Funded articles
- Page Path
- HOME > BROWSE ARTICLES > Funded articles
Original Articles
- Miscellaneous
- DN200434 Inhibits Vascular Smooth Muscle Cell Proliferation and Prevents Neointima Formation in Mice after Carotid Artery Ligation
- Sudeep Kumar, Jonghwa Jin, Hyeon Young Park, Mi-Jin Kim, Jungwook Chin, Sungwoo Lee, Jina Kim, Jung-Guk Kim, Yeon-Kyung Choi, Keun-Gyu Park
- Endocrinol Metab. 2022;37(5):800-809. Published online September 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1462
- Funded: National Research Foundation of Korea, Ministry of Science and ICT, Korea Health Industry Development Institute, Ministry of Health and Welfare
- 3,010 View
- 199 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
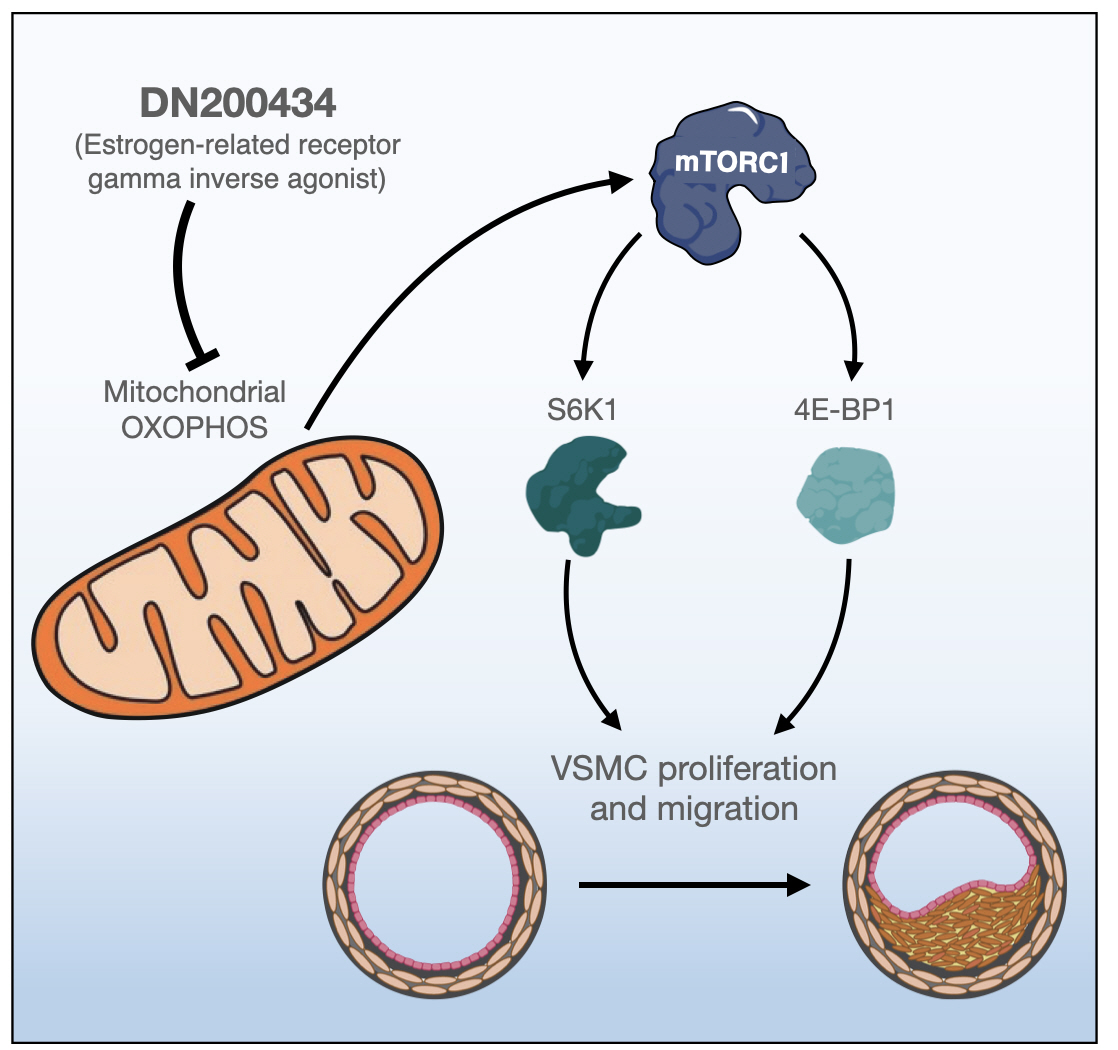
Excessive proliferation and migration of vascular smooth muscle cells (VSMCs), which contributes to the development of occlusive vascular diseases, requires elevated mitochondrial oxidative phosphorylation to meet the increased requirements for energy and anabolic precursors. Therefore, therapeutic strategies based on blockade of mitochondrial oxidative phosphorylation are considered promising for treatment of occlusive vascular diseases. Here, we investigated whether DN200434, an orally available estrogen receptor-related gamma inverse agonist, inhibits proliferation and migration of VSMCs and neointima formation by suppressing mitochondrial oxidative phosphorylation.
Methods
VSMCs were isolated from the thoracic aortas of 4-week-old Sprague-Dawley rats. Oxidative phosphorylation and the cell cycle were analyzed in fetal bovine serum (FBS)- or platelet-derived growth factor (PDGF)-stimulated VSMCs using a Seahorse XF-24 analyzer and flow cytometry, respectively. A model of neointimal hyperplasia was generated by ligating the left common carotid artery in male C57BL/6J mice.
Results
DN200434 inhibited mitochondrial respiration and mammalian target of rapamycin complex 1 activity and consequently suppressed FBS- or PDGF-stimulated proliferation and migration of VSMCs and cell cycle progression. Furthermore, DN200434 reduced carotid artery ligation-induced neointima formation in mice.
Conclusion
Our data suggest that DN200434 is a therapeutic option to prevent the progression of atherosclerosis. -
Citations
Citations to this article as recorded by- Jatrorrhizine inhibits Piezo1 activation and reduces vascular inflammation in endothelial cells
Tianying Hong, Xianmei Pan, Han Xu, Zhijuan Zheng, Lizhen Wen, Jing Li, Mingfeng Xia
Biomedicine & Pharmacotherapy.2023; 163: 114755. CrossRef
- Jatrorrhizine inhibits Piezo1 activation and reduces vascular inflammation in endothelial cells

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Recent Changes in the Incidence of Thyroid Cancer in Korea between 2005 and 2018: Analysis of Korean National Data
- Yun Mi Choi, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
- Endocrinol Metab. 2022;37(5):791-799. Published online October 11, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1533
- Funded: Korean Endocrine Society of EnM, Asan Institute for Life Sciences, Asan Medical Center
- 2,758 View
- 192 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
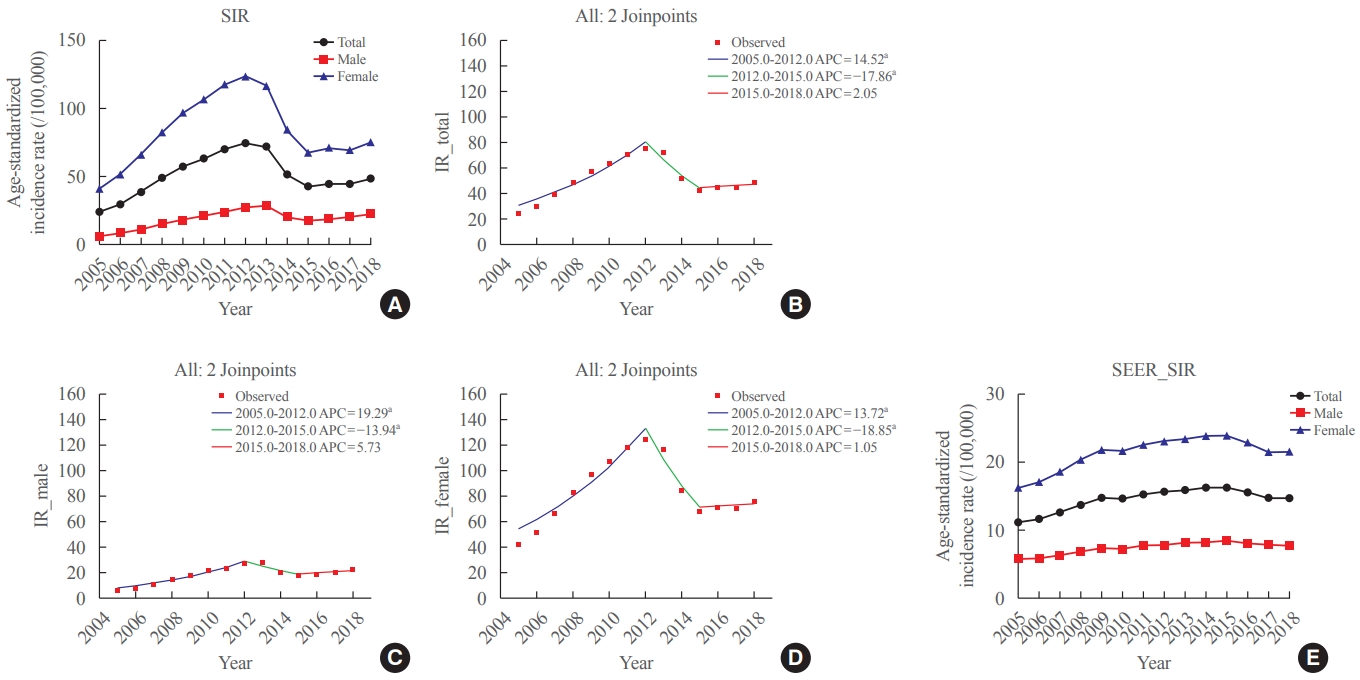
In this study, we evaluated the recent changes in the standardized, age-specific, stage-specific incidence rates (IRs) of thyroid cancer in Korea and compared them with the incidence data reported by the Surveillance, Epidemiology, and End Results Program.
Methods
The analysis was conducted using the incidence data (2005 to 2018) from the Statistics Korea and Korea Central Cancer Registry.
Results
The age-standardized IR (SIR) of thyroid cancer increased from 24.09 per 100,000 in 2005 to 74.83 in 2012 (annual percent change [APC], 14.5). From 2012 to 2015, the SIR decreased to 42.52 (APC, –17.9) and then remained stable until 2018 (APC, 2.1). This trend was similar in both men and women. Regarding age-specific IRs, the IRs for ages of 30 years and older showed a trend similar to that of the SIR; however, for ages below 30 years, no significant reduction was observed from the vertex of IR in 2015. Regarding stage-specific IRs, the increase was more prominent in those with regional disease (APC, 17.4) than in those with localized disease until 2012; then, the IR decreased until 2015 (APC, –16.1). The average APC from 2005 to 2018 increased in men, those under the age of 30 years, and those with regional disease.
Conclusion
The SIR in Korea peaked in 2012 and decreased until 2015 and then remained stable until 2018. However, in young individuals under the age of 30 years, the IR did not significantly decrease but tended to increase again. In terms of stage-specific IRs, the sharpest increase was seen among those with regional disease. -
Citations
Citations to this article as recorded by- Comparison of postoperative pain between transoral and conventional thyroidectomy: a propensity score-matched analysis
Min Kyu Park, Van Cuong Nguyen, Eugene Kim, Chang Myeon Song, Yong Bae Ji, Jin Hyeok Jeong, Kyung Tae
Surgical Endoscopy.2024; 38(3): 1512. CrossRef - Contents analysis of thyroid cancer-related information uploaded to YouTube by physicians in Korea: endorsing thyroid cancer screening, potentially leading to overdiagnosis
EunKyo Kang, HyoRim Ju, Soojeong Kim, Juyoung Choi
BMC Public Health.2024;[Epub] CrossRef - Bilateral axillo-breast approach robotic total thyroidectomy without isthmectomy: a case report
Hyeji Kim, Hyeonuk Hwang, Hyungju Kwon
The Ewha Medical Journal.2024;[Epub] CrossRef - Association between Consumption of Iodine-Rich Foods and Thyroid Cancer Prevalence: Findings from a Large Population-Based Study
Yu-Jin Kwon, Hye-Sun Lee, Sang-Wook Kang, Ji-Won Lee
Nutrients.2024; 16(7): 1041. CrossRef - Cancer and Mortality Risks of Graves’ Disease in South Korea Based on National Data from 2010 to 2019
Young Ju Choi, Kyungdo Han, Won Kyoung Cho, Min Ho Jung, Byung-Kyu Suh
Clinical Epidemiology.2023; Volume 15: 535. CrossRef - Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
Shinje Moon, Eun Kyung Lee, Hoonsung Choi, Sue K. Park, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 81. CrossRef - Cumulative exposure to metabolic syndrome increases thyroid cancer risk in young adults: a population-based cohort study
Jinyoung Kim, Kyungdo Han, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
The Korean Journal of Internal Medicine.2023; 38(4): 526. CrossRef - Cost-Effectiveness of Active Surveillance Compared to Early Surgery of Small Papillary Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Jaseong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chulmin Kim
Journal of Korean Medical Science.2023;[Epub] CrossRef - Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
Endocrinology and Metabolism.2023; 38(5): 588. CrossRef - Age and Post-Lobectomy Recurrence after Endoscopic or Robotic Thyroid Surgery: A Retrospective Cohort Study of 2348 Papillary Thyroid Carcinoma Patients
Jin-Seong Cho, Yong-Min Na, Hee Kyung Kim
Cancers.2023; 15(23): 5506. CrossRef
- Comparison of postoperative pain between transoral and conventional thyroidectomy: a propensity score-matched analysis

- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Association among Current Smoking, Alcohol Consumption, Regular Exercise, and Lower Extremity Amputation in Patients with Diabetic Foot: Nationwide Population-Based Study
- Yoon Jae Lee, Kyung-Do Han, Jun Hyeok Kim
- Endocrinol Metab. 2022;37(5):770-780. Published online October 12, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1519
- Funded: Yeouido St. Mary’s Hospital, The Catholic University of Korea
- 3,255 View
- 200 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
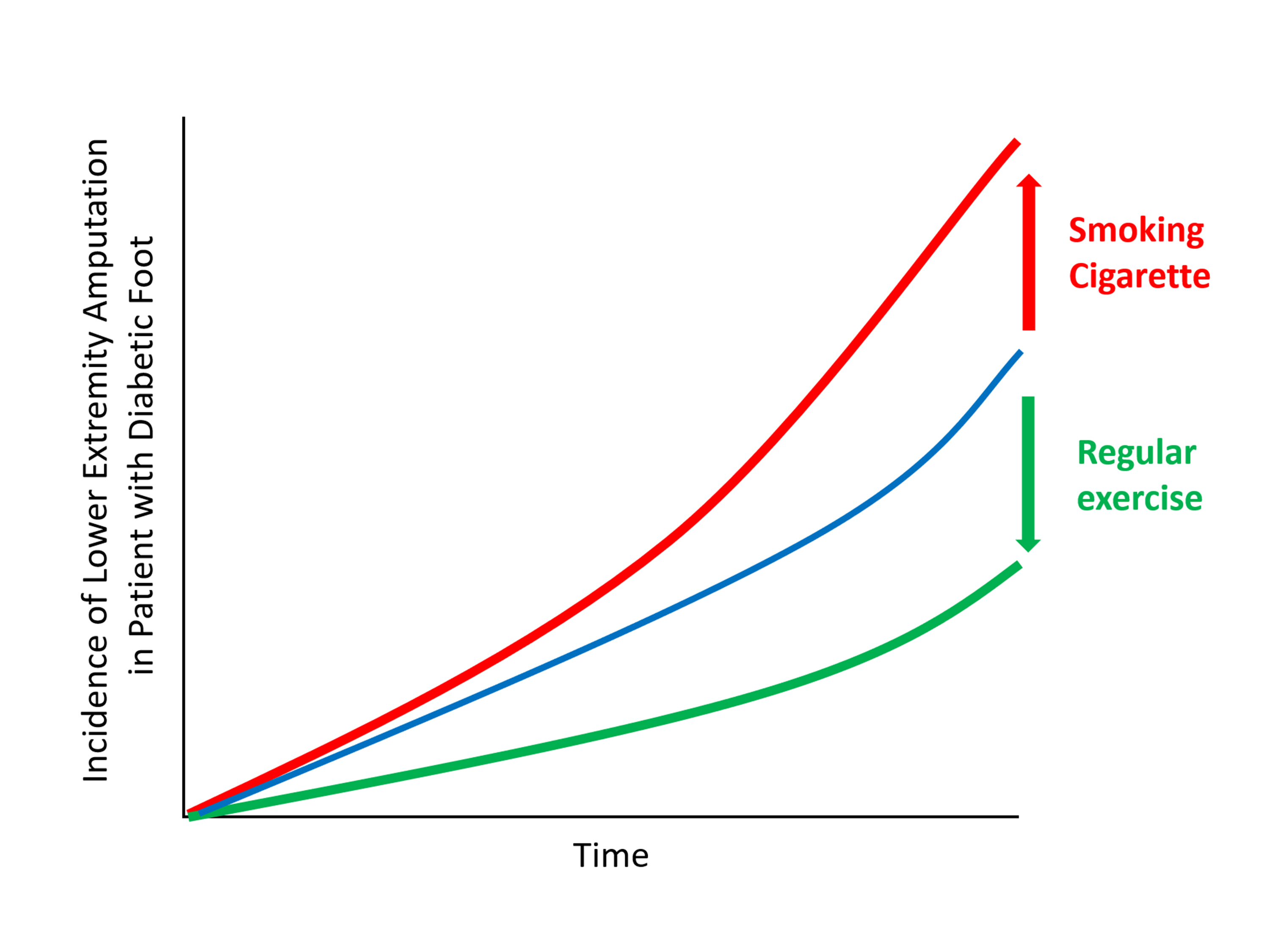
The present study investigates whether modifiable behavioral factors of current cigarette smoking, heavy alcohol consumption, and regular exercise are associated with risk of lower extremity amputation (LEA) in diabetic patients.
Methods
A total of 2,644,440 diabetic patients (aged ≥20 years) was analyzed using the database of the Korean National Health Insurance Service. Cox proportional hazard regression was used to assess adjusted hazard ratios (HRs) for the behavioral factors with risk of LEA under adjustment for potential confounders.
Results
The risk of LEA was significantly increased by current cigarette smoking and heavy alcohol consumption (HR, 1.436; 95% confidence interval [CI], 1.367 to 1.508 and HR, 1.082; 95% CI, 1.011 to 1.158) but significantly decreased with regular exercise (HR, 0.745; 95% CI, 0.706 to 0.786) after adjusting for age, sex, smoking, alcohol consumption, exercise, low income, hypertension, dyslipidemia, body mass index, using insulin or oral antidiabetic drugs, and diabetic duration. A synergistically increased risk of LEA was observed with larger number of risky behaviors.
Conclusion
Modification of behaviors of current smoking, heavy alcohol intake, and exercise prevents LEA and can improve physical, emotional, and social quality of life in diabetic patients. -
Citations
Citations to this article as recorded by- Adjuvant effect of antimicrobial photodynamic therapy (aPDT) in the treatment of diabetic foot ulcers: A case series
Rita de Cassia Ferreira, Rebeca Boltes Cecatto, Silvana Torres Perez, Raquel Agnelli Mesquita‐Ferrari, Sandra Kalil Bussadori, Cinthya Cosme Duran, Anna Carolina Tempestini Horliana, Kristianne Porta Santos Fernandes
Journal of Biophotonics.2024;[Epub] CrossRef - Factors associated with diabetic foot ulcers and lower limb amputations in type 1 and type 2 diabetes supported by real‐world data from the German/Austrian DPV registry
Alexander J. Eckert, Stefan Zimny, Marcus Altmeier, Ana Dugic, Anton Gillessen, Latife Bozkurt, Gabriele Götz, Wolfram Karges, Frank J. Wosch, Stephan Kress, Reinhard W. Holl
Journal of Diabetes.2024;[Epub] CrossRef - Investigating Diabetic Foot Pathophysiology and Amputation Prevention Strategies through Behavioral Modification
Jun Hyeok Kim
Journal of Wound Management and Research.2023; 19(3): 167. CrossRef
- Adjuvant effect of antimicrobial photodynamic therapy (aPDT) in the treatment of diabetic foot ulcers: A case series

Review Articles
- Calcium & Bone Metabolism
- Updates on Paget’s Disease of Bone
- Yong Jun Choi, Young Bae Sohn, Yoon-Sok Chung
- Endocrinol Metab. 2022;37(5):732-743. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1575
- Funded: National Research Foundation of Korea
- 3,735 View
- 315 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Paget’s disease of the bone is a prevalent bone disease characterized by disorganized bone remodeling; however, it is comparatively uncommon in East Asian countries, including China, Japan, and Korea. The exact cause still remains unknown. In genetically susceptible individuals, environmental triggers such as paramyxoviral infections are likely to cause the disease. Increased osteoclast activity results in increased bone resorption, which attracts osteoblasts and generates new bone matrix. Fast bone resorption and formation lead to the development of disorganized bone tissue. Increasing serum alkaline phosphatase or unique radiographic lesions may serve as the diagnostic indicators. Common symptoms include bone pain, bowing of the long bones, an enlarged skull, and hearing loss. The diagnosis is frequently confirmed by radiographic and nuclear scintigraphy of the bone. Further, bisphosphonates such as zoledronic acid and pamidronate are effective for its treatment. Moreover, biochemical monitoring is superior to the symptoms as a recurrence indicator. This article discusses the updates of Paget’s disease of bone with a clinical case.
-
Citations
Citations to this article as recorded by- Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats
Radina Vasileva, Tsvetan Chaprazov, Aneliya Milanova
Journal of Functional Biomaterials.2024; 15(4): 106. CrossRef - Newly Diagnosed Monostotic Paget’s Disease of Bone during Living Kidney Donor Candidate Evaluation
Diana Jędrzejuk, Paweł Poznański, Paweł Szewczyk, Oktawia Mazanowska, Marek Bolanowski, Magdalena Krajewska, Dorota Kamińska
Biomedicines.2023; 11(2): 401. CrossRef - Paget's disease of bone in the patient presented with a bowed leg
Mehrzad Hajialiloo, Sepideh Tahsini Tekantapeh
Clinical Case Reports.2023;[Epub] CrossRef
- Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats

- Hypothalamus and Pituitary Gland
- Independent Skeletal Actions of Pituitary Hormones
- Se-Min Kim, Farhath Sultana, Funda Korkmaz, Daria Lizneva, Tony Yuen, Mone Zaidi
- Endocrinol Metab. 2022;37(5):719-731. Published online September 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1573
- Funded: Icahn School of Medicine at Mount Sinai, National Institutes of Health
- 3,629 View
- 232 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
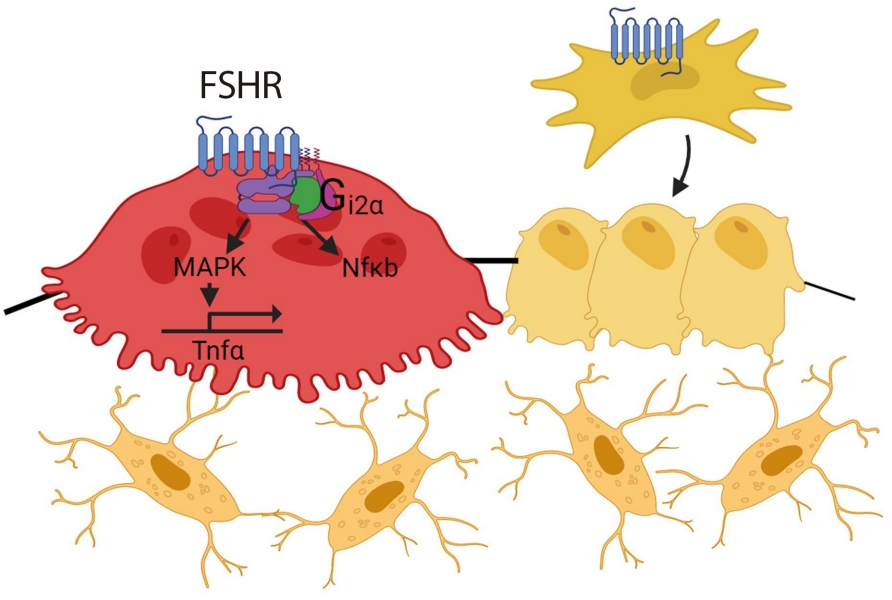
ePub - Over the past years, pituitary hormones and their receptors have been shown to have non-traditional actions that allow them to bypass the hypothalamus-pituitary-effector glands axis. Bone cells—osteoblasts and osteoclasts—express receptors for growth hormone, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), prolactin, oxytocin, and vasopressin. Independent skeletal actions of pituitary hormones on bone have been studied using genetically modified mice with haploinsufficiency and by activating or inactivating the receptors pharmacologically, without altering systemic effector hormone levels. On another front, the discovery of a TSH variant (TSH-βv) in immune cells in the bone marrow and skeletal action of FSHβ through tumor necrosis factor α provides new insights underscoring the integrated physiology of bone-immune-endocrine axis. Here we discuss the interaction of each pituitary hormone with bone and the potential it holds in understanding bone physiology and as a therapeutic target.
-
Citations
Citations to this article as recorded by- New tools for bone health assessment in secreting pituitary adenomas
Meliha Melin Uygur, Stefano Frara, Luigi di Filippo, Andrea Giustina
Trends in Endocrinology & Metabolism.2023; 34(4): 231. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - The mechanism of oxytocin and its receptors in regulating cells in bone metabolism
Liu Feixiang, Feng Yanchen, Li Xiang, Zhang Yunke, Miao Jinxin, Wang Jianru, Lin Zixuan
Frontiers in Pharmacology.2023;[Epub] CrossRef - To investigate the mechanism of Yiwei Decoction in the treatment of premature ovarian insufficiency-related osteoporosis using transcriptomics, network pharmacology and molecular docking techniques
Weisen Fan, Yan Meng, Jing Zhang, Muzhen Li, Yingjie Zhang, Xintian Qu, Xin Xiu
Scientific Reports.2023;[Epub] CrossRef
- New tools for bone health assessment in secreting pituitary adenomas

