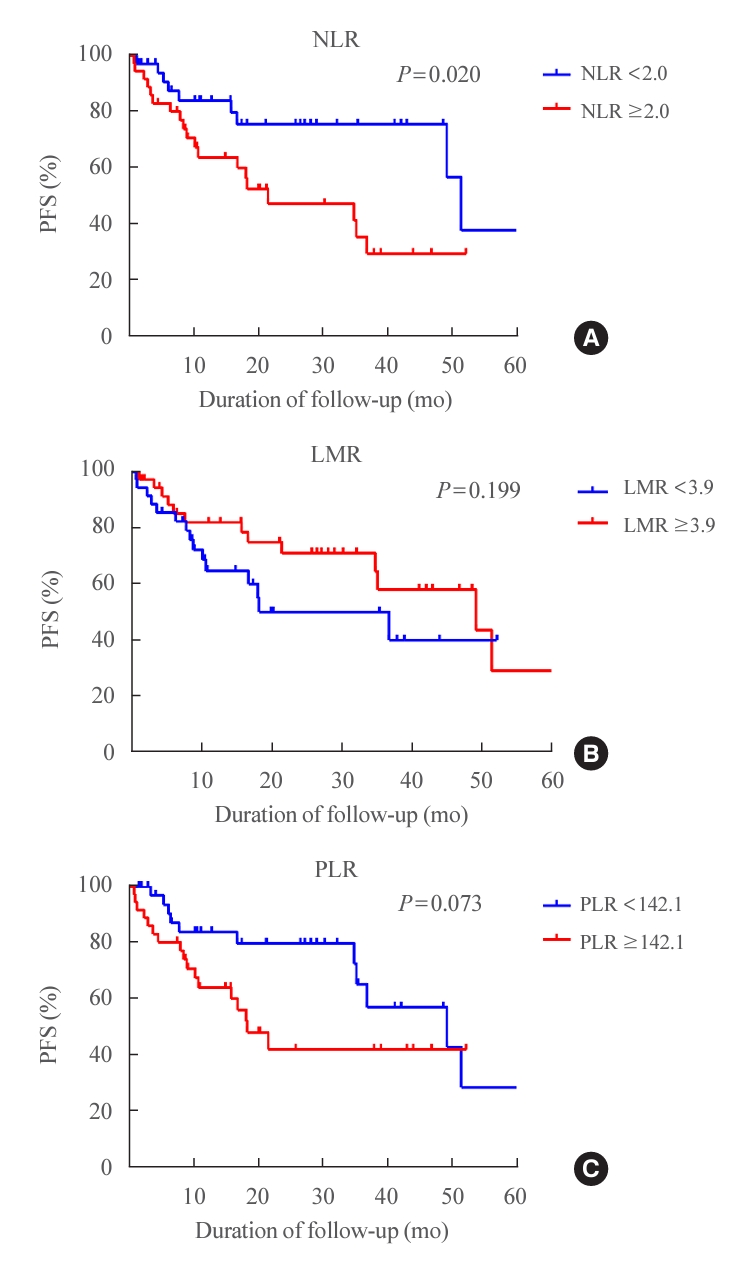
Progression-free survival (PFS) of patients who underwent lenvatinib treatment for refractory thyroid cancer based on inflammatory biomarkers. (A) PFS according to the neutrophil-to-lymphocyte ratio (NLR). (B) PFS according to the lymphocyte-to-monocyte ratio (LMR). (C) PFS according to the platelet-to-lymphocyte ratio (PLR).
We also evaluated OS according to three inflammatory biomarkers (Fig. 2). The median OS was 37.3 months (95% CI, 22.5 to 52.1). Patients in the high NLR group had significantly shorter OS (median OS, 26.2 months; 95% CI, 12.7 to 39.7 months) than those in the low NLR group (median OS, 54.9 months; 95% CI, 54.9 months to not available) (HR, 2.06; 95% CI, 1.24 to 3.42; P=0.017) (Fig. 2A). The median OS of patients with a lower LMR (<3.9) was 20.7 months (95% CI, 6.6 to 34.8), while that of patients with a higher LMR (LMR ≥3.9) was 54.9 months (95% CI, 24.4 to 85.4). A lower LMR (<3.9) was significantly associated with shorter OS (HR, 2.16; 95% CI, 1.03 to 4.52; P=0.036) (Fig. 2B). A high PLR (≥142.1) was also associated with shorter OS (median OS, 19.7 months; 95% CI, 7.6 to 33.8 months) than a low PLR (median OS, 53.9 months; 95% CI, 23.7 to 86.1 months) (HR, 2.23; 95% CI, 1.02 to 4.53; P=0.039) in patients who underwent lenvatinib treatment (Fig. 2C). In the multivariate analysis, a high NLR (NLR ≥2) was associated with poor OS after adjustment for multiple confounding factors, including age, sex, pathology, disease-related symptoms, cumulative RAI dose, time from diagnosis, longer diameter of the target lesion, serum Tg level, and the number of metastatic sites (HR, 3.0; 95% CI, 1.24 to 7.29; P=0.015).

