Search
- Page Path
- HOME > Search
Review Article
- Diabetes, Obesity and Metabolism
- Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
- Joon Ho Moon, Kyuho Kim, Sung Hee Choi
- Endocrinol Metab. 2022;37(4):575-586. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.402

- 7,601 View
- 434 Download
- 11 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
-
Citations
Citations to this article as recorded by- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the obesity medicine association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; : 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef
- The chylomicron saga: time to focus on postprandial metabolism

Original Articles
- Diabetes, Obesity and Metabolism
- The Effects of PPAR Agonists on Atherosclerosis and Nonalcoholic Fatty Liver Disease in ApoE−/−FXR−/− Mice
- Yenna Lee, Bo-Rahm Kim, Geun-Hyung Kang, Gwan Jae Lee, Young Joo Park, Haeryoung Kim, Hak Chul Jang, Sung Hee Choi
- Endocrinol Metab. 2021;36(6):1243-1253. Published online December 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1100

- 5,557 View
- 156 Download
- 11 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Farnesoid X receptor (FXR), a bile acid–activated nuclear receptor, is a potent regulator of glucose and lipid metabolism as well as of bile acid metabolism. Previous studies have demonstrated that FXR deficiency is associated with metabolic derangements, including atherosclerosis and nonalcoholic fatty liver disease (NAFLD), but its mechanism remains unclear. In this study, we investigated the role of FXR in atherosclerosis and NAFLD and the effect of peroxisome proliferator-activated receptor (PPAR) agonists in mouse models with FXR deficiency.
Methods
En face lipid accumulation analysis, liver histology, serum levels of glucose and lipids, and mRNA expression of genes related to lipid metabolism were compared between apolipoprotein E (ApoE)−/− and ApoE−/−FXR−/− mice. The effects of PPARα and PPARγ agonists were also compared in both groups of mice.
Results
Compared with ApoE−/− mice, ApoE−/−FXR−/− mice showed more severe atherosclerosis, hepatic steatosis, and higher levels of serum cholesterol, low-density lipoprotein cholesterol, and triglycerides, accompanied by increased mRNA expression of FAS, ApoC2, TNFα, IL-6 (liver), ATGL, TGH, HSL, and MGL (adipocytes), and decreased mRNA expressions of CPT2 (liver) and Tfam (skeletal muscle). Treatment with a PPARα agonist, but not with a PPARγ agonist, partly reversed atherosclerosis and hepatic steatosis, and decreased plasma triglyceride levels in the ApoE−/−FXR−/− mice, in association with increased mRNA expression of CD36 and FATP and decreased expression of ApoC2 and ApoC3 (liver).
Conclusion
Loss of FXR is associated with aggravation of atherosclerosis and hepatic steatosis in ApoE-deficient mice, which could be reversed by a PPARα agonist through induction of fatty acid uptake, β-oxidation, and triglyceride hydrolysis. -
Citations
Citations to this article as recorded by- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model
Shu-Yan Gao, Jing-Cheng Zhao, Qing Xia, Chen Sun, Maimaiti Aili, Ainiwaer Talifu, Shi-Xia Huo, Yun Zhang, Zhi-Jian Li
Frontiers in Pharmacology.2024;[Epub] CrossRef - Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics
Yuxiao Jiang, Lili Wu, Xiaopeng Zhu, Hua Bian, Xin Gao, Mingfeng Xia
Lipids in Health and Disease.2024;[Epub] CrossRef - Mitochondrial carnitine palmitoyltransferase-II dysfunction: A possible novel mechanism for nonalcoholic fatty liver disease in hepatocarcinogenesis
Min Yao, Ping Zhou, Yan-Yan Qin, Li Wang, Deng-Fu Yao
World Journal of Gastroenterology.2023; 29(12): 1765. CrossRef - Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases
Tess Yntema, Debby P. Y. Koonen, Folkert Kuipers
Nutrients.2023; 15(8): 1850. CrossRef - The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease
Alexandra C. Finney, Sandeep Das, Dhananjay Kumar, M. Peyton McKinney, Bishuang Cai, Arif Yurdagul, Oren Rom
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Targeting PPARs for therapy of atherosclerosis: A review
Miao Miao, Xue Wang, Tian Liu, Yan-Jie Li, Wen-Qian Yu, Tong-Mei Yang, Shou-Dong Guo
International Journal of Biological Macromolecules.2023; 242: 125008. CrossRef - Cabernet sauvignon dry red wine ameliorates atherosclerosis in mice by regulating inflammation and endothelial function, activating AMPK phosphorylation, and modulating gut microbiota
Xinlong Cheng, Xue Han, Liangfu Zhou, Yasai Sun, Qian Zhou, Xuan Lin, Zhe Gao, Jie Wang, Wen Zhao
Food Research International.2023; 169: 112942. CrossRef - Impacts of dietary lipids derived from animal or vegetable sources on healthy rats
Mostafa M Dalal, Gamal M Edrees, Hanaa A Hassan, Mamdouh Abdel-Mogib, Mai Alaa El-Dein
Egyptian Journal of Basic and Applied Sciences.2023; 10(1): 618. CrossRef - Whey protein hydrolysate alleviated atherosclerosis and hepatic steatosis by regulating lipid metabolism in apoE-/- mice fed a Western diet
Kai Wang, Zixin Fu, Xiaoyi Li, Hui Hong, Xin Zhan, Xiaohong Guo, Yongkang Luo, Yuqing Tan
Food Research International.2022; 157: 111419. CrossRef - Melatonin alleviates PM2.5‐induced glucose metabolism disorder and lipidome alteration by regulating endoplasmic reticulum stress
Zhou Du, Junjie Hu, Lisen Lin, Qingqing Liang, Mengqi Sun, Zhiwei Sun, Junchao Duan
Journal of Pineal Research.2022;[Epub] CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef - The role of the gut microbiota in health and cardiovascular diseases
Lu Wang, Shiqi Wang, Qing Zhang, Chengqi He, Chenying Fu, Quan Wei
Molecular Biomedicine.2022;[Epub] CrossRef
- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model