- Thyroid
- Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
- Chan Kwon Jung, Andrey Bychkov, Kennichi Kakudo
- Endocrinol Metab. 2022;37(5):703-718. Published online October 4, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1553
- Funded: National Research Foundation of Korea, Ministry of Science and ICT, Ministry of Health and Welfare
- 16,259 View
- 2,093 Download
- 47 Web of Science
- 64 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
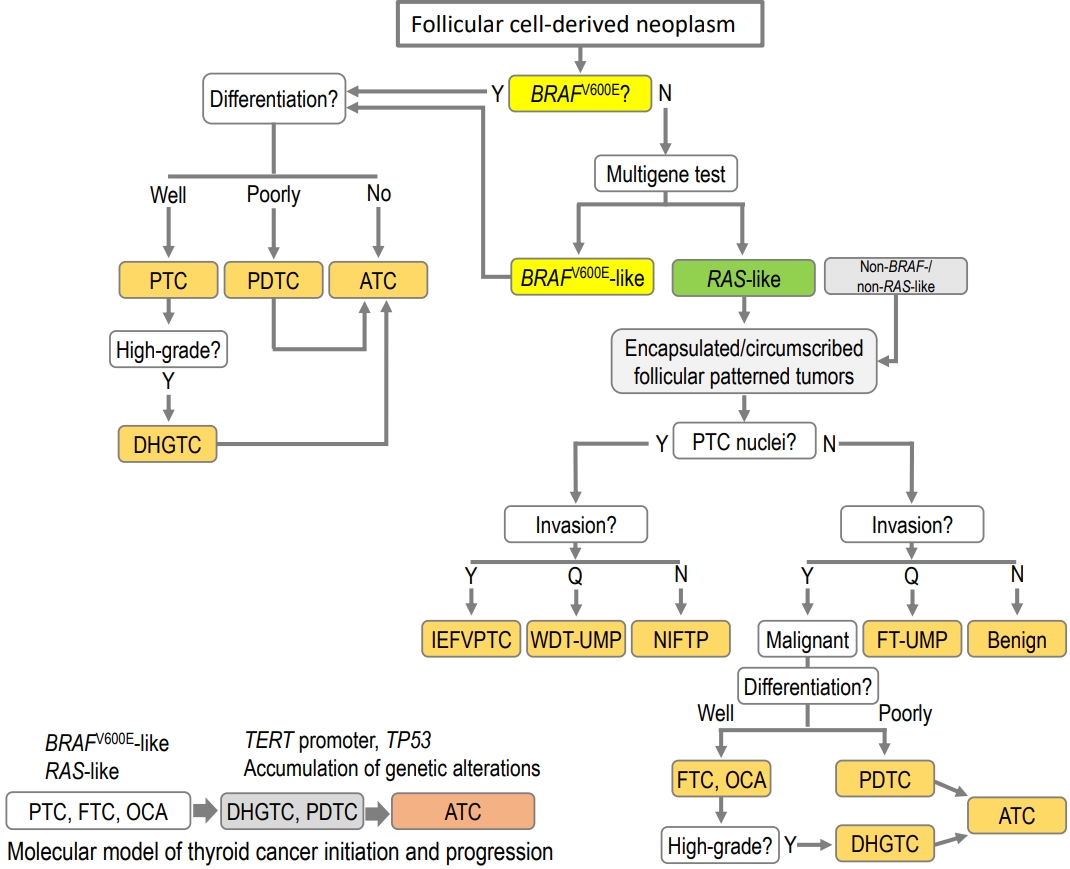
ePub - The fifth edition of the World Health Organization (WHO) histologic classification of thyroid neoplasms released in 2022 includes newly recognized tumor types, subtypes, and a grading system. Follicular cell-derived neoplasms are categorized into three families (classes): benign tumors, low-risk neoplasms, and malignant neoplasms. The terms “follicular nodular disease” and “differentiated high-grade thyroid carcinoma” are introduced to account for multifocal hyperplastic/neoplastic lesions and differentiated thyroid carcinomas with high-grade features, respectively. The term “Hürthle cells” is replaced with “oncocytic cells.” Invasive encapsulated follicular and cribriform morular variants of papillary thyroid carcinoma (PTC) are now redefined as distinct tumor types, given their different genetic alterations and clinicopathologic characteristics from other PTC subtypes. The term “variant” to describe a subclass of tumor has been replaced with the term “subtype.” Instead, the term “variant” is reserved to describe genetic alterations. A histologic grading system based on the mitotic count, necrosis, and/or the Ki67 index is used to identify high-grade follicular-cell derived carcinomas and medullary thyroid carcinomas. The 2022 WHO classification introduces the following new categories: “salivary gland-type carcinomas of the thyroid” and “thyroid tumors of uncertain histogenesis.” This review summarizes the major changes in the 2022 WHO classification and their clinical relevance.
-
Citations
Citations to this article as recorded by- Tumeurs de la thyroïde : nouveautés de la classification OMS 2022
Serge Guyétant, Myriam Decaussin Petrucci, Emmanuelle Leteurtre
Annales de Pathologie.2024; 44(1): 5. CrossRef - Diagnostic Performance of [18F]TFB PET/CT Compared with Therapeutic Activity [131I]Iodine SPECT/CT and [18F]FDG PET/CT in Recurrent Differentiated Thyroid Carcinoma
David Ventura, Matthias Dittmann, Florian Büther, Michael Schäfers, Kambiz Rahbar, Daniel Hescheler, Michael Claesener, Philipp Schindler, Burkhard Riemann, Robert Seifert, Wolfgang Roll
Journal of Nuclear Medicine.2024; 65(2): 192. CrossRef - Clinicopathological features of differentiated thyroid carcinoma as predictors of the effects of radioactive iodine therapy
Wen Liu, Beibei Jiang, Jingli Xue, Ruijing Liu, Yuqing Wei, Peifeng Li
Annals of Diagnostic Pathology.2024; 69: 152243. CrossRef - Management of aggressive variants of papillary thyroid cancer
Ying Ki Lee, Aleix Rovira, Paul V. Carroll, Ricard Simo
Current Opinion in Otolaryngology & Head & Neck Surgery.2024; 32(2): 125. CrossRef - Artificial Intelligence Detected the Relationship Between Nuclear Morphological Features and Molecular Abnormalities of Papillary Thyroid Carcinoma
Toui Nishikawa, Ibu Matsuzaki, Ayata Takahashi, Iwamoto Ryuta, Fidele Yambayamba Musangile, Kanako Sagan, Mizuki Nishikawa, Yurina Mikasa, Yuichi Takahashi, Fumiyoshi Kojima, Shin-ichi Murata
Endocrine Pathology.2024; 35(1): 40. CrossRef - Review of the 2023 World Congress on Thyroid Cancer (WCTC 2023, London): is there light at the end of the tunnel for patients with neglected cancer?
S.M. Cherenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2024; 19(8): 609. CrossRef - Molecular Alterations and Comprehensive Clinical Management of Oncocytic Thyroid Carcinoma
Lindsay A. Bischoff, Ian Ganly, Laura Fugazzola, Erin Buczek, William C. Faquin, Bryan R. Haugen, Bryan McIver, Caitlin P. McMullen, Kate Newbold, Daniel J. Rocke, Marika D. Russell, Mabel Ryder, Peter M. Sadow, Eric Sherman, Maisie Shindo, David C. Shonk
JAMA Otolaryngology–Head & Neck Surgery.2024; 150(3): 265. CrossRef - Malignancy in a Solitary Thyroid Nodule: A Retrospective Histopathological Evaluation
Hassan Alzahrani
International Journal of General Medicine.2024; Volume 17: 135. CrossRef - Pathologie thyroïdienne. Actualités et problèmes pratiques. Introduction
Serge Guyétant
Annales de Pathologie.2024; 44(2): 90. CrossRef - Diagnostic implication of thyroid spherules for cytological diagnosis of thyroid nodules
Heeseung Sohn, Kennichi Kakudo, Chan Kwon Jung
Cytopathology.2024; 35(3): 383. CrossRef - Aggressive Primary Thyroid Mucoepidermoid Carcinoma with Extensive Pulmonary Involvement
Marius Mitrache, Dana Terzea, Anca Sirbu, Simona Fica
Biomedicines.2024; 12(2): 285. CrossRef - Unravelling the role of long non-coding RNAs in modulating the Hedgehog pathway in cancer
Shailendra Singh Chandel, Anurag Mishra, Gaurav Dubey, Ravindra Pal Singh, Mithilesh Singh, Mohit Agarwal, Himmat Singh Chawra, Neelima Kukreti
Pathology - Research and Practice.2024; 254: 155156. CrossRef - Hürthle cell (Oncocytic) carcinoma – Is hemithyroidectomy enough?
Taylor O. Julsrud, Trenton R. Foster, Robert A. Vierkant, Melanie L. Lyden, Travis J. McKenzie, Mabel Ryder, Benzon M. Dy
Surgical Oncology Insight.2024; 1(1): 100010. CrossRef - Échographie des carcinomes thyroïdiens différenciés de souche folliculaire
P.Y. Marcy, M. Tassart, J.G. Marchand, J. Sanglier, A. Bizeau, E. Ghanassia
Journal d'imagerie diagnostique et interventionnelle.2024; 7(2): 54. CrossRef - Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Chankyung Kim, Shipra Agarwal, Andrey Bychkov, Jen-Fan Hang, Agnes Stephanie Harahap, Mitsuyoshi Hirokawa, Kennichi Kakudo, Somboon Keelawat, Chih-Yi Liu, Zhiyan Liu, Truong Phan-Xuan Nguyen, Chanchal Rana, Huy Gia Vuong, Yun Zhu, Chan Kwon Jung
Virchows Archiv.2024;[Epub] CrossRef - Cas no5. High-grade Tall cell papillary carcinoma
Myriam Decaussin-Petrucci
Annales de Pathologie.2024; 44(2): 114. CrossRef - Current and future of immunotherapy for thyroid cancer based on bibliometrics and clinical trials
Ke Wang, Ying Zhang, Yang Xing, Hong Wang, Minghua He, Rui Guo
Discover Oncology.2024;[Epub] CrossRef - Cas no6. Anaplastic thyroid carcinoma, epidermoid subtype
Myriam Decaussin-Petrucci
Annales de Pathologie.2024; 44(2): 120. CrossRef - Papillary Thyroid Carcinoma With Lymphoepithelial Features and Lacking Association With Epstein-Barr Virus (EBV): A Rare Case
Ahmed Bendari, Saroja Devi Geetha, Reham Al-Refai, Xuelin Zhong, Sunder Sham, Manju Harshan
Cureus.2024;[Epub] CrossRef - Clinical and morphological features and unsolved issues in diagnosis of aggressive forms of papillary thyroid carcinoma
Denis V. Korotovsky, Sergey V. Sergiiko, Sergey A. Lukyanov
Perm Medical Journal.2024; 41(1): 90. CrossRef - Finding possible diagnostic markers for differentiating benign and malignant thyroid tumors on example investigate of rheological properties1
I. Javakhishvili, T. Sanikidze, K. Mardaleishvili, N. Momtselidze, T. Urdulashvili, M. Mantskava, L. Prantl, F. Jung
Clinical Hemorheology and Microcirculation.2024; : 1. CrossRef - Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma
Lia Rodrigues, Arnaud Da Cruz Paula, Paula Soares, João Vinagre
Cells.2024; 13(7): 561. CrossRef - Management of Poorly Differentiated Thyroid Cancer and Differentiated High-Grade Thyroid Carcinoma
Iram S. Alam, Kepal N. Patel
Surgical Clinics of North America.2024;[Epub] CrossRef - What’s new in thyroid pathology 2024: updates from the new WHO classification and bethesda system
Andrey Bychkov, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(2): 98. CrossRef - Ultrasound–Histopathological Presentation of Thyroid and Ovary Lesions in Adolescent Patients with DICER1 Syndrome: Case Reports and Literature Overview
Dominika Januś, Monika Kujdowicz, Konrad Kaleta, Kamil Możdżeń, Jan Radliński, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Marcin Maślanka, Wojciech Górecki, Jerzy B. Starzyk
Children.2024; 11(4): 403. CrossRef - Surgical treatment of solid variant of papillary thyroid carcinoma: Fifteen-year experience of a tertiary center
Katarina Tausanović, Marina Stojanović, Milan Jovanović, Boban Stepanović, Jovan Ilić, Sara Ivaniš, Vladan Živaljević
Medicinska istrazivanja.2024; 57(1): 121. CrossRef - Critical appraisal of the WHO 2022 classification of thyroid cancer
Mithraa Devi Sekar, Debasis Gochhait, Sadishkumar Kamalanathan
Thyroid Research and Practice.2024; 20(1): 8. CrossRef - Update on C-Cell Neuroendocrine Neoplasm: Prognostic and Predictive Histopathologic and Molecular Features of Medullary Thyroid Carcinoma
Chan Kwon Jung, Shipra Agarwal, Jen-Fan Hang, Dong-Jun Lim, Andrey Bychkov, Ozgur Mete
Endocrine Pathology.2023; 34(1): 1. CrossRef - The 5th edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma
Fulvio Basolo, Elisabetta Macerola, Anello Marcello Poma, Liborio Torregrossa
Endocrine.2023; 80(3): 470. CrossRef - Preoperative Risk Stratification of Follicular-patterned Thyroid Lesions on Core Needle Biopsy by Histologic Subtyping and RAS Variant-specific Immunohistochemistry
Meejeong Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2023; 34(2): 247. CrossRef - Ultrasound evolution of parenchymal changes in the thyroid gland with autoimmune thyroiditis in children prior to the development of papillary thyroid carcinoma – a follow-up study
Dominika Januś, Monika Kujdowicz, Małgorzata Wójcik, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Wojciech Górecki, Jerzy B. Starzyk
Frontiers in Endocrinology.2023;[Epub] CrossRef - Papillary thyroid carcinoma with aggressive fused follicular and solid growth pattern: A unique histological subtype with high‐grade malignancy?
Shin‐ichi Murata, Ibu Matsuzaki, Mitsuo Kishimoto, Naomi Katsuki, Toshinori Onishi, Mitsuyoshi Hirokawa, Fumiyoshi Kojima
Pathology International.2023; 73(5): 207. CrossRef - Multi-Omics and Management of Follicular Carcinoma of the Thyroid
Thifhelimbilu Emmanuel Luvhengo, Ifongo Bombil, Arian Mokhtari, Maeyane Stephens Moeng, Demetra Demetriou, Claire Sanders, Zodwa Dlamini
Biomedicines.2023; 11(4): 1217. CrossRef - Molecular Genetics of Diffuse Sclerosing Papillary Thyroid Cancer
Meshael Alswailem, Balgees Alghamdi, Anwar Alotaibi, Abeer Aljomiah, Hindi Al-Hindi, Avaniyapuram Kannan Murugan, Mohamed Abouelhoda, Yufei Shi, Ali S Alzahrani
The Journal of Clinical Endocrinology & Metabolism.2023; 108(9): e704. CrossRef - Multifunctional Phase-Transition Nanoparticles for Effective Targeted Sonodynamic-Gene Therapy Against Thyroid Papillary Carcinoma
Shihui Guan, Dengke Teng, Hui Wang, Qimeihui Wang, Xi Zhen, Guoqing Sui, Yang Wang, Lingyu Zhu, Yuanqiang Lin, Dan Jiao, Feng Guo
International Journal of Nanomedicine.2023; Volume 18: 2275. CrossRef - Utilizing Dynamic Risk Stratification in Patients With Tall Cell Variant Papillary Thyroid Cancer
David Zimmer, Gilman Plitt, Brandon Prendes, Jamie Ku, Natalie Silver, Eric Lamarre, Emrullah Yilmaz, Jessica Geiger, Christian Nasr, Lea El Hage, Mario Skugor, Shauna Cambpell, Shlomo Koyfman, Jacob Miller, Neil Woody, Katherine Heiden, Nikhil Joshi, Tar
The Laryngoscope.2023; 133(9): 2430. CrossRef - Ultrasound, laboratory and histopathological insights in diagnosing papillary thyroid carcinoma in a paediatric population: a single centre follow-up study between 2000-2022
Dominika Januś, Małgorzata Wójcik, Anna Taczanowska-Niemczuk, Aleksandra Kiszka-Wiłkojć, Monika Kujdowicz, Małgorzata Czogała, Wojciech Górecki, Jerzy B. Starzyk
Frontiers in Endocrinology.2023;[Epub] CrossRef - Medullary Thyroid Carcinoma: A Single Institute Experience
Sonal Trivedi, T. Salahuddin, Mohamed Taher Mithi, Priyank Rathod, Arpit Bandi, Shashank J. Pandya, Mohit Sharma, Shailesh Patel, Vikas Warikoo, Ketul Puj, Abhijeet Salunkhe, Keval Patel, Shivam Pandya
Indian Journal of Otolaryngology and Head & Neck Surgery.2023; 75(4): 2884. CrossRef - FNAC (Fine needle aspiration cytology) and histopathological correlation and reclassification of thyroid neoplasm in accordance with WHO classification 2022
Fareeda Joshi, Shreya Hegde
Indian Journal of Pathology and Oncology.2023; 10(2): 132. CrossRef - Papillary thyroid carcinoma associated with non‑functioning parathyroid carcinoma with Warthin's tumor of the parotid gland: A case report and brief literature review
Ari Abdullah, Aras Qaradakhy, Yadgar Saeed, Abdulwahid Salih, Seema Karim, Osama Ali, Shko Hassan, Shalaw Nasraldeen, Shvan Mohammed, Fahmi Kakamad
Medicine International.2023;[Epub] CrossRef - Warthin-like papillary thyroid carcinoma: a case report and comprehensive review of the literature
Abdel Mouhaymen Missaoui, Fatma Hamza, Wafa Belabed, Manel Mellouli, Mohamed Maaloul, Slim Charfi, Issam Jardak, Tahya Sellami-Boudawara, Nabila Rekik, Mohamed Abid
Frontiers in Endocrinology.2023;[Epub] CrossRef - Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy
Chan Kwon Jung
Journal of Pathology and Translational Medicine.2023; 57(4): 208. CrossRef - Cancer Predisposition Syndromes and Thyroid Cancer: Keys for a Short Two-Way Street
Ioana Balinisteanu, Monica-Cristina Panzaru, Lavinia Caba, Maria-Christina Ungureanu, Andreea Florea, Ana Maria Grigore, Eusebiu Vlad Gorduza
Biomedicines.2023; 11(8): 2143. CrossRef - Deep learning prediction model for central lymph node metastasis in papillary thyroid microcarcinoma based on cytology
Wenhao Ren, Yanli Zhu, Qian Wang, Yuntao Song, Zhihui Fan, Yanhua Bai, Dongmei Lin
Cancer Science.2023; 114(10): 4114. CrossRef - Cytology and Histology of Thyroid Nodules: Exploring Novel Insights in the Molecular Era for Enhanced Patient Management
Beatrix Cochand-Priollet, Zahra Maleki
Current Oncology.2023; 30(8): 7753. CrossRef - Agrin is a novel oncogenic protein in thyroid cancer
Anna Adamiok‑Ostrowska, Małgorzata Grzanka, Barbara Czarnocka
Oncology Letters.2023;[Epub] CrossRef - Analysis of Pathological Features of Thyroid Carcinoma in the Western Yunnan
身吾 王
Advances in Clinical Medicine.2023; 13(10): 15820. CrossRef - Insight of novel biomarkers for papillary thyroid carcinoma through multiomics
Wei Liu, Junkan Zhu, Zhen Wu, Yongxiang Yin, Qiao Wu, Yiming Wu, Jiaojiao Zheng, Cong Wang, Hongyan Chen, Talal Jamil Qazi, Jun Wu, Yuqing Zhang, Houbao Liu, Jingmin Yang, Daru Lu, Xumin Zhang, Zhilong Ai
Frontiers in Oncology.2023;[Epub] CrossRef - Identification of NIFTP-Specific mRNA Markers for Reliable Molecular Diagnosis of Thyroid Tumors
So-Yeon Lee, Jong-Lyul Park, Kwangsoon Kim, Ja Seong Bae, Jae-Yoon Kim, Seon-Young Kim, Chan Kwon Jung
Endocrine Pathology.2023; 34(3): 311. CrossRef - Somatostatin Receptor Type 2 and Thyroid-Stimulating Hormone Receptor Expression in Oncocytic Thyroid Neoplasms: Implications for Prognosis and Treatment
Andrea Gillis, Rui Zheng-Pywell, Chandler McLeod, Dezhi Wang, John M. Ness, Rachael Guenter, Jason Whitt, Tomas A. Prolla, Herbert Chen, Manuel Lora Gonzalez, Bart Rose, Ricardo V. Lloyd, Renata Jaskula-Sztul, Diana Lin
Modern Pathology.2023; 36(12): 100332. CrossRef - Comparison of Treatment and Prognosis Between Follicular Variant
Papillary Thyroid Carcinoma and Classical Papillary Thyroid
Carcinoma
Bing Zhang, Wenming Wu, Jinjing Liu, Zhou Liang, Liang Zong
Hormone and Metabolic Research.2023; 55(12): 855. CrossRef - Sentinel lymph node mapping: current applications and future perspectives in thyroid carcinoma
Isabella Merante Boschin, Loris Bertazza, Carla Scaroni, Caterina Mian, Maria Rosa Pelizzo
Frontiers in Medicine.2023;[Epub] CrossRef - Utility of the Growth Differentiation Factor-15 in the Differential Diagnosis of Follicular-Patterned Lesions of the Thyroid on Cytopathologic and Histopathologic Samples
Prasanna V Perumal, Neelaiah Siddaraju, Sunil K Saxena, Soundravally Rajendiran, Ramachandra V Bhat
Cureus.2023;[Epub] CrossRef - Investigation of pre‐operative demographic, biochemical, sonographic and cytopathological findings in low‐risk thyroid neoplasms
Muzaffer Serdar Deniz, Didem Özdemir, Narin Nasıroğlu İmga, Hüsniye Başer, Fatma Neslihan Çuhacı Seyrek, Ayşegül Aksoy Altınboğa, Oya Topaloğlu, Reyhan Ersoy, Bekir Çakır
Clinical Endocrinology.2023; 99(5): 502. CrossRef - Applications of machine and deep learning to thyroid cytology and histopathology: a review
Greg Slabaugh, Luis Beltran, Hasan Rizvi, Panos Deloukas, Eirini Marouli
Frontiers in Oncology.2023;[Epub] CrossRef - The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
Journal of Pathology and Translational Medicine.2023; 57(6): 289. CrossRef - Anaplastic thyroid cancer: Pathogenesis, prognostic factors and genetic landscape (Review)
Abdul-Mohsen Alhejaily, Omar Alhuzim, Yazeed Alwelaie
Molecular and Clinical Oncology.2023;[Epub] CrossRef - Simultaneous Occurrence of Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma: A Case Series with Literature Review
Poupak Fallahi, Armando Patrizio, Giulio Stoppini, Giusy Elia, Francesca Ragusa, Sabrina Rosaria Paparo, Eugenia Balestri, Valeria Mazzi, Chiara Botrini, Gilda Varricchi, Salvatore Ulisse, Marco Ghionzoli, Alessandro Antonelli, Silvia Martina Ferrari
Current Oncology.2023; 30(12): 10237. CrossRef - Construction and validation of a nomogram for predicting cervical lymph node metastasis in diffuse sclerosing variant of papillary thyroid carcinoma
Xunyi Lin, Jiaxing Huo, Huan Zhang, Hang Su, Fenghua Zhang
Langenbeck's Archives of Surgery.2023;[Epub] CrossRef - Canine follicular cell and medullary thyroid carcinomas: Immunohistochemical characterization
Jana Jankovic, Eve Tièche, Martina Dettwiler, Kerstin Hahn, Stephanie Scheemaeker, Martin Kessler, Sylvie Daminet, Sven Rottenberg, Miguel Campos
Veterinary Pathology.2023;[Epub] CrossRef - A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma
Julia I. Staubitz-Vernazza, Sina Schwind, Oana Lozan, Thomas J. Musholt
Cancers.2023; 16(1): 163. CrossRef - Reprogramming of Cellular Metabolism and Its Therapeutic Applications in Thyroid Cancer
Yuji Nagayama, Koichiro Hamada
Metabolites.2022; 12(12): 1214. CrossRef - Developments to improve outcomes in thyroid surgery
Thomas J. Musholt
Innovative Surgical Sciences.2022; 7(3-4): 77. CrossRef - The relationship of the clinicopathological characteristics and treatment results of post-Chornobyl papillary thyroid microcarcinomas with the latency period and radiation exposure
Tetiana Bogdanova, Serhii Chernyshov, Liudmyla Zurnadzhy, Tatiana I. Rogounovitch, Norisato Mitsutake, Mykola Tronko, Masahiro Ito, Michael Bolgov, Sergii Masiuk, Shunichi Yamashita, Vladimir A. Saenko
Frontiers in Endocrinology.2022;[Epub] CrossRef
- Tumeurs de la thyroïde : nouveautés de la classification OMS 2022