Overall survival (OS) of patients who underwent lenvatinib treatment for refractory thyroid cancer based on inflammatory biomarkers. (A) OS according to the neutrophil-to-lymphocyte ratio (NLR). (B) OS according to the lymphocyte-to-monocyte ratio (LMR). (C) OS according to the platelet-to-lymphocyte ratio (PLR).
Due to the association between a poor prognosis and high NLR, low LMR, and high PLR in our cohort, we propose a predictive model using these three inflammatory biomarkers. Each inflammatory biomarker was considered as one risk factor. Patients with two or more risk factors were classified as high-risk, while those with fewer than two were classified as low-risk. The low-risk group comprised 33 patients, and the high-risk group included 38 patients. The median PFS of the high-risk group (≥2 risk factors) was 18.4 months (95% CI, 12.8 to 24.0) and that of the low-risk group (<2 risk factors) was 49.3 months (95% CI, 33.2 to 65.4) (Fig. 3A). There was no significant difference in the PFS according to the inflammatory risk group (P=0.082). The median OS of the high-risk group (≥2 risk factors) was 20.2 months (95% CI, 5.4 to 36.0) and that of the low-risk group (<2 risk factors) was 54.5 months (95% CI, 24.5 to 85.3) (Fig. 3B). The high-risk group had significantly shorter OS (HR, 2.34; 95% CI, 1.17 to 3.14; P=0.016) than the low-risk group.

Clinical course of patients who underwent lenvatinib treatment for refractory thyroid cancer based on the presence of inflammatory risk factors. (A) Progression-free survival of patients in the high- and low-risk groups. (B) Overall survival of patients in the high- and low-risk groups. RF, risk factor.
DISCUSSION
This multicenter cohort study evaluated the prognostic role of inflammatory biomarkers from the baseline CBCs of patients with RAI-refractory thyroid cancer who underwent lenvatinib treatment. A high NLR, low LMR, and high PLR were significantly associated with shorter OS in our cohort. Patients with a high NLR also had shorter PFS than those with a low NLR. Furthermore, in the multivariate analysis, a high NLR was significantly associated with poor OS after adjustment for confounding factors. Consistent with previous results in the post hoc analysis of the SELECT study, the NLR was associated with PFS and OS in patients with RAI-refractory thyroid cancer who underwent lenvatinib treatment. In our study, we proposed a predictive model using inflammatory biomarkers, and the patients in the high-risk group (with two or three inflammatory risk factors) had significantly shorter OS than their low-risk counterparts. Our findings indicate that peripheral biomarkers using baseline CBC values are useful for predicting the effectiveness of lenvatinib treatment for progressive RAI-refractory thyroid cancer.
Several studies have found that the prognosis of malignancy is associated with peripheral inflammatory indices based on the host immune response [24,25]. The balance between anti- and pro-tumorigenic inflammatory responses may also be important for thyroid cancer, although it is quite complex [2]. Within the thyroid tumor microenvironment, pro-tumorigenic cells include neutrophils, mast cells, and M2 macrophages (immunosuppression promoter), all of which secrete reactive oxygen species and innate immune system components. The innate immune system plays a role in the subsequent interaction of adaptive immune response through lymphocytes [26]. Anti-tumorigenic cells include TILs, dendritic cells, and M1 macrophages (phagocytosis promoter) [27]. In thyroid cancer, TAMs generally express M2 polarization, demonstrating a positive correlation between macrophage density and tumor growth [5,6,28,29]. Neutrophils have been reported to produce inflammatory cytokines, which exacerbate tumor progression [20]. In TILs, CD4+ T lymphocytes differentiate into Th1, Th2, Th17, or Treg to control the immune response, which was found to be correlated with tumor size. The levels of CD8+ cytotoxic T lymphocytes are markedly elevated in malignant thyroid cells, where they produce cytotoxins that help protect against cancer progression [26].
Inflammatory biomarkers can be easily calculated from information on peripheral leukocyte levels, which reflect systemic inflammation of host immune cells. The NLR was calculated as the ratio of neutrophil and lymphocyte percentages. Marked neutrophilia was found in the tumor microenvironment, which is considered to be a paraneoplastic manifestation. An association between a high NLR and increased recurrence has been observed in thyroid cancer [13,14]. The LMR was calculated as the ratio of lymphocyte and monocyte percentages, which reflects TILs and TAMs. Lymphopenia refers to a weakened immune response against a tumor, while increased TAM infiltration supports tumorigenesis. Therefore, an association between a low LMR and poor clinical outcomes is explicable based on the infiltration of leukocytes in malignancies [9,10]. Platelets, which are present at elevated levels, release platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), supporting tumor-related blood vessel formation; however, the exact mechanism is unknown [30]. The PLR, corresponding to the ratio of platelet and lymphocyte counts, has been associated with the prognosis of various cancers, including gastric cancer, colorectal cancer, and medullary thyroid carcinoma [15,16,31]. All these inflammatory biomarkers, which are highly applicable to tumors, can be easily measured from a routine CBC. However, there is little evidence supporting a correlation between peripheral inflammatory biomarkers and the tumor-infiltrating immune cell composition. Therefore, more research is needed to fully understand the complicated interactions between inflammatory biomarkers and the tumor microenvironment in refractory thyroid cancer.
