Search
- Page Path
- HOME > Search
- Calcium & bone metabolism
- New Insights into Calorie Restriction Induced Bone Loss
- Linyi Liu, Clifford J. Rosen
- Endocrinol Metab. 2023;38(2):203-213. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1673

- 3,285 View
- 175 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Caloric restriction (CR) is now a popular lifestyle choice due to its ability in experimental animals to improve lifespan, reduce body weight, and lessen oxidative stress. However, more and more emerging evidence suggests this treatment requires careful consideration because of its detrimental effects on the skeletal system. Experimental and clinical studies show that CR can suppress bone growth and raise the risk of fracture, but the specific mechanisms are poorly understood. Reduced mechanical loading has long been thought to be the primary cause of weight loss-induced bone loss from calorie restriction. Despite fat loss in peripheral depots with calorie restriction, bone marrow adipose tissue (BMAT) increases, and this may play a significant role in this pathological process. Here, we update recent advances in our understanding of the effects of CR on the skeleton, the possible pathogenic role of BMAT in CR-induced bone loss, and some strategies to mitigate any potential side effects on the skeletal system.
-
Citations
Citations to this article as recorded by- Obesity, diabetes and risk of bone fragility: How BMAT behavior is affected by metabolic disturbances and its influence on bone health
Gregório Corrêa Guimarães, João Bosco Costa Coelho, João Gabriel Oliveira Silva, Ana Carolina Chalfun de Sant’Ana, Cássia Alves Carrilho de Sá, Júlia Marques Moreno, Lívia Marçal Reis, Camila Souza de Oliveira Guimarães
Osteoporosis International.2024; 35(4): 575. CrossRef - Bone Marrow Adipose Tissue Is Not Required for Reconstitution of the Immune System Following Irradiation in Male Mice
Jessica A. Keune, Carmen P. Wong, Adam J. Branscum, Scott A. Menn, Urszula T. Iwaniec, Russell T. Turner
International Journal of Molecular Sciences.2024; 25(4): 1980. CrossRef - Dietary restriction plus exercise change gene expression of Cxcl12 abundant reticular cells in female mice
Aoi Ikedo, Yuuki Imai
Journal of Bone and Mineral Metabolism.2024;[Epub] CrossRef
- Obesity, diabetes and risk of bone fragility: How BMAT behavior is affected by metabolic disturbances and its influence on bone health

