Most cited
- Page Path
- HOME > BROWSE ARTICLES > Most cited
From articles published in Endocrinology and Metabolism during the past two years (2022 ~ ).
Original Articles
- Hypothalamus and Pituitary Gland
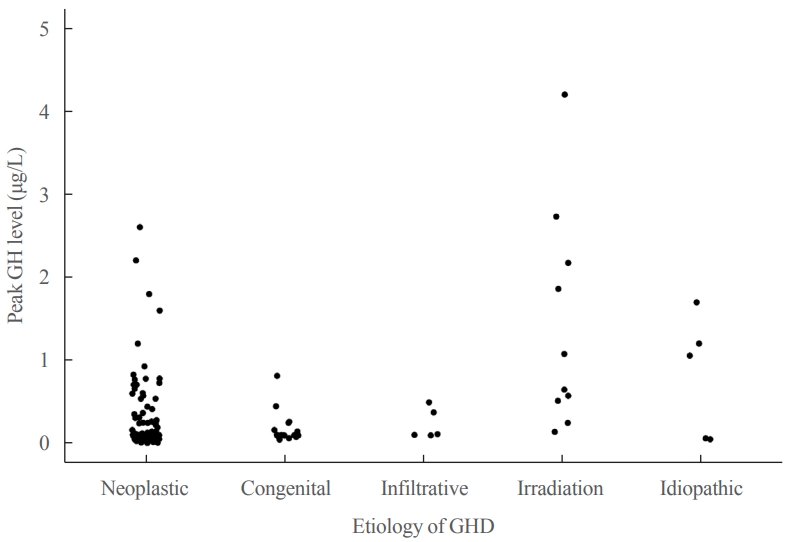
- Metabolic Impacts of Discontinuation and Resumption of Recombinant Human Growth Hormone Treatment during the Transition Period in Patients with Childhood-Onset Growth Hormone Deficiency
- Yun Jeong Lee, Yunha Choi, Han-Wook Yoo, Young Ah Lee, Choong Ho Shin, Han Saem Choi, Ho-Seong Kim, Jae Hyun Kim, Jung Eun Moon, Cheol Woo Ko, Moon Bae Ahn, Byung-Kyu Suh, Jin-Ho Choi
- Endocrinol Metab. 2022;37(2):359-368. Published online April 25, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1384
- 4,350 View
- 180 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Discontinuing growth hormone (GH) treatment during the transition to adulthood has been associated with adverse health outcomes in patients with childhood-onset growth hormone deficiency (CO-GHD). This study investigated the metabolic changes associated with interrupting GH treatment in adolescents with CO-GHD during the transition period.
Methods
This study included 187 patients with CO-GHD who were confirmed to have adult GHD and were treated at six academic centers in Korea. Data on clinical parameters, including anthropometric measurements, metabolic profiles, and bone mineral density (BMD) at the end of childhood GH treatment, were collected at the time of re-evaluation for GHD and 1 year after treatment resumption.
Results
Most patients (n=182, 97.3%) had organic GHD. The median age at treatment discontinuation and re-evaluation was 15.6 and 18.7 years, respectively. The median duration of treatment interruption was 2.8 years. During treatment discontinuation, body mass index Z-scores and total cholesterol, low-density lipoprotein, and non-high-density lipoprotein (HDL) cholesterol levels increased, whereas fasting glucose levels decreased. One year after GH treatment resumption, fasting glucose levels, HDL cholesterol levels, and femoral neck BMD increased significantly. Longer GH interruption (>2 years, 60.4%) resulted in worse lipid profiles at re-evaluation. The duration of interruption was positively correlated with fasting glucose and non-HDL cholesterol levels after adjusting for covariates.
Conclusion
GH treatment interruption during the transition period resulted in worse metabolic parameters, and a longer interruption period was correlated with poorer outcomes. GH treatment should be resumed early in patients with CO-GHD during the transition period. -
Citations
Citations to this article as recorded by- Ghrelin regulating liver activity and its potential effects on liver fibrosis and Echinococcosis
Jiang Zhu, Tanfang Zhou, Meng Menggen, Kalibixiati Aimulajiang, Hao Wen
Frontiers in Cellular and Infection Microbiology.2024;[Epub] CrossRef - Relationship between the Stimulated Peak Growth Hormone Level and Metabolic Parameters in Children with Growth Hormone Deficiency
Seong Yong Lee
The Ewha Medical Journal.2023;[Epub] CrossRef - Dyslipidaemia and growth hormone deficiency – A comprehensive review
Matthias Hepprich, Fahim Ebrahimi, Emanuel Christ
Best Practice & Research Clinical Endocrinology & Metabolism.2023; 37(6): 101821. CrossRef
- Ghrelin regulating liver activity and its potential effects on liver fibrosis and Echinococcosis

- Diabetes, Obesity and Metabolism
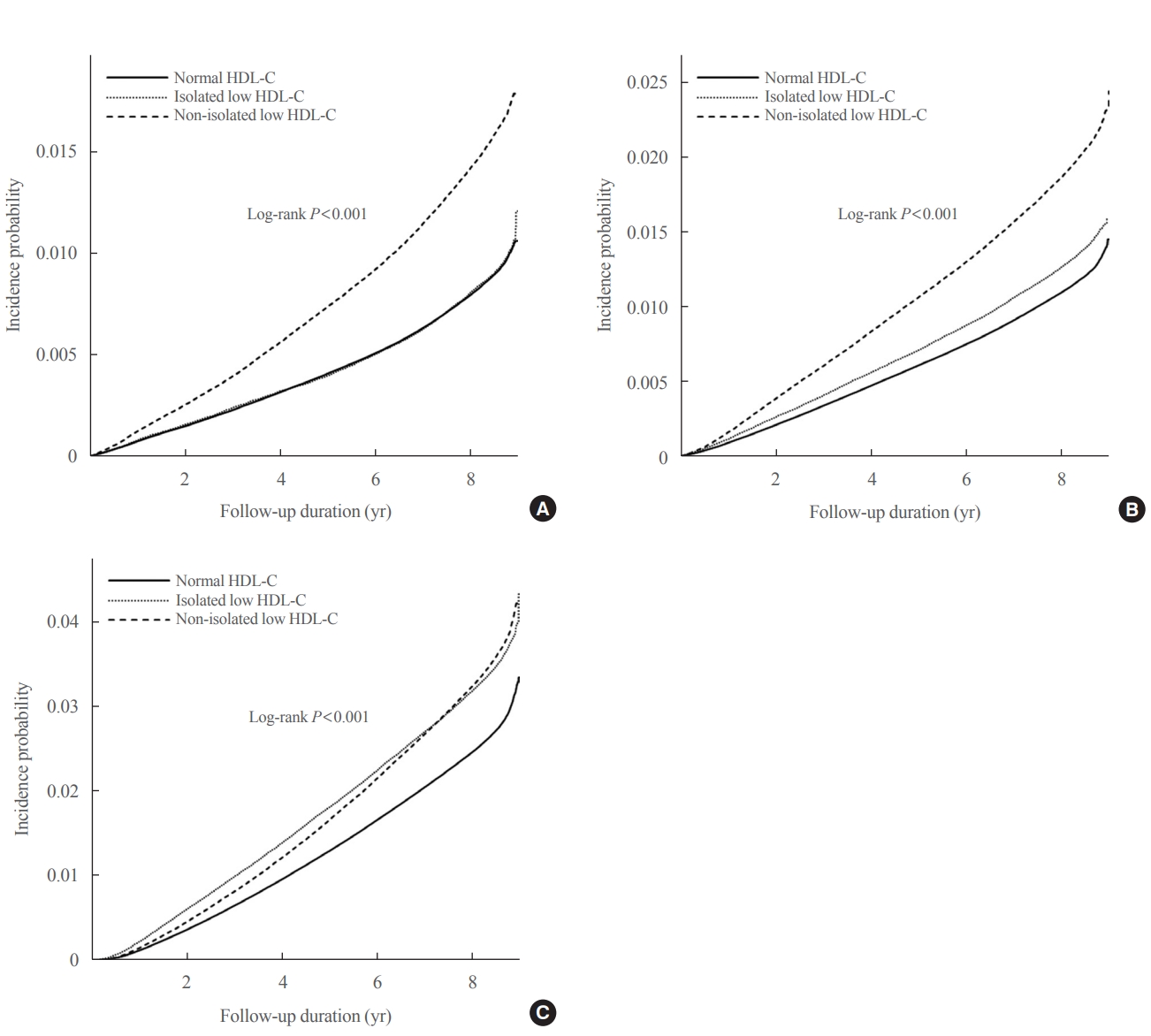
Big Data Articles (National Health Insurance Service Database) - Association of High-Density Lipoprotein Cholesterol Phenotypes with the Risk of Cardiovascular Diseases and Mortality: A Cohort Study in Korea
- Ga Eun Nam, Youn Huh, Jin-Hyung Jung, Kyungdo Han, Seon Mee Kim, on Behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity
- Endocrinol Metab. 2022;37(2):261-271. Published online April 25, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1259
- 3,470 View
- 139 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated whether low high-density lipoprotein cholesterol (HDL-C) and isolated and non-isolated low HDL-C levels are associated with the risk of cardiovascular diseases and all-cause mortality among Korean adults.
Methods
We included 8,665,841 individuals aged ≥20 years who had undergone a health examination provided by the Korean National Health Insurance Service (NHIS) in 2009 and were followed up until the end of 2018. The hazard ratios (HRs) and 95% confidence intervals (CIs) for study outcomes were calculated using multivariable Cox proportional hazard regression analysis.
Results
During the 8.2 years of mean follow-up, myocardial infarction (MI), stroke, and all-cause mortality occurred in 81,431, 110,996, and 244,309 individuals, respectively. After adjusting for confounding variables (model 3), individuals with low HDL-C and lower HDL quartiles were associated with significantly increased risks of all three outcomes, compared to those with normal HDL-C and highest HDL-C quartile (all P<0.001), respectively. HRs for incident MI (1.28; 95% CI, 1.26 to 1.30), stroke (1.13; 95% CI, 1.11 to 1.15), and all-cause mortality (1.07; 95% CI, 1.05 to 1.08) increased in the non-isolated low HDL-C group compared to the normal HDL-C group. Isolated low HDL-C also showed an increase in the HRs of incident stroke (1.06; 95% CI, 1.04 to 1.08) and all-cause mortality (1.30; 95% CI, 1.28 to 1.32).
Conclusion
Low HDL-C and non-isolated low HDL-C were associated with increased risk of MI, stroke, and all-cause mortality, and isolated low HDL-C was associated with incident stroke and all-cause mortality risk. -
Citations
Citations to this article as recorded by- Association between HDL levels and stroke outcomes in the Arab population
Aizaz Ali, Omar Obaid, Naveed Akhtar, Rahul Rao, Syed Haroon Tora, Ashfaq Shuaib
Scientific Reports.2024;[Epub] CrossRef - Association of adiposity and fitness with triglyceride-to-high-density lipoprotein cholesterol ratio in youth
Danladi Ibrahim Musa, Abel Lamina Toriola, Nurudeen O Abubakar, Sunday Omachi, Victor B Olowoleni, Kolade B Ayodele
Annals of Pediatric Cardiology.2023; 16(3): 194. CrossRef - Association between cholesterol levels and dementia risk according to the presence of diabetes and statin use: a nationwide cohort study
You-Bin Lee, Min Young Kim, Kyungdo Han, Bongsung Kim, Jiyun Park, Gyuri Kim, Kyu Yeon Hur, Jae Hyeon Kim, Sang-Man Jin
Scientific Reports.2022;[Epub] CrossRef
- Association between HDL levels and stroke outcomes in the Arab population