Letter
- Thyroid
- Efficient Dissociation Protocol for Generation of Single Cell Suspension from Human Thyroid Tissue for Single Cell RNA Sequencing
- Shinae Yi, Hyun Jung Kim, Bon Seok Koo, Seong Eun Lee, Jahyun Choi, Yea Eun Kang
- Endocrinol Metab. 2022;37(4):698-700. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1536
- Funded: Ministry of Health and Welfare, National Research Foundation of Korea
- 1,814 View
- 119 Download
- 1 Web of Science
- 1 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses
Seong Eun Lee, Seongyeol Park, Shinae Yi, Na Rae Choi, Mi Ae Lim, Jae Won Chang, Ho-Ryun Won, Je Ryong Kim, Hye Mi Ko, Eun-Jae Chung, Young Joo Park, Sun Wook Cho, Hyeong Won Yu, June Young Choi, Min-Kyung Yeo, Boram Yi, Kijong Yi, Joonoh Lim, Jun-Young K
Nature Communications.2024;[Epub] CrossRef
- Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses

Original Articles
- Miscellaneous
- Protective Effect of Delta-Like 1 Homolog Against Muscular Atrophy in a Mouse Model
- Ji Young Lee, Minyoung Lee, Dong-Hee Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
- Endocrinol Metab. 2022;37(4):684-697. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1446
- Funded: Dong-A ST Research Center

- 3,164 View
- 127 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Muscle atrophy is caused by an imbalance between muscle growth and wasting. Delta-like 1 homolog (DLK1), a protein that modulates adipogenesis and muscle development, is a crucial regulator of myogenic programming. Thus, we investigated the effect of exogenous DLK1 on muscular atrophy.
Methods
We used muscular atrophy mouse model induced by dexamethasone (Dex). The mice were randomly divided into three groups: (1) control group, (2) Dex-induced muscle atrophy group, and (3) Dex-induced muscle atrophy group treated with DLK1. The effects of DLK1 were also investigated in an in vitro model using C2C12 myotubes.
Results
Dex-induced muscular atrophy in mice was associated with increased expression of muscle atrophy markers and decreased expression of muscle differentiation markers, while DLK1 treatment attenuated these degenerative changes together with reduced expression of the muscle growth inhibitor, myostatin. In addition, electron microscopy revealed that DLK1 treatment improved mitochondrial dynamics in the Dex-induced atrophy model. In the in vitro model of muscle atrophy, normalized expression of muscle differentiation markers by DLK1 treatment was mitigated by myostatin knockdown, implying that DLK1 attenuates muscle atrophy through the myostatin pathway.
Conclusion
DLK1 treatment inhibited muscular atrophy by suppressing myostatin-driven signaling and improving mitochondrial biogenesis. Thus, DLK1 might be a promising candidate to treat sarcopenia, characterized by muscle atrophy and degeneration.