RAI-refractory thyroid cancer is related to poor survival outcomes, with a reported 10-year survival rate of 10% [32]. There are few prognostic biomarkers; for example, the V600E mutation of the BRAF gene and the TERT promoter mutation have been associated with the aggressive phenotype [33]. However, it is difficult to use these mutations as a predictive biomarker of lenvatinib treatment because lenvatinib is a multitargeted TKI that improved PFS in the SELECT study [34]. Lenvatinib exerts its antitumor effect mainly by inhibiting VEGF receptors, fibroblast growth factor (FGF) receptors, PDGF receptor α, rearranged during transfection receptor (RET), and tyrosine kinase receptor (KIT) [19]. VEGF significantly influences the tumor microenvironment, i.e., it promotes tumor growth by evading the immune system and increasing angiogenesis. This process is facilitated by the recruitment of TAMs, regulatory T cells, and myeloid-derived suppressor cells, while mitigating the responses of effector T cells [35]. Compared with sorafenib, lenvatinib exerts a more potent effect on VEGF receptors and FGF receptors, which might improve immunity through TAM modulation and cytotoxic T-cell activation [36]. Several recent studies have proposed the immune response as a prognostic biomarker in thyroid cancer. One study demonstrated that a higher M1/M2 macrophage ratio was associated with a high proliferation rate, which reiterates the local proinflammatory state [37]. The other study observed that the infiltration of CD8+ TILs and the expression of cyclooxygenase2 (COX2) expression predicted relapse in patients with thyroid cancer [38]. Another study also showed that the selective depletion of TAMs induced tumor regression in advanced stages of PTC, meaning that TAMs can be potential therapeutic targets [39]. These findings suggest that the immunomodulatory effects of lenvatinib on tumor microenvironment and peripheral inflammatory biomarkers may serve as prognostic biomarkers in advanced thyroid cancer. By inhibiting VEGF signaling, lenvatinib administration can lead to a reduction in TAMs and an increase in cytotoxic T cells [40]. Therefore, a low LMR throughout lenvatinib treatment might indicate a reduced treatment response and disease progression. Furthermore, because neutrophils are known to promote tumor cell proliferation, an increase in the NLR during lenvatinib treatment, suggesting a relative increase in neutrophils and a decrease in lymphocytes, may indicate a poor prognosis. Further research is needed to investigate the dynamics of the tumor microenvironment before and after therapy with lenvatinib or other targeted agents.
This study had several limitations. First, since this was a retrospective cohort study, there was a possibility of selection bias. Second, the number of patients included in the study was relatively small. Third, the cutoff of each inflammatory biomarker was determined by the median value in this study. Therefore, further research involving more cases and large-scale prospective studies is necessary to establish appropriate cutoff values for clinical application. Fourth, we did not have access to tissue specimens that would allow us to explore the relationship between immune cells in the tumor microenvironment and peripheral inflammatory cells. Despite these limitations, this multicenter cohort study has validated the previous hypothesis that the NLR might serve as a prognostic biomarker and shown that other inflammatory indices might also serve as prognostic markers.
In conclusion, peripheral inflammatory biomarkers of the host immune system, such as a low NLR, high LMR, and low PLR, are associated with prolonged OS in patients with progressive RAI-refractory thyroid cancer before lenvatinib treatment. Baseline inflammatory biomarkers, which are inexpensive to obtain and readily available in clinical practices, can serve as simple prognostic biomarkers and may help individualize the treatment.
Supplementary Material
Supplemental Fig. S1.
Baseline number of metastatic organs by (A) lymphocyte-to-monocyte ratio (LMR) and (B) platelet-to-lymphocyte ratio Basel
Notes
CONFLICTS OF INTEREST
Won Bae Kim and Won Gu Kim were advisory board members of Bayer, Eisai, Lilly, and Hanmi Pharmaceuticals. The others have no competing financial interests.
AUTHOR CONTRIBUTIONS
Conception or design: M.J.J., D.J.L., D.Y.S., W.G.K. Acquisition, analysis, or interpretation of data: C.A.K., M.K., M.J., H. K.K., D.J.L., B.H.K., H.C.K., W.B.K., D.Y.S., W.G.K. Drafting the work or revising: C.A.K., M.K., M.J.J., W.B.K., D.Y.S., W.G.K. Final approval of the manuscript: C.A.K., M.K., M.J., H.K.K., M.J.J., D.J.L., B.H.K., H.C.K., W.B.K., D.Y.S., W.G.K.
Acknowledgements
This study was supported by a grant (2024IL0008) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. A part of this study was presented as an abstract at a meeting of the Seoul International Congress of Endocrinology and Metabolism in 2022.