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Graves’ Disease and the Risk of End-Stage Renal Disease: A Korean Population-Based Study
- Yoon Young Cho, Bongseong Kim, Dong Wook Shin, Hye Ryoun Jang, Bo-Yeon Kim, Chan-Hee Jung, Jae Hyeon Kim, Sun Wook Kim, Jae Hoon Chung, Kyungdo Han, Tae Hyuk Kim
- Endocrinol Metab. 2022;37(2):281-289. Published online April 6, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1333

- 3,844 View
- 133 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Hyperthyroidism is associated with an increased glomerular filtration rate (GFR) in the hyperdynamic state, which is reversible after restoring euthyroidism. However, long-term follow-up of renal dysfunction in patients with hyperthyroidism has not been performed.
Methods
This was a retrospective cohort study using the Korean National Health Insurance database and biannual health checkup data. We included 41,778 Graves’ disease (GD) patients and 41,778 healthy controls, matched by age and sex. The incidences of end-stage renal disease (ESRD) were calculated in GD patients and controls. The cumulative dose and duration of antithyroid drugs (ATDs) were calculated for each patient and categorized into the highest, middle, and lowest tertiles.
Results
Among 41,778 GD patients, 55 ESRD cases occurred during 268,552 person-years of follow-up. Relative to the controls, regardless of smoking, drinking, or comorbidities, including chronic kidney disease, GD patients had a 47% lower risk of developing ESRD (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.37 to 0.76). In particular, GD patients with a higher baseline GFR (≥90 mL/min/1.73 m2; HR, 0.33; 95% CI, 0.11 to 0.99), longer treatment duration (>33 months; HR, 0.31; 95% CI, 0.17 to 0.58) or higher cumulative dose (>16,463 mg; HR, 0.29; 95% CI, 0.15 to 0.57) of ATDs had a significantly reduced risk of ESRD.
Conclusion
This was the first epidemiological study on the effect of GD on ESRD, and we demonstrated that GD population had a reduced risk for developing ESRD. -
Citations
Citations to this article as recorded by- Renal function changes in patients with subclinical hyperthyroidism: a novel postulated mechanism
Magdy Mohamed Allam, Hanaa Tarek El-Zawawy, Tarek Hussein El-Zawawy
Endocrine.2023; 82(1): 78. CrossRef - Effect of Hyperthyroidism on Preventing Renal Insufficiency
Tae Yong Kim
Endocrinology and Metabolism.2022; 37(2): 220. CrossRef - Effects and Clinical Value of Peritoneal Dialysis on Water and Water Balance, Adverse Reactions, Quality of Life, and Clinical Prognosis in Patients with Decompensated Chronic Nephropathy: A Systematic Review and Meta-Analysis
Xichao Wang, Miaomiao Zhang, Na Sun, Wenxiu Chang, Gang Chen
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef
- Renal function changes in patients with subclinical hyperthyroidism: a novel postulated mechanism

Review Article
- Diabetes, Obesity and Metabolism
- State-of-the-Art Overview of the Pharmacological Treatment of Non-Alcoholic Steatohepatitis
- Yongin Cho, Yong-ho Lee
- Endocrinol Metab. 2022;37(1):38-52. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.102
- 4,230 View
- 282 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide, and non-alcoholic steatohepatitis (NASH), a subtype of NAFLD, can progress to cirrhosis, hepatocellular carcinoma, and death. Nevertheless, the current treatment for NAFLD/NASH is limited to lifestyle modifications, and no drugs are currently officially approved as treatments for NASH. Many global pharmaceutical companies are pursuing the development of medications for the treatment of NASH, and results from phase 2 and 3 clinical trials have been published in recent years. Here, we review data from these recent clinical trials and reports on the efficacy of newly developed antidiabetic drugs in NASH treatment.
-
Citations
Citations to this article as recorded by- Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Soyeon Shin, Jaeyoung Kim, Ju Yeon Lee, Jun Kim, Chang-Myung Oh
Journal of Obesity & Metabolic Syndrome.2023; 32(4): 289. CrossRef - Sodium-glucose cotransporter 2 inhibitors for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: A nationwide propensity-score matched cohort study
Jinyoung Kim, Kyungdo Han, Bongsung Kim, Ki-Hyun Baek, Ki-Ho Song, Mee Kyoung Kim, Hyuk-Sang Kwon
Diabetes Research and Clinical Practice.2022; 194: 110187. CrossRef
- Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study