- Clinical Study
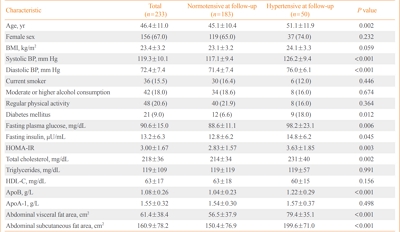
- Apolipoprotein B Levels Predict Future Development of Hypertension Independent of Visceral Adiposity and Insulin Sensitivity
- Seung Jin Han, Wilfred Y. Fujimoto, Steven E. Kahn, Donna L. Leonetti, Edward J. Boyko
- Endocrinol Metab. 2020;35(2):351-358. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.351
- 5,762 View
- 130 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
High plasma apolipoprotein B (apoB) levels have been shown to be associated with hypertension, central obesity, and insulin resistance in cross-sectional research. However, it is unclear whether apoB levels predict future hypertension independent of body composition and insulin sensitivity. Therefore, we prospectively investigated whether plasma apoB concentrations independently predicted the risk of hypertension in a cohort of Japanese Americans.
Methods
A total of 233 normotensive Japanese Americans (77 men, 156 women; mean age, 46.4±11.0 years) were followed over 10 years to monitor them for the development of hypertension. Fasting plasma concentrations of apoB, glucose, and insulin were measured at baseline. Insulin sensitivity was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR). The abdominal visceral and subcutaneous fat areas were measured at baseline using computed tomography. Logistic regression analysis was used to estimate the association between apoB concentrations and the odds of incident hypertension.
Results
The 10-year cumulative incidence of hypertension was 21.5%. The baseline apoB level was found to be positively associated with the odds of incident hypertension over 10 years after adjustment for age, sex, body mass index, systolic blood pressure, abdominal visceral fat area, abdominal subcutaneous fat area, total plasma cholesterol concentration, diabetes status, and HOMA-IR at baseline (odds ratio and 95% confidence interval for a 1-standard deviation increase, 1.89 [1.06 to 3.37]; P=0.030).
Conclusion
Higher apoB concentrations predicted greater risks of future hypertension independent of abdominal visceral fat area and insulin sensitivity in Japanese Americans. -
Citations
Citations to this article as recorded by- Correlation between Central Obesity and Liver Function in Young Adults—A Cross-Sectional Study
John Alvin, Damodara Gowda KM
Journal of Health and Allied Sciences NU.2023; 13(02): 273. CrossRef - Serum amyloid A in children and adolescents: association with overweight and carotid intima-media thickness
Maria Vitória Mareschi Barbosa, João Carlos Pina Faria, Stephanie Ramos Coelho, Fernando Luiz Affonso Fonseca, Andrea Paula Kafejian Haddad, Fabíola Isabel Suano de Souza, Roseli Oselka Saccardo Sarni
einstein (São Paulo).2023;[Epub] CrossRef - The association of the apolipoprotein B/A1 ratio and the metabolic syndrome in children and adolescents: a systematic review and meta-analysis
Kayhan Dinpanah, Toba Kazemi, Sameep Shetty, Saeede Khosravi Bizhaem, Ali Fanoodi, Seyed Mohammad Riahi
Journal of Diabetes & Metabolic Disorders.2023;[Epub] CrossRef - Current Data and New Insights into the Genetic Factors of Atherogenic Dyslipidemia Associated with Metabolic Syndrome
Lăcramioara Ionela Butnariu, Eusebiu Vlad Gorduza, Elena Țarcă, Monica-Cristina Pânzaru, Setalia Popa, Simona Stoleriu, Vasile Valeriu Lupu, Ancuta Lupu, Elena Cojocaru, Laura Mihaela Trandafir, Ștefana Maria Moisă, Andreea Florea, Laura Stătescu, Minerva
Diagnostics.2023; 13(14): 2348. CrossRef - Sex-Based Differences and Risk Factors for Comorbid Nonalcoholic Fatty Liver Disease in Patients with Bipolar Disorder: A Cross-Sectional Retrospective Study
Ying Wang, Yiyi Liu, Xun Zhang, Qing Wu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3533. CrossRef - Apolipoprotein B Displays Superior Predictive Value Than Other Lipids for Long-Term Prognosis in Coronary Atherosclerosis Patients and Particular Subpopulations: A Retrospective Study
Chunyan Zhang, Jingwei Ni, Zhenyue Chen
Clinical Therapeutics.2022; 44(8): 1071. CrossRef - Genetics of Cholesterol-Related Genes in Metabolic Syndrome: A Review of Current Evidence
Sok Kuan Wong, Fitri Fareez Ramli, Adli Ali, Nurul ‘Izzah Ibrahim
Biomedicines.2022; 10(12): 3239. CrossRef
- Correlation between Central Obesity and Liver Function in Young Adults—A Cross-Sectional Study