- Calcium & Bone Metabolism
- Development of a Spine X-Ray-Based Fracture Prediction Model Using a Deep Learning Algorithm
- Sung Hye Kong, Jae-Won Lee, Byeong Uk Bae, Jin Kyeong Sung, Kyu Hwan Jung, Jung Hee Kim, Chan Soo Shin
- Endocrinol Metab. 2022;37(4):674-683. Published online August 5, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1461
- Funded: National Research Foundation of Korea
- 3,847 View
- 209 Download
- 12 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
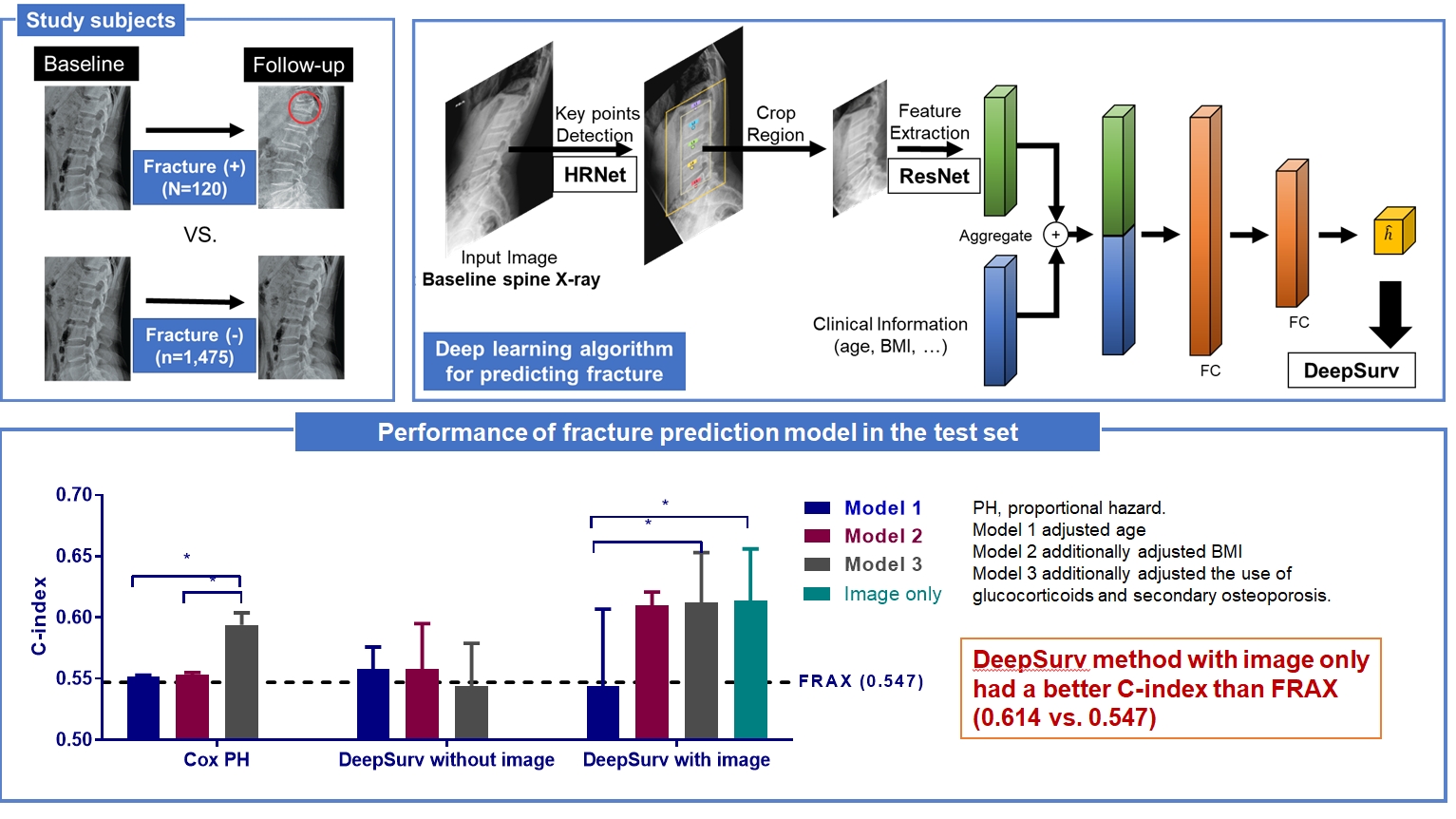
Since image-based fracture prediction models using deep learning are lacking, we aimed to develop an X-ray-based fracture prediction model using deep learning with longitudinal data.
Methods
This study included 1,595 participants aged 50 to 75 years with at least two lumbosacral radiographs without baseline fractures from 2010 to 2015 at Seoul National University Hospital. Positive and negative cases were defined according to whether vertebral fractures developed during follow-up. The cases were divided into training (n=1,416) and test (n=179) sets. A convolutional neural network (CNN)-based prediction algorithm, DeepSurv, was trained with images and baseline clinical information (age, sex, body mass index, glucocorticoid use, and secondary osteoporosis). The concordance index (C-index) was used to compare performance between DeepSurv and the Fracture Risk Assessment Tool (FRAX) and Cox proportional hazard (CoxPH) models.
Results
Of the total participants, 1,188 (74.4%) were women, and the mean age was 60.5 years. During a mean follow-up period of 40.7 months, vertebral fractures occurred in 7.5% (120/1,595) of participants. In the test set, when DeepSurv learned with images and clinical features, it showed higher performance than FRAX and CoxPH in terms of C-index values (DeepSurv, 0.612; 95% confidence interval [CI], 0.571 to 0.653; FRAX, 0.547; CoxPH, 0.594; 95% CI, 0.552 to 0.555). Notably, the DeepSurv method without clinical features had a higher C-index (0.614; 95% CI, 0.572 to 0.656) than that of FRAX in women.
Conclusion
DeepSurv, a CNN-based prediction algorithm using baseline image and clinical information, outperformed the FRAX and CoxPH models in predicting osteoporotic fracture from spine radiographs in a longitudinal cohort. -
Citations
Citations to this article as recorded by- Automated detection of vertebral fractures from X-ray images: A novel machine learning model and survey of the field
Li-Wei Cheng, Hsin-Hung Chou, Yu-Xuan Cai, Kuo-Yuan Huang, Chin-Chiang Hsieh, Po-Lun Chu, I-Szu Cheng, Sun-Yuan Hsieh
Neurocomputing.2024; 566: 126946. CrossRef - Application of radiomics model based on lumbar computed tomography in diagnosis of elderly osteoporosis
Baisen Chen, Jiaming Cui, Chaochen Li, Pengjun Xu, Guanhua Xu, Jiawei Jiang, Pengfei Xue, Yuyu Sun, Zhiming Cui
Journal of Orthopaedic Research.2024;[Epub] CrossRef - Machine Learning and Deep Learning in Spinal Injury: A Narrative Review of Algorithms in Diagnosis and Prognosis
Satoshi Maki, Takeo Furuya, Masahiro Inoue, Yasuhiro Shiga, Kazuhide Inage, Yawara Eguchi, Sumihisa Orita, Seiji Ohtori
Journal of Clinical Medicine.2024; 13(3): 705. CrossRef - A CT-based Deep Learning Model for Predicting Subsequent Fracture Risk in Patients with Hip Fracture
Yisak Kim, Young-Gon Kim, Jung-Wee Park, Byung Woo Kim, Youmin Shin, Sung Hye Kong, Jung Hee Kim, Young-Kyun Lee, Sang Wan Kim, Chan Soo Shin
Radiology.2024;[Epub] CrossRef - A Novel QCT-Based Deep Transfer Learning Approach for Predicting Stiffness Tensor of Trabecular Bone Cubes
Pengwei Xiao, Tinghe Zhang, Yufei Huang, Xiaodu Wang
IRBM.2024; 45(2): 100831. CrossRef - Development and Validation of a Convolutional Neural Network Model to Predict a Pathologic Fracture in the Proximal Femur Using Abdomen and Pelvis CT Images of Patients With Advanced Cancer
Min Wook Joo, Taehoon Ko, Min Seob Kim, Yong-Suk Lee, Seung Han Shin, Yang-Guk Chung, Hong Kwon Lee
Clinical Orthopaedics & Related Research.2023; 481(11): 2247. CrossRef - Automated Opportunistic Trabecular Volumetric Bone Mineral Density Extraction Outperforms Manual Measurements for the Prediction of Vertebral Fractures in Routine CT
Sophia S. Goller, Jon F. Rischewski, Thomas Liebig, Jens Ricke, Sebastian Siller, Vanessa F. Schmidt, Robert Stahl, Julian Kulozik, Thomas Baum, Jan S. Kirschke, Sarah C. Foreman, Alexandra S. Gersing
Diagnostics.2023; 13(12): 2119. CrossRef - Machine learning‐based prediction of osteoporosis in postmenopausal women with clinical examined features: A quantitative clinical study
Kainat A. Ullah, Faisal Rehman, Muhammad Anwar, Muhammad Faheem, Naveed Riaz
Health Science Reports.2023;[Epub] CrossRef - Skeletal Fracture Detection with Deep Learning: A Comprehensive Review
Zhihao Su, Afzan Adam, Mohammad Faidzul Nasrudin, Masri Ayob, Gauthamen Punganan
Diagnostics.2023; 13(20): 3245. CrossRef - Deep learning system for automated detection of posterior ligamentous complex injury in patients with thoracolumbar fracture on MRI
Sang Won Jo, Eun Kyung Khil, Kyoung Yeon Lee, Il Choi, Yu Sung Yoon, Jang Gyu Cha, Jae Hyeok Lee, Hyunggi Kim, Sun Yeop Lee
Scientific Reports.2023;[Epub] CrossRef - Vertebra Segmentation Based Vertebral Compression Fracture Determination from Reconstructed Spine X-Ray Images
Srinivasa Rao Gadu, Chandra Sekhar Potala
International Journal of Electrical and Electronics Research.2023; 11(4): 1225. CrossRef - Computer Vision in Osteoporotic Vertebral Fracture Risk Prediction: A Systematic Review
Anthony K. Allam, Adrish Anand, Alex R. Flores, Alexander E. Ropper
Neurospine.2023; 20(4): 1112. CrossRef - A Meaningful Journey to Predict Fractures with Deep Learning
Jeonghoon Ha
Endocrinology and Metabolism.2022; 37(4): 617. CrossRef - New Horizons: Artificial Intelligence Tools for Managing Osteoporosis
Hans Peter Dimai
The Journal of Clinical Endocrinology & Metabolism.2022;[Epub] CrossRef
- Automated detection of vertebral fractures from X-ray images: A novel machine learning model and survey of the field

- Thyroid
- Quality of Life of Survivors of Thyroid Cancer Is Not Inferior to That in Subjects without Cancer: Long-Term after Over 5 Years
- Jeongmin Lee, Youn-Ju Lee, Dong-Jun Lim, Jung-Min Lee, Sang-Ah Chang, Min-Hee Kim
- Endocrinol Metab. 2022;37(4):664-673. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1499
- Funded: Clinical Trials Center of Eunpyeong St. Mary’s Hospital, Catholic University of Korea