Original Articles
- Diabetes, Obesity and Metabolism
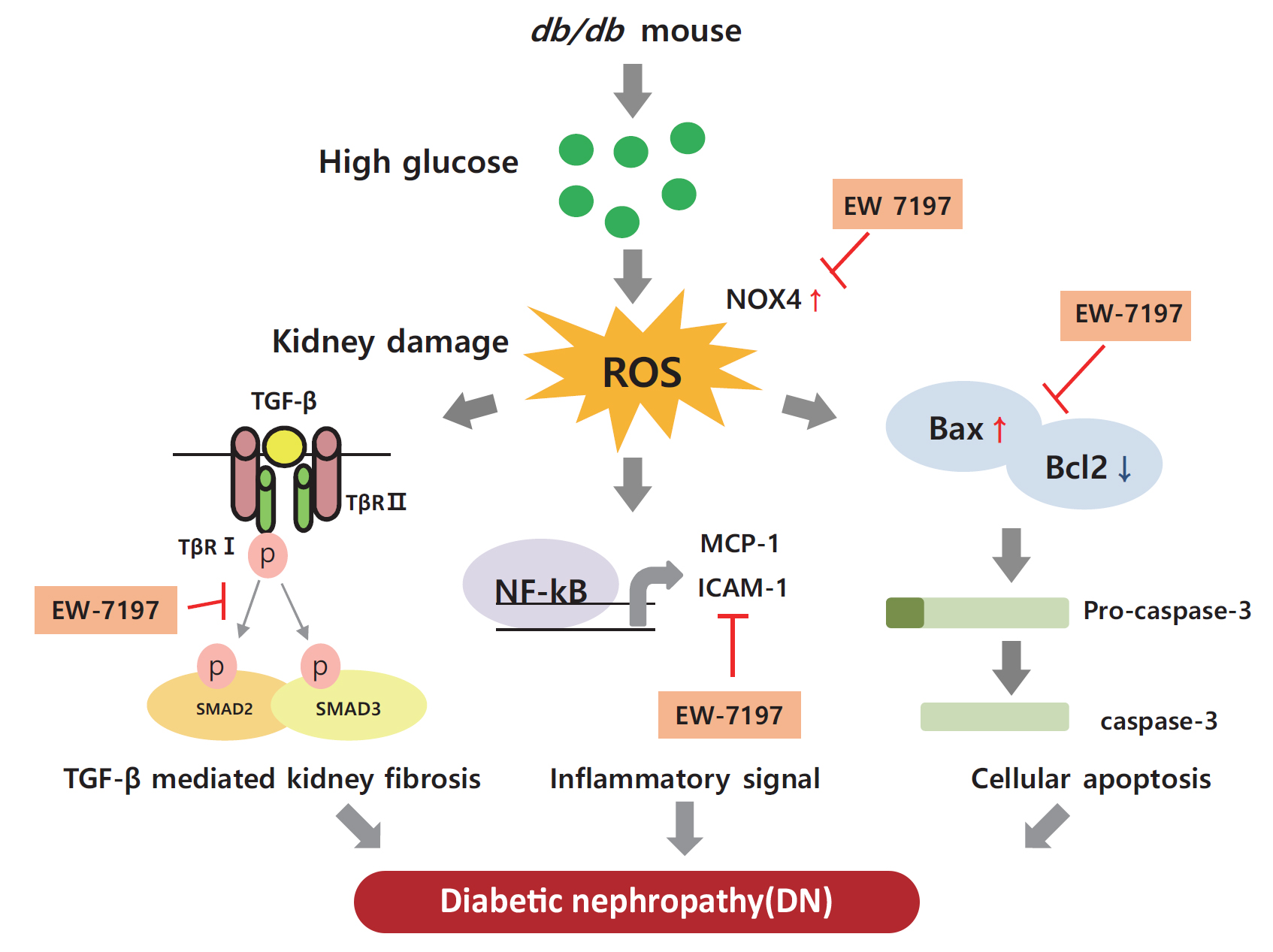
- EW-7197 Attenuates the Progression of Diabetic Nephropathy in db/db Mice through Suppression of Fibrogenesis and Inflammation
- Kyung Bong Ha, Weerapon Sangartit, Ah Reum Jeong, Eun Soo Lee, Hong Min Kim, Soyeon Shim, Upa Kukongviriyapan, Dae-Kee Kim, Eun Young Lee, Choon Hee Chung
- Endocrinol Metab. 2022;37(1):96-111. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1305
- 3,935 View
- 179 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Diabetic nephropathy (DN) is characterized by albuminuria and accumulation of extracellular matrix (ECM) in kidney. Transforming growth factor-β (TGF-β) plays a central role in promoting ECM accumulation. We aimed to examine the effects of EW-7197, an inhibitor of TGF-β type 1 receptor kinase (ALK5), in retarding the progression of DN, both in vivo, using a diabetic mouse model (db/db mice), and in vitro, in podocytes and mesangial cells.
Methods
In vivo study: 8-week-old db/db mice were orally administered EW-7197 at a dose of 5 or 20 mg/kg/day for 10 weeks. Metabolic parameters and renal function were monitored. Glomerular histomorphology and renal protein expression were evaluated by histochemical staining and Western blot analyses, respectively. In vitro study: DN was induced by high glucose (30 mM) in podocytes and TGF-β (2 ng/mL) in mesangial cells. Cells were treated with EW-7197 (500 nM) for 24 hours and the mechanism associated with the attenuation of DN was investigated.
Results
Enhanced albuminuria and glomerular morphohistological changes were observed in db/db compared to that of the nondiabetic (db/m) mice. These alterations were associated with the activation of the TGF-β signaling pathway. Treatment with EW-7197 significantly inhibited TGF-β signaling, inflammation, apoptosis, reactive oxygen species, and endoplasmic reticulum stress in diabetic mice and renal cells.
Conclusion
EW-7197 exhibits renoprotective effect in DN. EW-7197 alleviates renal fibrosis and inflammation in diabetes by inhibiting downstream TGF-β signaling, thereby retarding the progression of DN. Our study supports EW-7197 as a therapeutically beneficial compound to treat DN. -
Citations
Citations to this article as recorded by- TGF-β signaling in health, disease, and therapeutics
Ziqin Deng, Tao Fan, Chu Xiao, He Tian, Yujia Zheng, Chunxiang Li, Jie He
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols
Qi Jin, Tongtong Liu, Yuan Qiao, Donghai Liu, Liping Yang, Huimin Mao, Fang Ma, Yuyang Wang, Liang Peng, Yongli Zhan
Frontiers in Immunology.2023;[Epub] CrossRef - Beneficial Effects of a Curcumin Derivative and Transforming Growth Factor-β Receptor I Inhibitor Combination on Nonalcoholic Steatohepatitis
Kyung Bong Ha, Eun Soo Lee, Na Won Park, Su Ho Jo, Soyeon Shim, Dae-Kee Kim, Chan Mug Ahn, Choon Hee Chung
Diabetes & Metabolism Journal.2023; 47(4): 500. CrossRef
- TGF-β signaling in health, disease, and therapeutics