- Hypothalamus and Pituitary Gland
- Independent Skeletal Actions of Pituitary Hormones
- Se-Min Kim, Farhath Sultana, Funda Korkmaz, Daria Lizneva, Tony Yuen, Mone Zaidi
- Endocrinol Metab. 2022;37(5):719-731. Published online September 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1573
- 3,636 View
- 234 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
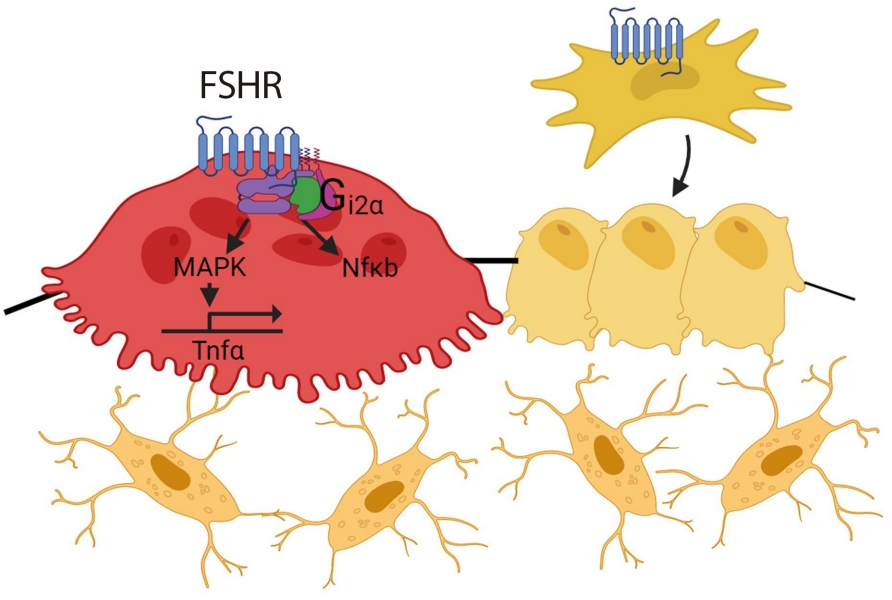
ePub - Over the past years, pituitary hormones and their receptors have been shown to have non-traditional actions that allow them to bypass the hypothalamus-pituitary-effector glands axis. Bone cells—osteoblasts and osteoclasts—express receptors for growth hormone, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), prolactin, oxytocin, and vasopressin. Independent skeletal actions of pituitary hormones on bone have been studied using genetically modified mice with haploinsufficiency and by activating or inactivating the receptors pharmacologically, without altering systemic effector hormone levels. On another front, the discovery of a TSH variant (TSH-βv) in immune cells in the bone marrow and skeletal action of FSHβ through tumor necrosis factor α provides new insights underscoring the integrated physiology of bone-immune-endocrine axis. Here we discuss the interaction of each pituitary hormone with bone and the potential it holds in understanding bone physiology and as a therapeutic target.
-
Citations
Citations to this article as recorded by- New tools for bone health assessment in secreting pituitary adenomas
Meliha Melin Uygur, Stefano Frara, Luigi di Filippo, Andrea Giustina
Trends in Endocrinology & Metabolism.2023; 34(4): 231. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - The mechanism of oxytocin and its receptors in regulating cells in bone metabolism
Liu Feixiang, Feng Yanchen, Li Xiang, Zhang Yunke, Miao Jinxin, Wang Jianru, Lin Zixuan
Frontiers in Pharmacology.2023;[Epub] CrossRef - To investigate the mechanism of Yiwei Decoction in the treatment of premature ovarian insufficiency-related osteoporosis using transcriptomics, network pharmacology and molecular docking techniques
Weisen Fan, Yan Meng, Jing Zhang, Muzhen Li, Yingjie Zhang, Xintian Qu, Xin Xiu
Scientific Reports.2023;[Epub] CrossRef
- New tools for bone health assessment in secreting pituitary adenomas

- Bone Metabolism
- Operationalizing Treat-to-Target for Osteoporosis
- E. Michael Lewiecki
- Endocrinol Metab. 2021;36(2):270-278. Published online March 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.970

- 5,512 View
- 296 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Treat-to-target (TTT) for osteoporosis is a concept for individualizing patient treatment decisions that focuses on achieving an acceptable level of fracture risk rather than response to treatment alone. While a response to treatment is essential in order to achieve an acceptable level of risk, it is not necessarily sufficient. Some patients have a good response to treatment yet remain at high level of fracture risk. Since there is no way to directly measure bone strength in patients treated for osteoporosis, a surrogate measurement must be used. Bone mineral density (BMD) is commonly used to select patients for treatment and has emerged as the most useful surrogate for assessing reduction of fracture risk after treatment is started. Recent large meta-regression studies have shown a robust correlation between larger increases in BMD with treatment and greater reductions in fracture risk. Application of TTT for osteoporosis involves assessing fracture risk before starting treatment and initiating treatment with an agent that is most likely to reduce fracture risk to an acceptable level, represented by a target BMD T-score, over a reasonable period of time. This review offers suggestions for implementing TTT for osteoporosis in clinical practice and managing patients who fail or succeed in reaching the target. More study is needed to fully validate the use of TTT for osteoporosis for initiating and modifying treatments to reduce fracture risk.
-
Citations
Citations to this article as recorded by- Treatment sequencing using the dual amylin and calcitonin receptor agonist KBP-336 and semaglutide results in durable weight loss
Anna Thorsø Larsen, Morten A. Karsdal, Kim Henriksen
European Journal of Pharmacology.2023; 954: 175837. CrossRef - Osteoporosis: Spotlight on current approaches to pharmacological treatment
Dilşad Sindel
Turkish Journal of Physical Medicine and Rehabilitation.2023; 69(2): 140. CrossRef - Postmenopausal Osteoporosis
Caren G. Solomon, Marcella Donovan Walker, Elizabeth Shane
New England Journal of Medicine.2023; 389(21): 1979. CrossRef - Prevalence and Risk Factors of T-Score Spine-Hip Discordance in Patients with Osteoporotic Vertebral Compression Fracture
Byung-Ho Yoon, Ho Won Kang, Su Min Kim, Young Do Koh
Journal of Bone Metabolism.2022; 29(1): 43. CrossRef - Pharmacological treatment of osteoporosis: 2022 update
Yunkyung Jeon, In-Joo Kim
Journal of the Korean Medical Association.2022; 65(4): 241. CrossRef
- Treatment sequencing using the dual amylin and calcitonin receptor agonist KBP-336 and semaglutide results in durable weight loss