- 3,134 View
- 181 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Patients with thyroid cancer undergo less extensive surgery and additional therapies compared to those with other cancers. We aimed to compare the quality of life (QoL) between patients with thyroid cancer and healthy subjects using representative data from Korea. Differences in QoL of thyroid cancer survivors according to the duration after cancer diagnosis was also evaluated.
Methods
This population-based cohort study included 50,278 subjects who participated in the Korea National Health and Nutrition Examination Survey between 2007 and 2017. QoL was compared between patients with thyroid cancer and healthy subjects using self-reported data from the EuroQoL (EQ)-5 dimension (5D) and EQ-visual analog scale (VAS). Propensity score matching was used to match thyroid cancer survivors to healthy subjects (1:5 matching).
Results
Linear regression with univariate analysis showed that the presence of thyroid cancer was positively correlated with better EQ-5D index scores (β-coefficient=0.010, p=0.046). After adjusting for multiple covariables, statistical significance was maintained. EQ-VAS fails to demonstrate any significant correlation. Among the EQ-5D categories, patients with thyroid cancer showed better self-care than healthy subjects. Thyroid cancer duration did not correlate with the EQ-5D index score. In subgroup analyses, compared to patients with thyroid cancer duration of <5 years, no significant difference was observed in the correlation between the EQ-5D index score and survival duration in those with thyroid cancer duration of 5 to 9 years and ≥10 years.
Conclusion
Using a large-scale nationwide population-based database, our study demonstrated better QoL, especially in terms of self-care, among thyroid cancer survivors than among healthy subjects without cancer. -
Citations
Citations to this article as recorded by- Quality of Life Considerations in Patients Treated for Differentiated Thyroid Cancer
Jie Liu
Clinical Thyroidology.2023; 35(4): 160. CrossRef - The psychosocial impact of thyroid cancer
Parker Haymart, Nina Jackson Levin, Megan R. Haymart
Current Opinion in Endocrinology, Diabetes & Obesity.2023; 30(5): 252. CrossRef - Longitudinal Changes in Quality of Life Before and After Thyroidectomy in Patients With Differentiated Thyroid Cancer
Byung Hun Kim, Soo Rack Ryu, Jin Won Lee, Chang Myeon Song, Yong Bae Ji, Seok Hyun Cho, Seung Hwan Lee, Kyung Tae
The Journal of Clinical Endocrinology & Metabolism.2023;[Epub] CrossRef
- Quality of Life Considerations in Patients Treated for Differentiated Thyroid Cancer

- Diabetes, Obesity and Metabolism
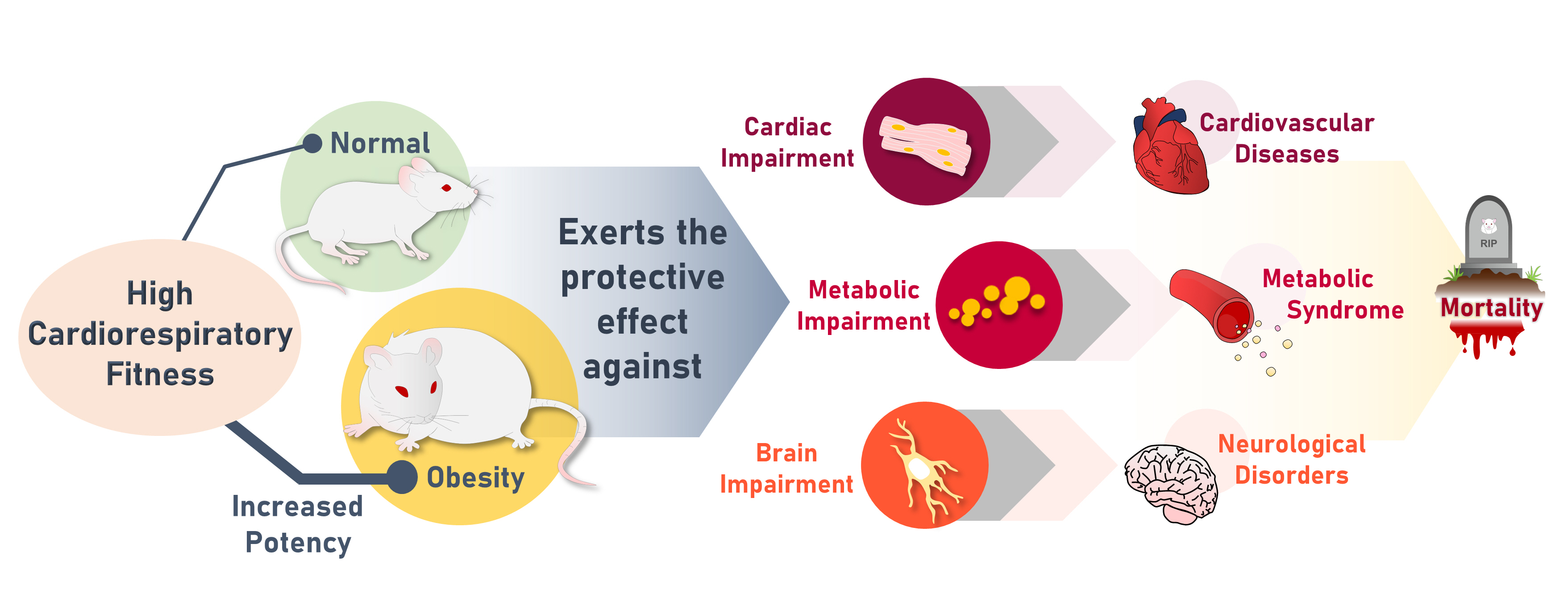
- High Cardiorespiratory Fitness Protects against Molecular Impairments of Metabolism, Heart, and Brain with Higher Efficacy in Obesity-Induced Premature Aging
- Patcharapong Pantiya, Chanisa Thonusin, Natticha Sumneang, Benjamin Ongnok, Titikorn Chunchai, Sasiwan Kerdphoo, Thidarat Jaiwongkam, Busarin Arunsak, Natthaphat Siri-Angkul, Sirawit Sriwichaiin, Nipon Chattipakorn, Siriporn C. Chattipakorn
- Endocrinol Metab. 2022;37(4):630-640. Published online August 5, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1430
- Funded: Faculty of Medicine Chiang Mai University Endowment Fund, National Research Council of Thailand, Chiang Mai University, National Research Council of Thailand, Royal Golden Jubilee Ph.D. program, National Science and Technology Development Agency, Chiang Mai University Center of Excellence Award, Thailand Science Research and Innovation
- 4,002 View
- 120 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
High cardiorespiratory fitness (CRF) protects against age-related diseases. However, the mechanisms mediating the protective effect of high intrinsic CRF against metabolic, cardiac, and brain impairments in non-obese versus obese conditions remain incompletely understood. We aimed to identify the mechanisms through which high intrinsic CRF protects against metabolic, cardiac, and brain impairments in non-obese versus obese untrained rats.
Methods
Seven-week-old male Wistar rats were divided into two groups (n=8 per group) to receive either a normal diet or a highfat diet (HFD). At weeks 12 and 28, CRF, carbohydrate and fatty acid oxidation, cardiac function, and metabolic parameters were evaluated. At week 28, behavior tests were performed. At the end of week 28, rats were euthanized to collect heart and brain samples for molecular studies.
Results
The obese rats exhibited higher values for aging-related parameters than the non-obese rats, indicating that they experienced obesity-induced premature aging. High baseline CRF levels were positively correlated with several favorable metabolic, cardiac, and brain parameters at follow-up. Specifically, the protective effects of high CRF against metabolic, cardiac, and brain impairments were mediated by the modulation of body weight and composition, the lipid profile, substrate oxidation, mitochondrial function, insulin signaling, autophagy, apoptosis, inflammation, oxidative stress, cardiac function, neurogenesis, blood-brain barrier, synaptic function, accumulation of Alzheimer’s disease-related proteins, and cognition. Interestingly, this effect was more obvious in HFD-fed rats.
Conclusion
The protective effect of high CRF is mediated by the modulation of several mechanisms. These effects exhibit greater efficacy under conditions of obesity-induced premature aging. -
Citations
Citations to this article as recorded by- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty
KAYLONI OLSON, DENISE K. HOUSTON, JOHNATHAN ROSS, RENA R. WING, FELICIA R. SIMPSON, AMBARISH PANDEY, MICHAEL P. WALKUP, MIA YANG, MARK A. ESPELAND
Medicine & Science in Sports & Exercise.2024; 56(4): 717. CrossRef - Interplay between obesity and aging on myocardial geometry and function: Role of leptin-STAT3-stress signaling
Wei Jin, Fei Tu, Feng Dong, Qinqin Deng, Miyesaier Abudureyimu, Wei Yu, Guo-jun Cai, Jian-ming Pei, Zhaohui Pei, Jun Ren
Biochimica et Biophysica Acta (BBA) - General Subjects.2023; 1867(2): 130281. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef
- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty

- Diabetes, Obesity and Metabolism
- The Effects of Irisin on the Interaction between Hepatic Stellate Cell and Macrophage in Liver Fibrosis
- Dinh Vinh Do, So Young Park, Giang Thi Nguyen, Dae Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2022;37(4):620-629. Published online July 22, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1412
- Funded: Kangwon National University

- 4,478 View
- 195 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Hepatic stellate cells (HSCs) are the central players interacting with multiple cell types in liver fibrosis. The crosstalk between HSCs and macrophages has recently become clearer. Irisin, an exercise-responsive myokine, was known to have a potentially protective role in liver and renal fibrosis, especially in connection with stellate cells. This study investigated the effects of irisin on the interaction between HSCs and macrophages.
Methods
Tamm-Horsfall protein-1 (THP-1) human monocytes were differentiated into macrophages, polarized into the inflammatory M1 phenotype with lipopolysaccharide. Lieming Xu-2 (LX-2) cells, human HSCs, were treated with conditioned media (CM) from M1 macrophages, with or without recombinant irisin. HSCs responses to CM from M1 macrophages were evaluated regarding activation, proliferation, wound healing, trans-well migration, contractility, and related signaling pathway.
Results
CM from M1 macrophages significantly promoted HSC proliferation, wound healing, transwell migration, and contractility, but not activation of HSCs. Irisin co-treatment attenuated these responses of HSCs to CM. However, CM and irisin treatment did not induce any changes in HSC activation. Further, irisin co-treatment alleviated CM-induced increase of phopho-protein kinase B (pAKT), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinases-1 (TIMP-1).
Conclusion
These findings suggested that irisin may play a protective role in the pathogenesis of liver fibrosis, especially when working in the crosstalk between HSCs and macrophages. -
Citations
Citations to this article as recorded by- Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis
Liang Shan, Fengling Wang, Dandan Zhai, Xiangyun Meng, Jianjun Liu, Xiongwen Lv
Biomedicine & Pharmacotherapy.2023; 161: 114472. CrossRef - Potential role of irisin in digestive system diseases
Yueming Zhang, Linxian Zhao, Huan Gao, Jinghui Zhai, Yanqing Song
Biomedicine & Pharmacotherapy.2023; 166: 115347. CrossRef - The effect of sarcopenia and serum myokines on prognosis and survival in cirrhotic patients: a multicenter cross-sectional study
Salih Boga, Abdullah Emre Yildirim, Enver Ucbilek, Ali Riza Koksal, Sevil Tokdemir Sisman, Ibrahim Durak, Ilker Sen, Beril Dogu, Erdinc Serin, Ayse Bolat Ucbilek, Makbule Ozge Yildirim, Sukru Mehmet Erturk, Huseyin Alkim, Canan Alkim
European Journal of Gastroenterology & Hepatology.2022; 34(12): 1261. CrossRef
- Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis

Review Articles
- Adrenal Gland
- Clinical and Technical Aspects in Free Cortisol Measurement
- Man Ho Choi
- Endocrinol Metab. 2022;37(4):599-607. Published online August 19, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1549
- Funded: Korea Health Industry Development Institute, Ministry of Health and Welfare