- Diabetes, Obesity and Metabolism
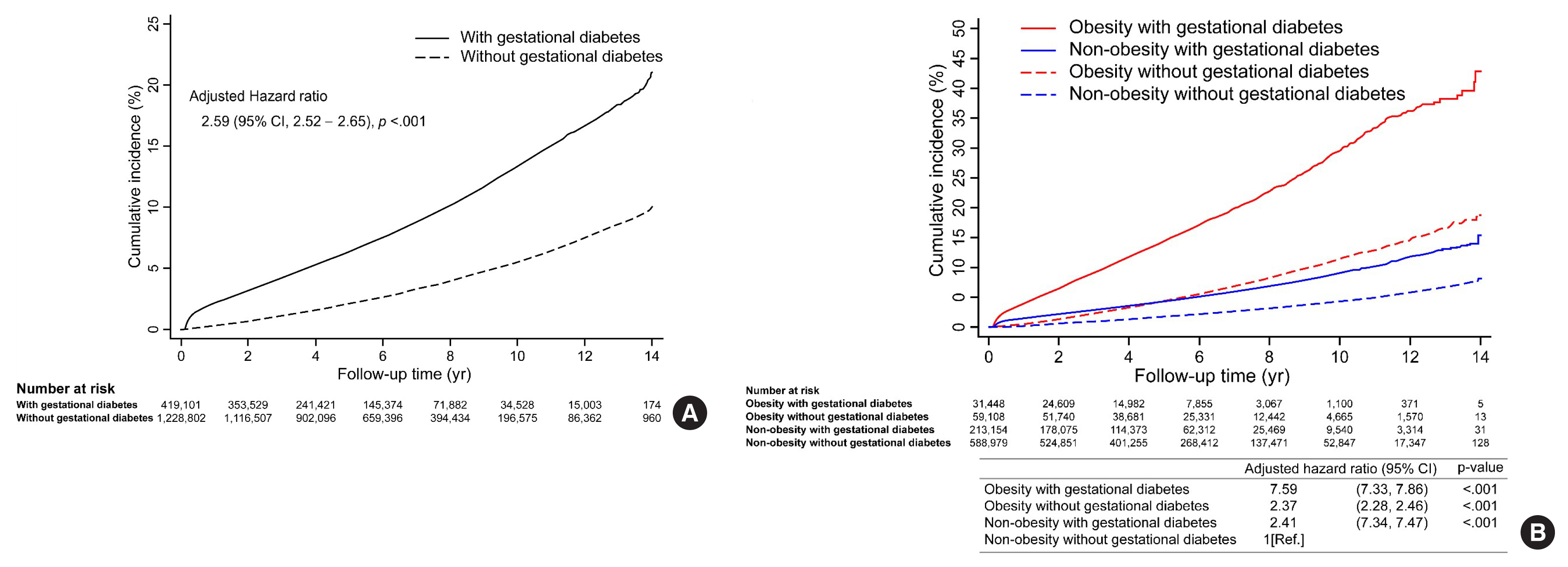
Big Data Articles (National Health Insurance Service Database) - Risk and Risk Factors for Postpartum Type 2 Diabetes Mellitus in Women with Gestational Diabetes: A Korean Nationwide Cohort Study
- Mi Jin Choi, Jimi Choi, Chae Weon Chung
- Endocrinol Metab. 2022;37(1):112-123. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1276
- 4,050 View
- 171 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There are differences in risk and risk factor findings of postpartum type 2 diabetes mellitus (T2DM) after gestational diabetes depending on study design and subjects of previous studies. This study aimed to assess these risk and risk factors more accurately through a population-based study to provide basic data for prevention strategies.
Methods
This open retrospective cohort included data of 419,101 women with gestational diabetes and matched 1,228,802 control women who delivered between 2004 and 2016 from the South Korea National Health Information Database of the National Health Insurance Service. Following 14 (median 5.9) years of follow-up, the incidence and hazard ratio (HR) of postpartum T2DM were evaluated using Kaplan-Meier curves and Cox proportional regression models.
Results
The incidence and HR of postpartum T2DM in women with gestational diabetes (compared to women without gestational diabetes) after the 14-year follow-up was 21.3% and 2.78 (95% confidence interval [CI], 2.74 to 2.82), respectively. Comorbid obesity (body mass index [BMI] ≥25 kg/m2) increased postpartum T2DM risk 7.59 times (95% CI, 7.33 to 7.86). Significant risk factors for postpartum T2DM were fasting glucose level, BMI, age, family history of diabetes, hypertension, and insulin use during pregnancy.
Conclusion
This population-based study showed higher postpartum T2DM risk in women with gestational diabetes than in those without, which was further increased by comorbid obesity. BMI and fasting glucose level were important postpartum risk factors. The management of obesity and glycemic control may be important strategies to prevent the incidence of diabetes after delivery. -
Citations
Citations to this article as recorded by- Antenatal factors and risk of postpartum hyperglycemia in women with gestational diabetes mellitus: A central India prospective cohort study
Nilajkumar Bagde, Madhuri Bagde, Vijayalakshmi Shanbhag, Pragati Trigunait, Nagma Sheikh, Sarita Agrawal
Journal of Family Medicine and Primary Care.2024; 13(1): 59. CrossRef - Integration of nutrigenomics, melatonin, serotonin and inflammatory cytokines in the pathophysiology of pregnancy-specific urinary incontinence in women with gestational diabetes mellitus
Danielle Cristina Honorio França, Eduardo Luzía França, Luis Sobrevia, Angélica Mércia Pascon Barbosa, Adenilda Cristina Honorio-França, Marilza Vieira Cunha Rudge
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2023; 1869(6): 166737. CrossRef - Risk factors associated with early postpartum glucose intolerance in women with a history of gestational diabetes mellitus: a systematic review and meta-analysis
Zhe Liu, Qianghuizi Zhang, Leyang Liu, Weiwei Liu
Endocrine.2023; 82(3): 498. CrossRef
- Antenatal factors and risk of postpartum hyperglycemia in women with gestational diabetes mellitus: A central India prospective cohort study

- Thyroid
- Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
- Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
- Endocrinol Metab. 2023;38(5):588-595. Published online September 8, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1723

- 1,416 View
- 85 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid cancer mortality has been largely overlooked as relatively stable given the large gap between thyroid cancer incidence and mortality. This study evaluated long-term trends in age-standardized mortality rates (ASMRs) throughout Korea and compared them with mortality data reported by the Surveillance, Epidemiology, and End Results (SEER).
Methods
Cancer-specific mortality data from 1985 to 2020 were obtained from Statistics Korea. ASMRs from thyroid cancer were calculated based on the Korean mid-year resident registration population of 2005. We assessed SEER*Explorer and downloaded the mortality data.
Results
The ASMR increased from 0.19 to 0.77/100,000 between 1985 and 2002 but decreased continuously to 0.36/100,000 in 2020. The annual percent change (APC) in the ASMR between 1985 and 2003 and between 2003 and 2020 was 6.204 and −4.218, respectively, with similar patterns observed in both men and women. The ASMR of the SEER showed a modest increase from 1988 to 2016 and then stabilized. In subgroup analysis, the ASMR of the old age group (≥55 years) increased significantly from 0.82 in 1985 to 3.92/100,000 in 2002 (APC 6.917) but then decreased again to 1.86/100,000 in 2020 (APC −4.136). ASMRs according to the age group in the SEER showed a relatively stable trend even in the elderly group.
Conclusion
The ASMR of thyroid cancer in Korea had increased from 1985 to 2002 but has since been steadily decreasing. This trend was mainly attributed to elderly people aged 55 or over. The absolute APC value of Korea was much higher than that of the SEER. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef - A Clinical Audit of Thyroid Hormonal Replacement After Total Thyroidectomy
Islam Mansy, Abdelfatah M Elsenosy, Eslam M Hassan, Mujtaba Zakria
Cureus.2023;[Epub] CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

- Thyroid
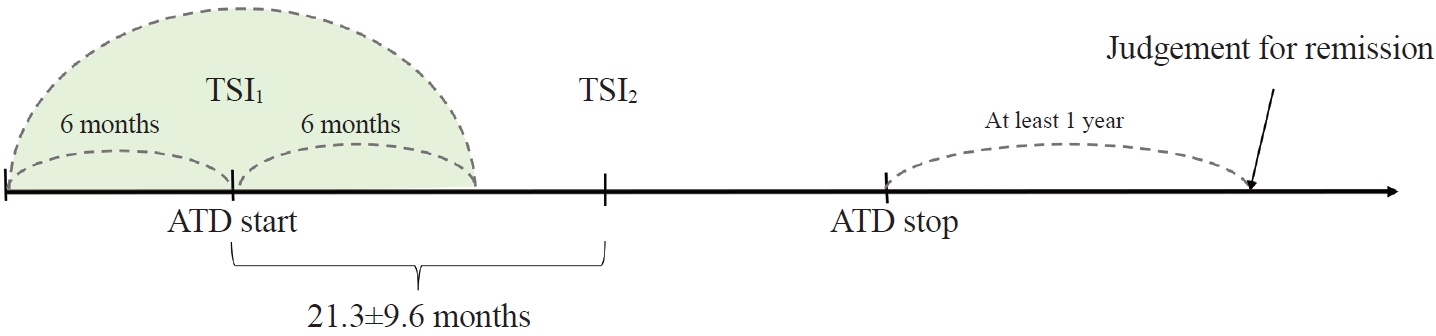
- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
- Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
- Endocrinol Metab. 2023;38(3):338-346. Published online June 9, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1664
- 1,688 View
- 100 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To determine whether baseline thyroid-stimulating immunoglobulin (TSI) bioassay or its early response upon treatment with an anti-thyroid drug (ATD) can predict prognosis of Graves’ disease (GD) in real-world practice.
Methods
This retrospective study enrolled GD patients who had previous ATD treatment with TSI bioassay checked at baseline and at follow-up from April 2010 to November 2019 in one referral hospital. The study population were divided into two groups: patients who experienced relapse or continued ATD (relapse/persistence), and patients who experienced no relapse after ATD discontinuation (remission). The slope and area under the curve at 1st year (AUC1yr) of thyroid-stimulating hormone receptor antibodies including TSI bioassay and thyrotropin-binding inhibitory immunoglobulin (TBII) were calculated as differences between baseline and second values divided by time duration (year).
Results
Among enrolled 156 study subjects, 74 (47.4%) had relapse/persistence. Baseline TSI bioassay values did not show significant differences between the two groups. However, the relapse/persistence group showed less decremental TSI bioassay in response to ATD than the remission group (–84.7 [TSI slope, –198.2 to 8.2] vs. –120.1 [TSI slope, –204.4 to –45.9], P=0.026), whereas the TBII slope was not significantly different between the two groups. The relapse/persistence group showed higher AUC1yr of TSI bioassay and TBII in the 1st year during ATD treatment than the remission group (AUC1yr for TSI bioassay, P=0.0125; AUC1yr for TBII,P =0.001).
Conclusion
Early changes in TSI bioassay can better predict prognosis of GD than TBII. Measurement of TSI bioassay at beginning and follow-up could help predict GD prognosis. -
Citations
Citations to this article as recorded by- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Long-term effect of thyrotropin-binding inhibitor immunoglobulin on atrial fibrillation in euthyroid patients
Jung-Chi Hsu, Kang-Chih Fan, Ting-Chuan Wang, Shu-Lin Chuang, Ying-Ting Chao, Ting-Tse Lin, Kuan-Chih Huang, Lian-Yu Lin, Lung-Chun Lin
Endocrine Practice.2024;[Epub] CrossRef
- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study