- APOA5 Polymorphism Is Associated with Metabolic Syndrome in Korean Postmenopausal Women.
- Doh Hee Kim, Seung Hee Lee, Kyung Hoon Han, Chae Bong Kim, Kwan Young Song, Sook Cho, Kye Heui Lee
- Endocrinol Metab. 2012;27(4):276-281. Published online December 20, 2012
- DOI: https://doi.org/10.3803/EnM.2012.27.4.276
- 4,637 View
- 23 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Menopause is an independent risk factor in metabolic syndrome which induced an alteration of the lipid metabolism by hormonal changes. Apolipoprotein A5 gene (APOA5) was related to the regulation of triglyceride and high density lipoprotein cholesterol (HDL-C) level with biosynthesis and decomposition. This study was conducted to investigate the relationship between APOA5 polymorphism and metabolic syndrome in Korean postmenopausal women. METHODS: This study included 307 postmenopausal women with anthropometric and biochemical measurement in 2010-2011. The polymorphism of APOA5 was analyzed by polymerase chain reaction-restriction fragment length polymorphism method with MseI restriction enzyme. RESULTS: The metabolic syndrome prevalence with TT genotype was significantly lower than the frequency in those with TC/CC (27.09%, 38.46%, and 45.71% for TT, TC, and CC, respectively; P < 0.05). Multiple regression analysis of metabolic syndrome risk factors indicated that postmenopausal women with CC genotype had a higher risk with 3 times than that in TT genotype (P < 0.05). APOA5 C carriers showed an increased risk of triglyceride level (odd ratio, 2.93 and 1.85 for CC and TC+CC, respectively; P < 0.05). Interestingly, HDL-C was related to triglyceride directly in comparison to APOA5. CONCLUSION: The results of this study indicate that APOA5 has an influence on serum triglyceride and HDL-C, which contribute to metabolic syndrome in Korean postmenopausal women. -
Citations
Citations to this article as recorded by- Effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein A-V levels in patients with impaired fasting glucose or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism
Minjoo Kim, Jey Sook Chae, Miri Kim, Sang-Hyun Lee, Jong Ho Lee
Nutrition Journal.2014;[Epub] CrossRef - APOA5Polymorphism Is Associated with Metabolic Syndrome in Korean Postmenopausal Women
Mi Hae Seo, Won Young Lee
Endocrinology and Metabolism.2012; 27(4): 274. CrossRef
- Effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein A-V levels in patients with impaired fasting glucose or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism


 KES
KES

 First
First Prev
Prev