- Endocrine Research
- Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
- Kyoung Min Kim, Hyun-Jin Jin, Seo Yeon Lee, Hyo Jin Maeng, Gha Young Lee, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang, Soo Lim
- Endocrinol Metab. 2017;32(3):389-395. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.389
- 4,752 View
- 50 Download
- 11 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Bone strength is impaired in patients with type 2 diabetes mellitus despite an increase in bone mineral density (BMD). Thiazolidinedione (TZD), a peroxisome proliferator activated receptor γ agonist, promotes adipogenesis, and suppresses osteoblastogenesis. Therefore, its use is associated with an increased risk of fracture. The aim of this study was to examine the
in vitro andin vivo effects of lobeglitazone, a new TZD, on bone.Methods MC3T3E1 and C3H10T1/2 cells were cultured in osteogenic medium and exposed to lobeglitazone (0.1 or 1 µM), rosiglitazone (0.4 µM), or pioglitazone (1 µM) for 10 to 14 days. Alkaline phosphatase (ALP) activity, Alizarin red staining, and osteoblast marker gene expression were analyzed. For
in vivo experiments, 6-month-old C57BL/6 mice were treated with vehicle, one of two doses of lobeglitazone, rosiglitazone, or pioglitazone. BMD was assessed using a PIXImus2 instrument at the baseline and after 12 weeks of treatment.Results As expected,
in vitro experiments showed that ALP activity was suppressed and the mRNA expression of osteoblast marker genes RUNX2 (runt-related transcription factor 2) and osteocalcin was significantly attenuated after rosiglitazone treatment. By contrast, lobeglitazone at either dose did not inhibit these variables. Rosiglitazone-treated mice showed significantly accelerated bone loss for the whole bone and femur, but BMD did not differ significantly between the lobeglitazone-treated and vehicle-treated mice.Conclusion These findings suggest that lobeglitazone has no detrimental effects on osteoblast biology and might not induce side effects in the skeletal system.
-
Citations
Citations to this article as recorded by- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Effect of lobeglitazone on motor function in rat model of Parkinson’s disease with diabetes co-morbidity
Kambiz Hassanzadeh, Arman Rahimmi, Mohammad Raman Moloudi, Rita Maccarone, Massimo Corbo, Esmael Izadpanah, Marco Feligioni
Brain Research Bulletin.2021; 173: 184. CrossRef - Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
Endocrinology and Metabolism.2021; 36(4): 895. CrossRef - Xenogeneic native decellularized matrix carrying PPARγ activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration
Xue Han, Lijun Liao, Tian Zhu, Yuchan Xu, Fei Bi, Li Xie, Hui Li, Fangjun Huo, Weidong Tian, Weihua Guo
Materials Science and Engineering: C.2020; 116: 111224. CrossRef - Diabetes pharmacotherapy and effects on the musculoskeletal system
Evangelia Kalaitzoglou, John L. Fowlkes, Iuliana Popescu, Kathryn M. Thrailkill
Diabetes/Metabolism Research and Reviews.2019;[Epub] CrossRef - The effects of diabetes therapy on bone: A clinical perspective
Karim G. Kheniser, Carmen M. Polanco Santos, Sangeeta R. Kashyap
Journal of Diabetes and its Complications.2018; 32(7): 713. CrossRef
- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis

- Bone Metabolism
- Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
- Hyo Min Jeong, Sun Wook Cho, Serk In Park
- Endocrinol Metab. 2016;31(4):485-492. Published online December 20, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.4.485
- 3,866 View
- 47 Download
- 13 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The tumor microenvironment is comprised of diverse stromal cell populations in addition to tumor cells. Increasing evidence now clearly supports the role of microenvironment stromal cells in tumor progression and metastasis, yet the regulatory mechanisms and interactions among tumor and stromal cells remain to be elucidated. Bone metastasis is the major problem in many types of human malignancies including prostate, breast and lung cancers, and the biological basis of bone metastasis let alone curative approaches are largely undetermined. Among the many types of stromal cells in bone, osteoblasts are shown to be an important player. In this regard, osteoblasts are a key target cell type in the development of bone metastasis, but there are currently no drugs or therapeutic approaches are available that specifically target osteoblasts. This review paper summarizes the current knowledge on osteoblasts in the metastatic tumor microenvironment, aiming to provide clues and directions for future research endeavor.
-
Citations
Citations to this article as recorded by- Bone marrow adipocytes and lung cancer bone metastasis: unraveling the role of adipokines in the tumor microenvironment
Jian Li, Jialu Wu, Yanni Xie, Xijie Yu
Frontiers in Oncology.2024;[Epub] CrossRef - Circulating biomarkers for diagnosis and therapeutic monitoring in bone metastasis
Min-Kyoung Song, Serk In Park, Sun Wook Cho
Journal of Bone and Mineral Metabolism.2023; 41(3): 337. CrossRef - Mobilization of monocytic myeloid-derived suppressor cells is regulated by PTH1R activation in bone marrow stromal cells
Eun Jung Lee, Kyoung Jin Lee, Seungpil Jung, Kyong Hwa Park, Serk In Park
Bone Research.2023;[Epub] CrossRef - 2E‐Decene‐4,6‐diyn‐1‐ol‐acetate inhibits osteoclastogenesis through mitogen‐activated protein kinase‐c‐Fos‐NFATc1 signalling pathways
Young Ran Park, Xiang‐Dong Su, Saroj Kumar Shrestha, Seo Young Yang, Yunjo Soh
Clinical and Experimental Pharmacology and Physiology.2022; 49(3): 341. CrossRef - The let-7f-5p–Nme4 pathway mediates tumor necrosis factor α-induced impairment in osteogenesis of bone marrow-derived mesenchymal stem cells
Ying-Jie Zhao, Zheng-Chao Gao, Xi-Jing He, Jing Li
Biochemistry and Cell Biology.2021; 99(4): 488. CrossRef - Circulating Osteocalcin-Positive Cells as a Novel Diagnostic Biomarker for Bone Metastasis in Breast Cancer Patients
Kyung-Hun Lee, Kyoung Jin Lee, Tae-Yong Kim, Febby Hutomo, Hyun Jin Sun, Gi Jeong Cheon, Serk In Park, Sun Wook Cho, Seock-Ah Im
Journal of Bone and Mineral Research.2020; 35(10): 1838. CrossRef - The Early Results of Vertebral Pathological Compression Fracture of Extra- nodal Lymphoma with HIV-positive Patients Treated by Percutaneous Kyphoplasty
Sheng Sun, Biao Xu, Qiang Zhang, Chang-song Zhao, Rui Ma, Jie He, Yao Zhang
Current HIV Research.2020; 18(4): 248. CrossRef - Sema4D expression and secretion are increased by HIF-1α and inhibit osteogenesis in bone metastases of lung cancer
Wu-gui Chen, Jing Sun, Wei-wei Shen, Si-zhen Yang, Ying Zhang, Xu Hu, Hao Qiu, Shang-cheng Xu, Tong-wei Chu
Clinical & Experimental Metastasis.2019; 36(1): 39. CrossRef - Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis
Changki Lee, Young Mi Whang, Preston Campbell, Patrick L. Mulcrone, Florent Elefteriou, Sun Wook Cho, Serk In Park
Cancer Letters.2018; 414: 205. CrossRef - Targeting the tumour stroma to improve cancer therapy
Kenneth C. Valkenburg, Amber E. de Groot, Kenneth J. Pienta
Nature Reviews Clinical Oncology.2018; 15(6): 366. CrossRef - Bone Microenvironment and Role of Rank-Rankl-Opg in Breast Cancer Metastasis in Bone
Hongwei Zhang
Journal of Cancer Prevention & Current Research.2017;[Epub] CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Bone marrow adipocytes and lung cancer bone metastasis: unraveling the role of adipokines in the tumor microenvironment


 KES
KES

 First
First Prev
Prev