- Diabetes, obesity and metabolism
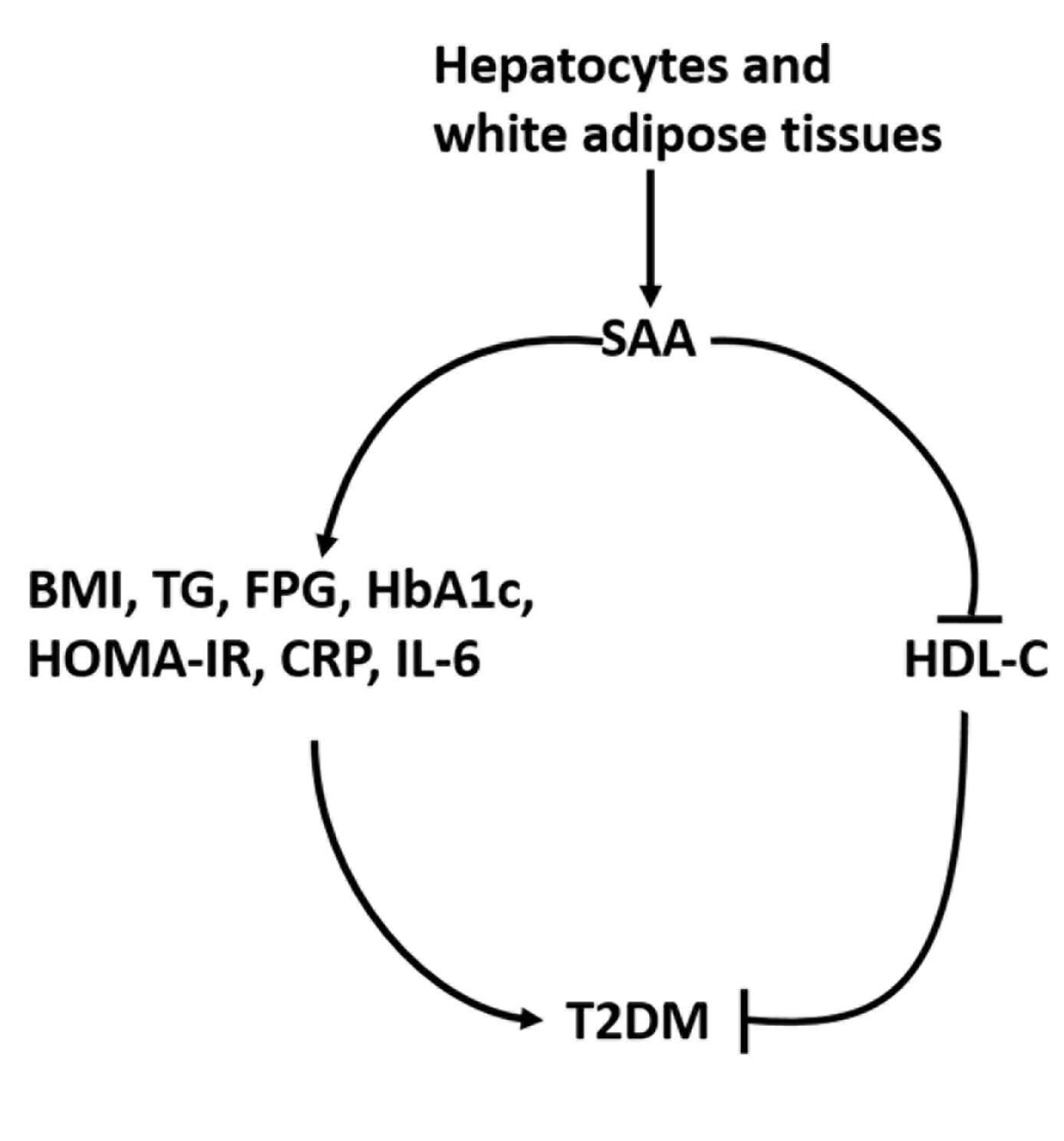
- Association between Serum Amyloid A Levels and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- Ting Liu, Meng Li, Chunying Cui, Jielin Zhou
- Endocrinol Metab. 2023;38(3):315-327. Published online June 7, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1621
- 2,092 View
- 103 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
To date, consistent data have not been reported on the association between serum amyloid A (SAA) levels and type 2 diabetes mellitus (T2DM). The purpose of this study was to systematically summarize their relationship.
Methods
Databases including PubMed, Cochrane Library, Embase, Web of Science, and MEDLINE were searched until August 2021. Cross-sectional and case-control studies were included.
Results
Twenty-one studies with 1,780 cases and 2,070 controls were identified. SAA levels were significantly higher in T2DM patients than in healthy groups (standardized mean difference [SMD], 0.68; 95% confidence interval [CI], 0.39 to 0.98). A subgroup analysis showed that the mean age of participants and the continent that participants were from were related to differences in SAA levels between cases and controls. Furthermore, in T2DM patients, SAA levels were positively associated with body mass index (r=0.34; 95% CI, 0.03 to 0.66), triglycerides (r=0.12; 95% CI, 0.01 to 0.24), fasting plasma glucose (r=0.26; 95% CI, 0.07 to 0.45), hemoglobin A1c (r=0.24; 95% CI, 0.16 to 0.33), homeostasis model assessment for insulin resistance (r=0.22; 95% CI, 0.10 to 0.34), C-reactive protein (r=0.77; 95% CI, 0.62 to 0.91), and interleukin-6 (r=0.42; 95% CI, 0.31 to 0.54), but negatively linked with highdensity lipoprotein cholesterol (r=–0.23; 95% CI, –0.44 to –0.03).
Conclusion
The meta-analysis suggests that high SAA levels may be associated with the presence of T2DM, as well as lipid metabolism homeostasis and the inflammatory response. -
Citations
Citations to this article as recorded by- Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients
Yuanyuan Zhang, Huaizhen Liu
BMC Endocrine Disorders.2024;[Epub] CrossRef - Antioxidant and Anti-Inflammatory Functions of High-Density Lipoprotein in Type 1 and Type 2 Diabetes
Damien Denimal
Antioxidants.2023; 13(1): 57. CrossRef
- Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients

Review Article
- Thyroid
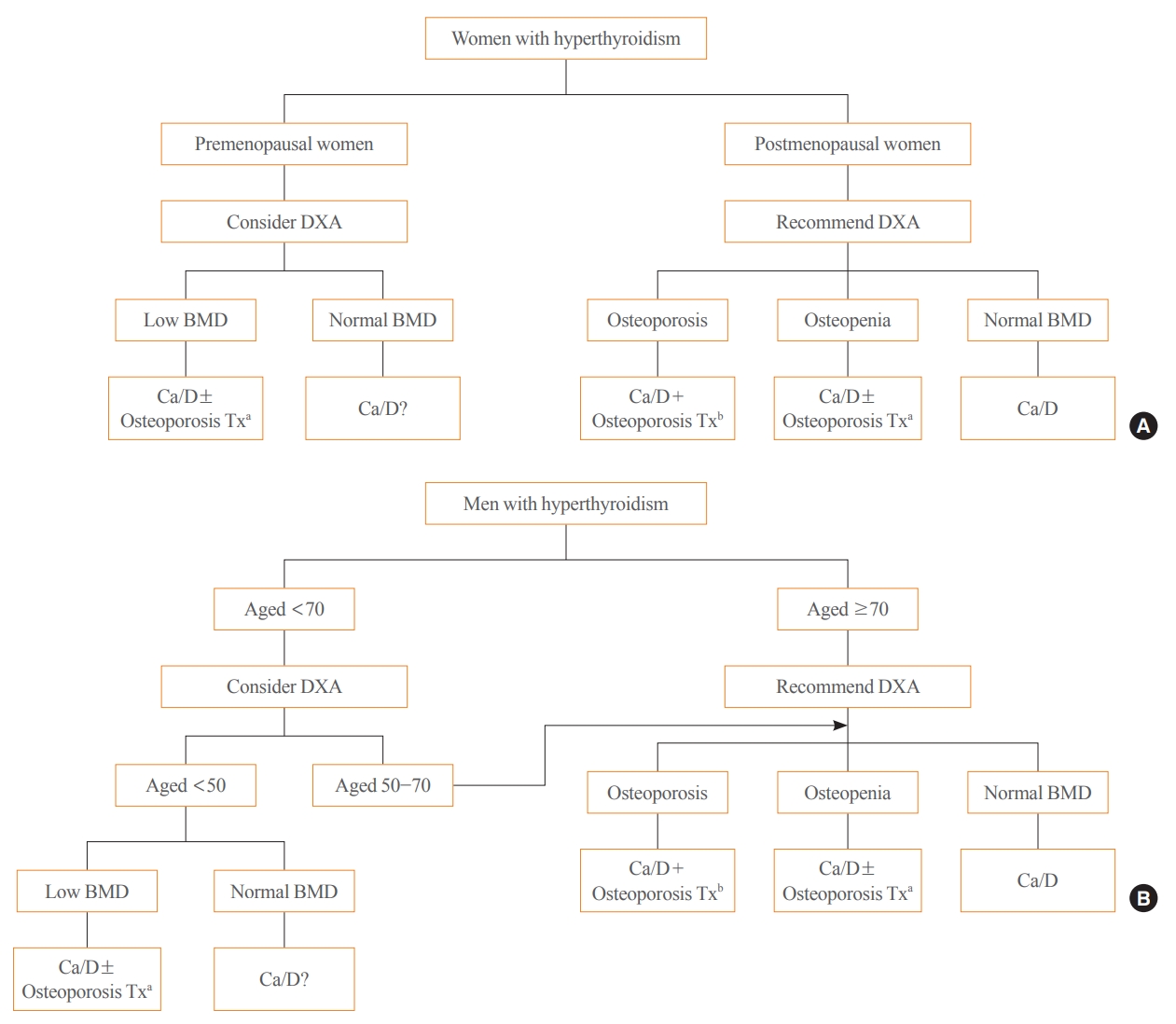
- Evaluation and Management of Bone Health in Patients with Thyroid Diseases: A Position Statement of the Korean Thyroid Association
- A Ram Hong, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(2):175-189. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1701
- 3,725 View
- 244 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Thyroid hormones play an important physiological role in maintaining adult bone structure and strength. Consequently, thyroid dysfunction is related to skeletal outcomes. Overt hyperthyroidism is an established cause of high bone turnover with accelerated bone loss, leading to osteoporosis and increased fracture risk. Hyperthyroidism induced by thyroid-stimulating hormone-suppressive therapy in patients with differentiated thyroid cancer is a cause of secondary osteoporosis. In contrast, there is a lack of evidence on the negative impact of hypothyroidism on bone health. Considering the clinical updates on the importance of bone health in thyroid dysfunction, the Task Force from the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association recently developed a position statement on the evaluation and management of bone health of patients with thyroid diseases, particularly focused on endogenous hyperthyroidism and thyroid-stimulating hormone-suppressive therapy-associated hyperthyroidism in patients with differentiated thyroid cancer. Herein, we review the Korean Thyroid Association’s position statement on the evaluation and management of bone health associated with thyroid diseases.
-
Citations
Citations to this article as recorded by- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism
Justyna Kuliczkowska-Płaksej, Aleksandra Zdrojowy-Wełna, Aleksandra Jawiarczyk-Przybyłowska, Łukasz Gojny, Marek Bolanowski
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - Review on the protective activity of osthole against the pathogenesis of osteoporosis
Jincai Chen, Xiaofei Liao, Juwen Gan
Frontiers in Pharmacology.2023;[Epub] CrossRef
- Diagnosis and therapeutic approach to bone health in patients with hypopituitarism

Original Articles
- Diabetes, obesity and metabolism
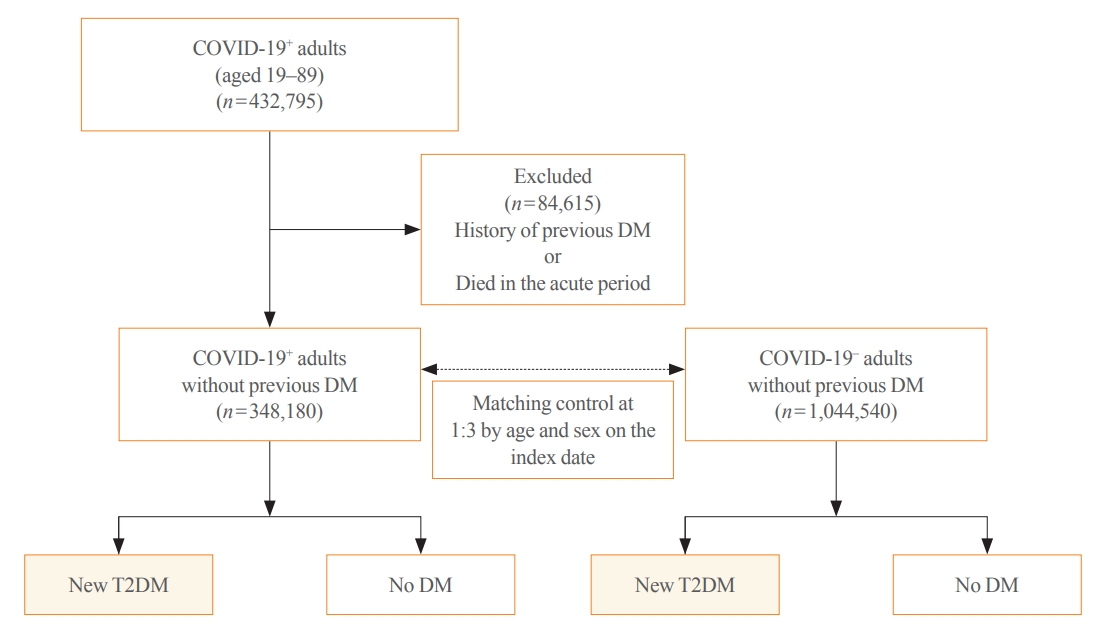
Big Data Articles (National Health Insurance Service Database) - Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
- Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
- Endocrinol Metab. 2023;38(2):245-252. Published online April 5, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1662
- 2,209 View
- 118 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
Methods
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
Conclusion
COVID-19 may increase the risk of developing T2DM beyond the acute period. The higher the severity of COVID-19 in the acute phase, the higher the risk of newly diagnosed T2DM. Therefore, T2DM should be included as a component of managing long-term COVID-19. -
Citations
Citations to this article as recorded by- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review
Anca Pantea Stoian, Ioana-Cristina Bica, Teodor Salmen, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez,
Diabetes Therapy.2024; 15(1): 33. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review