- 4,661 View
- 288 Download
- 9 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Accurate measurement of cortisol is critical in adrenal insufficiency as it reduces the risk associated with misdiagnosis and supports the optimization of stress dose. Comprehensive assays have been developed to determine the levels of bioactive free cortisol and their clinical and analytical efficacies have been extensively discussed because the level of total cortisol is affected by changes in the structure or circulating levels of corticoid-binding globulin and albumin, which are the main reservoirs of cortisol in the human body. Antibody-based immunoassays are routinely used in clinical laboratories; however, the lack of molecular specificity in cortisol assessment limits their applicability to characterize adrenocortical function. Improved specificity and sensitivity can be achieved by mass spectrometry coupled with chromatographic separation methods, which is a cutting-edge technology to measure individual as well as a panel of steroids in a single analytical run. The purpose of this review is to introduce recent advances in free cortisol measurement from the perspectives of clinical specimens and issues associated with prospective analytical technologies.
-
Citations
Citations to this article as recorded by- Highly Responsive Bioassay for Quantification of Glucocorticoids
Mathias Flensted Poulsen, Martin Overgaard, Christian Brix Folsted Andersen, Andreas Lodberg
Analytical Chemistry.2024; 96(5): 2000. CrossRef - An LC-MS/MS Method for the Simultaneous Quantification of Insulin, Cortisol, Glucagon-like Peptide 1, Ghrelin, and Osteocalcin
Zhichao Zhang, Hareem Siddiqi, Yu-Ping Huang, Shannon McClorry, Peng Ji, Daniela Barile, Carolyn M. Slupsky
Separations.2024; 11(2): 41. CrossRef - Determination of cortisol cut-off limits and steroid dynamics in the ACTH stimulation test: a comparative analysis using Roche Elecsys Cortisol II immunoassay and LC-MS/MS
Sema Okutan, Nanna Thurmann Jørgensen, Lars Engers Pedersen, Stina Willemoes Borresen, Linda Hilsted, Lennart Friis Hansen, Ulla Feldt-Rasmussen, Marianne Klose
Endocrine.2024;[Epub] CrossRef - Advancements in Cortisol Detection: From Conventional Methods to Next-Generation Technologies for Enhanced Hormone Monitoring
Visesh Vignesh, Bernardo Castro-Dominguez, Tony D. James, Julie M. Gamble-Turner, Stafford Lightman, Nuno M. Reis
ACS Sensors.2024;[Epub] CrossRef - Comparative analysis of salivary cortisol measurements using different assay methods in relation to serum-free cortisol measurement
Anna Lee, Sooah Jang, Sanghoo Lee, Hyun-Kyung Park, In-Young Kim, Ryunsup Ahn, Jeong-Ho Seok, Kyoung-Ryul Lee
Practical Laboratory Medicine.2024; 40: e00393. CrossRef - A dilute and shoot method for urinary free cortisol analysis by LC-MS/MS
Ying Shen, Xia Luo, Qing Guan, Liming Cheng
Journal of Chromatography B.2024; 1239: 124127. CrossRef - Osteopathic Manipulation as a Method of Cortisol Modification: A Systematic Review
Dylan Thibaut, Valentine Santarlas, Joseph Hoppes, Alejandra Vásquez-Castillo, Alexa Morrow, Eddie Oviedo, James Toldi
Cureus.2023;[Epub] CrossRef - Pitfalls in the Diagnosis and Management of Hypercortisolism (Cushing Syndrome) in Humans; A Review of the Laboratory Medicine Perspective
Kade C. Flowers, Kate E. Shipman
Diagnostics.2023; 13(8): 1415. CrossRef - Electrochemical sensors for cortisol detection: Principles, designs, fabrication, and characterisation
Gopi Karuppaiah, Min-Ho Lee, Shekhar Bhansali, Pandiaraj Manickam
Biosensors and Bioelectronics.2023; 239: 115600. CrossRef - The role of the hypothalamic-pituitary-adrenal axis in depression across the female reproductive lifecycle: current knowledge and future directions
Liisa Hantsoo, Kathleen M. Jagodnik, Andrew M. Novick, Ritika Baweja, Teresa Lanza di Scalea, Aysegul Ozerdem, Erin C. McGlade, Diana I. Simeonova, Sharon Dekel, Sara L. Kornfield, Michelle Nazareth, Sandra J. Weiss
Frontiers in Endocrinology.2023;[Epub] CrossRef - РІВЕНЬ СТРЕСУ В ДІТЕЙ ШКІЛЬНОГО ВІКУ З COVID-19
Г. А. Павлишин, О. І. Панченко
Здобутки клінічної і експериментальної медицини.2023; (4): 119. CrossRef - Corticotropin-stimulated steroid profiles to predict shock development and mortality in sepsis: From the HYPRESS study
Josef Briegel, Patrick Möhnle, Didier Keh, Johanna M. Lindner, Anna C. Vetter, Holger Bogatsch, Dorothea Lange, Sandra Frank, Ludwig C. Hinske, Djillali Annane, Michael Vogeser, Michael Bauer, Thorsten Brenner, Patrick Meybohm, Markus Weigand, Matthias Gr
Critical Care.2022;[Epub] CrossRef
- Highly Responsive Bioassay for Quantification of Glucocorticoids

- Adrenal Gland
- Long-Term Outcomes of Congenital Adrenal Hyperplasia
- Anna Nordenström, Svetlana Lajic, Henrik Falhammar
- Endocrinol Metab. 2022;37(4):587-598. Published online July 8, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1528
- Funded: Magnus Bergvall foundation, Stockholm County Council, Swedish Research Council, Region Stockholm, Karolinska Institutet, Stiftelsen Frimurare Barnhuset i Stockholm
- 30,194 View
- 274 Download
- 12 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - A plethora of negative long-term outcomes have been associated with congenital adrenal hyperplasia (CAH). The causes are multiple and involve supra-physiological gluco- and mineralocorticoid replacement, excess adrenal androgens both intrauterine and postnatal, elevated steroid precursor and adrenocorticotropic hormone levels, living with a congenital condition as well as the proximity of the cytochrome P450 family 21 subfamily A member 2 (CYP21A2) gene to other genes. This review aims to discuss the different long-term outcomes of CAH.
-
Citations
Citations to this article as recorded by- Increased Prevalence of Accidents and Injuries in Congenital Adrenal Hyperplasia: A Population-based Cohort Study
Henrik Falhammar, Angelica Lindén Hirschberg, Agneta Nordenskjöld, Henrik Larsson, Anna Nordenström
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1175. CrossRef - International Newborn Screening Practices for the Early Detection of Congenital Adrenal Hyperplasia
Tracey A. Conlon, Colin P. Hawkes, Jennifer J. Brady, J. Gerard Loeber, Nuala Murphy
Hormone Research in Paediatrics.2024; 97(2): 113. CrossRef - Low renin forms of monogenic hypertension: review of the evidence
Ugochi Chinenye Okorafor, Uchechi Chioma Okorafor
Journal of Clinical Medicine of Kazakhstan.2024; 21(1): 14. CrossRef - Increased risk of nephrolithiasis: an emerging issue in children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency
Mariangela Chiarito, Crescenza Lattanzio, Vito D’Ascanio, Donatella Capalbo, Paolo Cavarzere, Anna Grandone, Francesca Aiello, Giorgia Pepe, Malgorzata Wasniewska, Thomas Zoller, Mariacarolina Salerno, Maria Felicia Faienza
Endocrine.2024;[Epub] CrossRef - Congenital adrenal hyperplasia: New biomarkers and adult treatments
Bleuenn Dreves, Yves Reznik, Antoine Tabarin
Annales d'Endocrinologie.2023; 84(4): 472. CrossRef - Interpretation of Steroid Biomarkers in 21-Hydroxylase Deficiency and Their Use in Disease Management
Kyriakie Sarafoglou, Deborah P Merke, Nicole Reisch, Hedi Claahsen-van der Grinten, Henrik Falhammar, Richard J Auchus
The Journal of Clinical Endocrinology & Metabolism.2023; 108(9): 2154. CrossRef - Impact of Newborn Screening on Adult Height in Patients With Congenital Adrenal Hyperplasia (CAH)
Heike Hoyer-Kuhn, Alexander J Eckert, Gerhard Binder, Walter Bonfig, Angelika Dübbers, Stefan Riedl, Joachim Woelfle, Helmuth G Dörr, Reinhard W Holl
The Journal of Clinical Endocrinology & Metabolism.2023; 108(11): e1199. CrossRef - Specialty grand challenge in adrenal endocrinology
Henrik Falhammar
Frontiers in Endocrinology.2023;[Epub] CrossRef - Contexts of care for people with differences of sex development
Alexandra E. Kulle, Martina Jürgensen, Ulla Döhnert, Lisa Malich, Louise Marshall, Olaf Hiort
Medizinische Genetik.2023; 35(3): 181. CrossRef - Cardiovascular risk in Cuban adolescents and young adults with congenital adrenal hyperplasia
Tania M. Espinosa Reyes, Alba Katherine Pesántez Velepucha, Julio Oscar Cabrera Rego, Wendy Valdés Gómez, Emma Domínguez Alonso, Henrik Falhammar
BMC Endocrine Disorders.2023;[Epub] CrossRef - Landscape of Adrenal Tumours in Patients with Congenital Adrenal Hyperplasia
Mara Carsote, Ana-Maria Gheorghe, Claudiu Nistor, Alexandra-Ioana Trandafir, Oana-Claudia Sima, Anca-Pati Cucu, Adrian Ciuche, Eugenia Petrova, Adina Ghemigian
Biomedicines.2023; 11(11): 3081. CrossRef - Editorial: Recent advances in diagnosis and treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency
Semra Çaglar Çetinkaya
Frontiers in Endocrinology.2023;[Epub] CrossRef - Approach of Heterogeneous Spectrum Involving 3beta-Hydroxysteroid Dehydrogenase 2 Deficiency
Andreea Gabriela Nicola, Mara Carsote, Ana-Maria Gheorghe, Eugenia Petrova, Alexandru Dan Popescu, Adela Nicoleta Staicu, Mihaela Jana Țuculină, Cristian Petcu, Ionela Teodora Dascălu, Tiberiu Tircă
Diagnostics.2022; 12(9): 2168. CrossRef - Effetti di Crinecerfont sulla secrezione di ACTH nell’iperplasia surrenalica congenita: uno studio di fase 2
Marianna Rita Stancampiano, Silvia Laura Carla Meroni, Giovanna Weber, Gianni Russo
L'Endocrinologo.2022; 23(6): 662. CrossRef
- Increased Prevalence of Accidents and Injuries in Congenital Adrenal Hyperplasia: A Population-based Cohort Study

- Diabetes, Obesity and Metabolism
- Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
- Joon Ho Moon, Kyuho Kim, Sung Hee Choi
- Endocrinol Metab. 2022;37(4):575-586. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.402
- Funded: National Research Foundation of Korea

- 7,737 View
- 436 Download
- 11 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
-
Citations
Citations to this article as recorded by- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the obesity medicine association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; : 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef
- The chylomicron saga: time to focus on postprandial metabolism


 KES
KES

 First
First Prev
Prev