- Adrenal gland
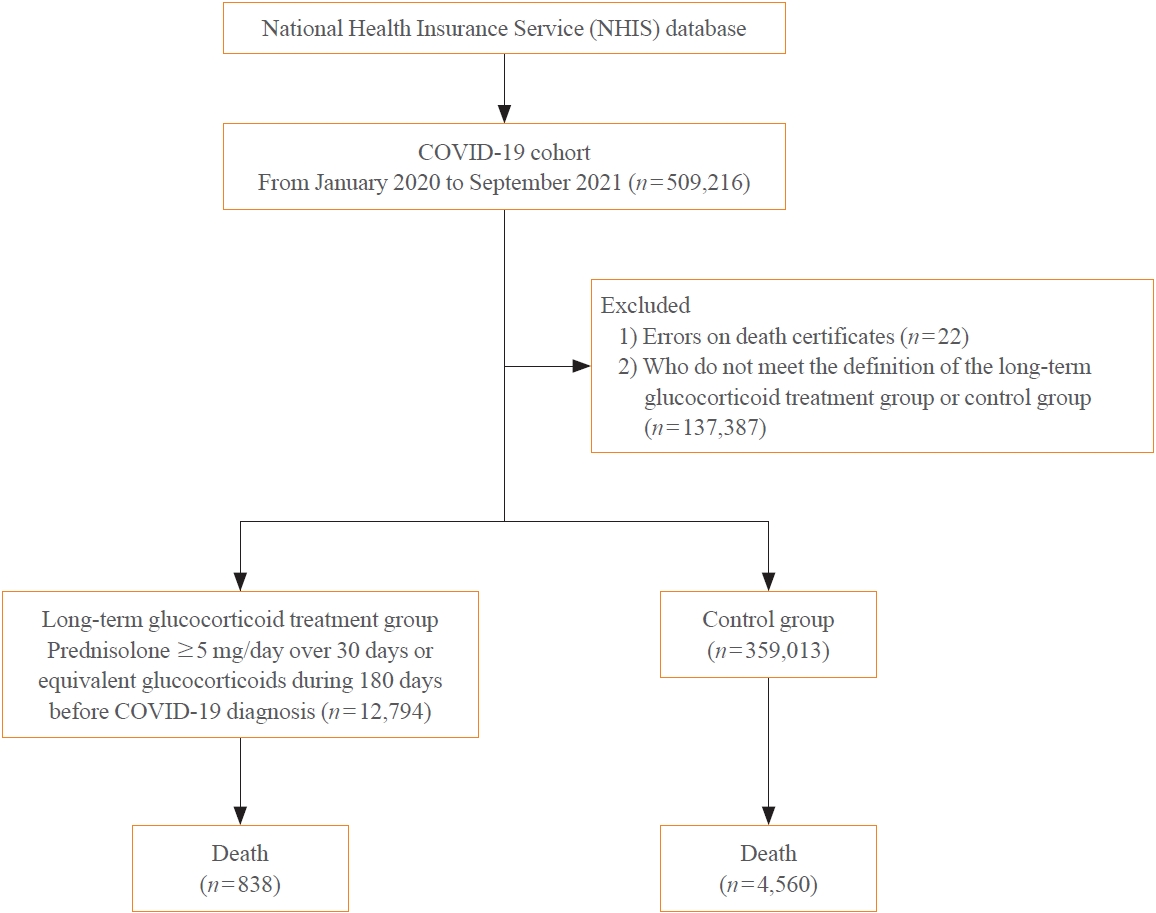
Big Data Articles (National Health Insurance Service Database) - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
- Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
- Endocrinol Metab. 2023;38(2):253-259. Published online March 21, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1607
- 2,537 View
- 101 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The severity of coronavirus disease 2019 (COVID-19) among patients with long-term glucocorticoid treatment (LTGT) has not been established. We aimed to evaluate the association between LTGT and COVID-19 prognosis.
Methods
A Korean nationwide cohort database of COVID-19 patients between January 2019 and September 2021 was used. LTGT was defined as exposure to at least 150 mg of prednisolone (≥5 mg/day and ≥30 days) or equivalent glucocorticoids 180 days before COVID-19 infection. The outcome measurements were mortality, hospitalization, intensive care unit (ICU) admission, length of stay, and mechanical ventilation.
Results
Among confirmed patients with COVID-19, the LTGT group (n=12,794) was older and had a higher proportion of comorbidities than the control (n=359,013). The LTGT group showed higher in-hospital, 30-day, and 90-day mortality rates than the control (14.0% vs. 2.3%, 5.9% vs. 1.1%, and 9.9% vs. 1.8%, respectively; all P<0.001). Except for the hospitalization rate, the length of stay, ICU admission, and mechanical ventilation proportions were significantly higher in the LTGT group than in the control (all P<0.001). Overall mortality was higher in the LTGT group than in the control group, and the significance remained in the fully adjusted model (odds ratio [OR], 5.75; 95% confidence interval [CI], 5.31 to 6.23) (adjusted OR, 1.82; 95% CI, 1.67 to 2.00). The LTGT group showed a higher mortality rate than the control within the same comorbidity score category.
Conclusion
Long-term exposure to glucocorticoids increased the mortality and severity of COVID-19. Prevention and early proactive measures are inevitable in the high-risk LTGT group with many comorbidities. -
Citations
Citations to this article as recorded by- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
Eun-Hee Cho
Endocrinology and Metabolism.2023; 38(2): 223. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy

Review Article
- Diabetes, Obesity and Metabolism
- Overcoming Therapeutic Inertia as the Achilles’ Heel for Improving Suboptimal Diabetes Care: An Integrative Review
- Boon-How Chew, Barakatun-Nisak Mohd-Yusof, Pauline Siew Mei Lai, Kamlesh Khunti
- Endocrinol Metab. 2023;38(1):34-42. Published online February 16, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1649
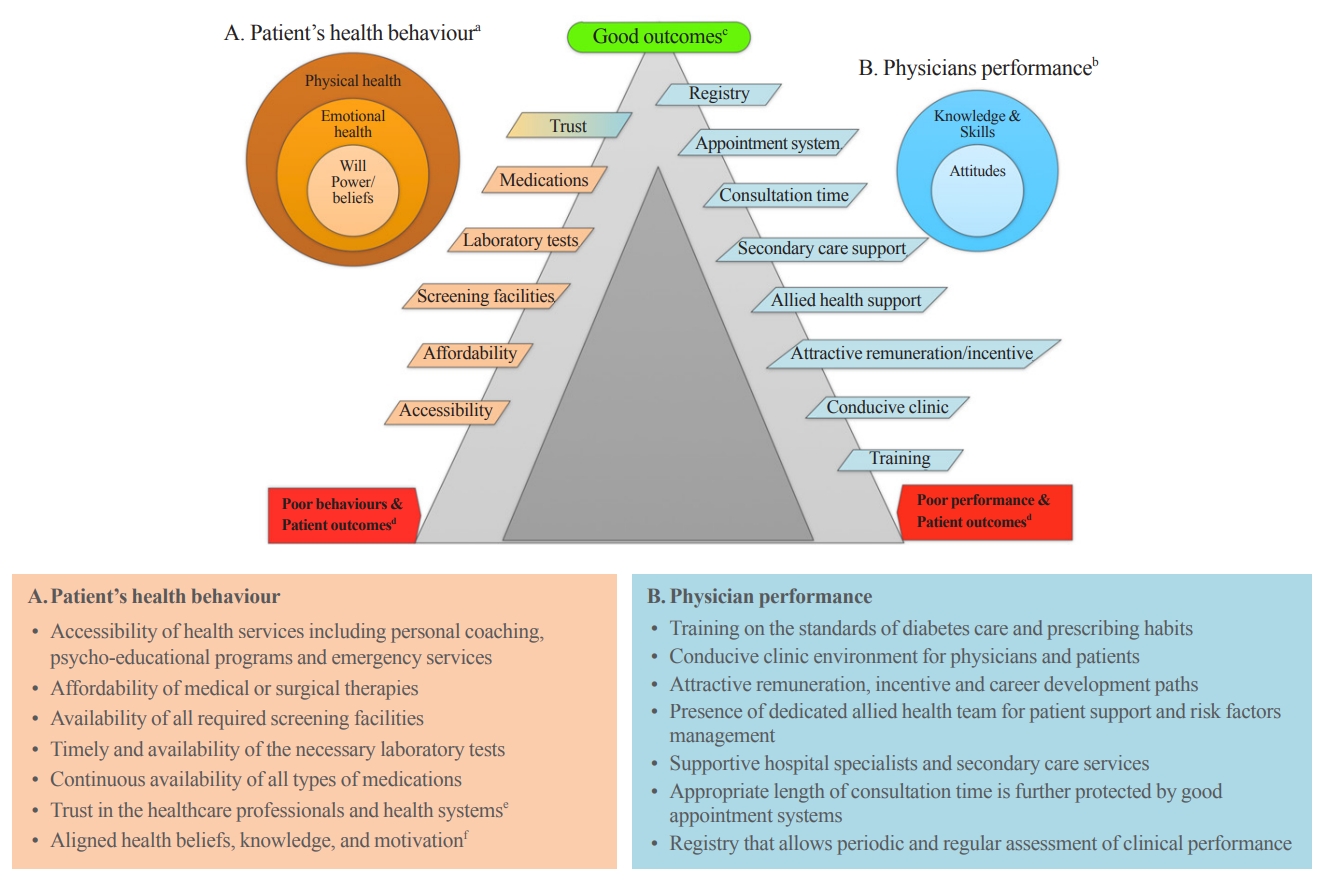
- 2,909 View
- 214 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The ultimate purpose of diabetes care is achieving the outcomes that patients regard as important throughout the life course. Despite advances in pharmaceuticals, nutraceuticals, psychoeducational programs, information technologies, and digital health, the levels of treatment target achievement in people with diabetes mellitus (DM) have remained suboptimal. This clinical care of people with DM is highly challenging, complex, costly, and confounded for patients, physicians, and healthcare systems. One key underlying problem is clinical inertia in general and therapeutic inertia (TI) in particular. TI refers to healthcare providers’ failure to modify therapy appropriately when treatment goals are not met. TI therefore relates to the prescribing decisions made by healthcare professionals, such as doctors, nurses, and pharmacists. The known causes of TI include factors at the level of the physician (50%), patient (30%), and health system (20%). Although TI is often multifactorial, the literature suggests that 28% of strategies are targeted at multiple levels of causes, 38% at the patient level, 26% at the healthcare professional level, and only 8% at the healthcare system level. The most effective interventions against TI are shorter intervals until revisit appointments and empowering nurses, diabetes educators, and pharmacists to review treatments and modify prescriptions.
-
Citations
Citations to this article as recorded by- Obesity management from the perspectives of people living with obesity in Canada: A mixed‐methods study
David C. W. Lau, Ian Patton, Reena Lavji, Adel Belloum, Ginnie Ng, Renuca Modi
Diabetes, Obesity and Metabolism.2024; 26(4): 1529. CrossRef - Bridging the gap in cardiovascular care in diabetic patients: are cardioprotective antihyperglycemic agents underutilized?
André J Scheen
Expert Review of Clinical Pharmacology.2023; 16(11): 1053. CrossRef
- Obesity management from the perspectives of people living with obesity in Canada: A mixed‐methods study

Special Article
- Miscellaneous
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
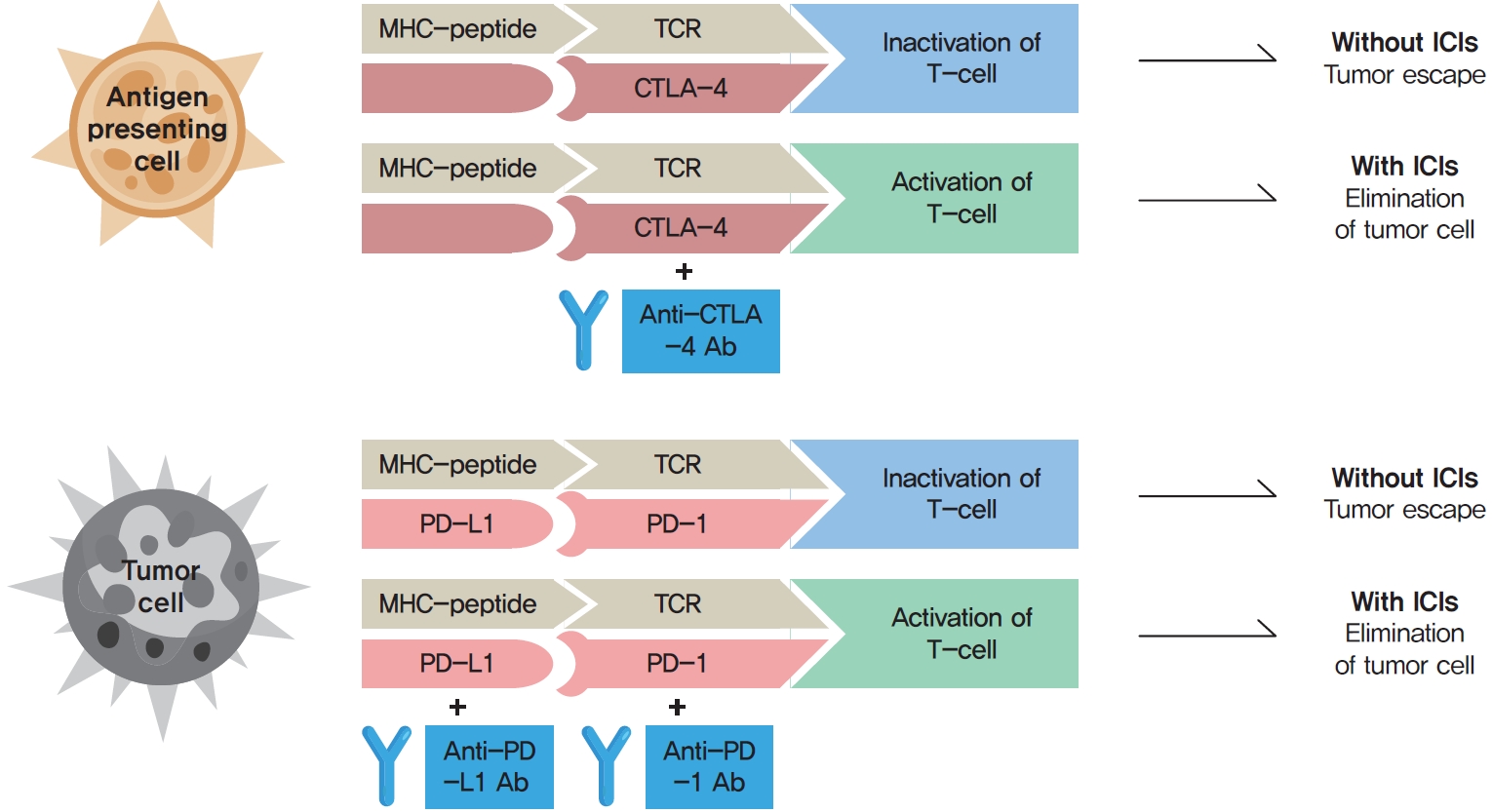
- 3,432 View
- 320 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

Original Article
- Diabetes, Obesity and Metabolism
- Inhibition of miR-146a-5p and miR-8114 in Insulin-Secreting Cells Contributes to the Protection of Melatonin against Stearic Acid-Induced Cellular Senescence by Targeting Mafa
- Shenghan Su, Qingrui Zhao, Lingfeng Dan, Yuqing Lin, Xuebei Li, Yunjin Zhang, Chunxiao Yang, Yimeng Dong, Xiaohan Li, Romano Regazzi, Changhao Sun, Xia Chu, Huimin Lu
- Endocrinol Metab. 2022;37(6):901-917. Published online December 7, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1565

- 2,323 View
- 219 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Chronic exposure to elevated levels of saturated fatty acids results in pancreatic β-cell senescence. However, targets and effective agents for preventing stearic acid-induced β-cell senescence are still lacking. Although melatonin administration can protect β-cells against lipotoxicity through anti-senescence processes, the precise underlying mechanisms still need to be explored. Therefore, we investigated the anti-senescence effect of melatonin on stearic acid-treated mouse β-cells and elucidated the possible role of microRNAs in this process.
Methods
β-Cell senescence was identified by measuring the expression of senescence-related genes and senescence-associated β-galactosidase staining. Gain- and loss-of-function approaches were used to investigate the involvement of microRNAs in stearic acid-evoked β-cell senescence and dysfunction. Bioinformatics analyses and luciferase reporter activity assays were applied to predict the direct targets of microRNAs.
Results
Long-term exposure to a high concentration of stearic acid-induced senescence and upregulated miR-146a-5p and miR- 8114 expression in both mouse islets and β-TC6 cell lines. Melatonin effectively suppressed this process and reduced the levels of these two miRNAs. A remarkable reversibility of stearic acid-induced β-cell senescence and dysfunction was observed after silencing miR-146a-5p and miR-8114. Moreover, V-maf musculoaponeurotic fibrosarcoma oncogene homolog A (Mafa) was verified as a direct target of miR-146a-5p and miR-8114. Melatonin also significantly ameliorated senescence and dysfunction in miR-146a-5pand miR-8114-transfected β-cells.
Conclusion
These data demonstrate that melatonin protects against stearic acid-induced β-cell senescence by inhibiting miR-146a- 5p and miR-8114 and upregulating Mafa expression. This not only provides novel targets for preventing stearic acid-induced β-cell dysfunction, but also points to melatonin as a promising drug to combat type 2 diabetes progression. -
Citations
Citations to this article as recorded by- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice
Yuxuan Chen, Rou Peng, Yi Qian, Yizhou Lu, Liyao Chen, Meiling Yu, Minjiao Jiang, Wei Wu, Shengfeng Lu
Gene.2024; 897: 148090. CrossRef - MiR-126 and miR-146a as Melatonin-Responsive Biomarkers for Neonatal Brain Ischemia
Maria Cristina Albertini, Tania Vanzolini, Serafina Perrone, Michael D. Weiss, Giuseppe Buonocore, Valentina Dell’Orto, Walter Balduini, Silvia Carloni
Journal of Molecular Neuroscience.2023; 73(9-10): 763. CrossRef
- Genome-wide analysis in PC6 electroacupuncture to ameliorate carfilzomib-induced cardiotoxicity in mice


 KES
KES

 First
First Prev
Prev



