Search
- Page Path
- HOME > Search
Original Article
- Diabetes, obesity and metabolism
- Efficacy and Safety of Omarigliptin, a Novel Once-Weekly Dipeptidyl Peptidase-4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- A.B.M. Kamrul-Hasan, Muhammad Shah Alam, Samir Kumar Talukder, Deep Dutta, Shahjada Selim
- Endocrinol Metab. 2024;39(1):109-126. Published online January 23, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1839

- 1,090 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
No recent meta-analysis has holistically analyzed and summarized the efficacy and safety of omarigliptin in type 2 diabetes mellitus (T2DM). We conducted a meta-analysis to address this knowledge gap.
Methods
Electronic databases were searched to identify randomized controlled trials (RCTs) that included patients with T2DM who received omarigliptin in the intervention arm. The control arm consisted of either a placebo (passive control group [PCG]) or an active comparator (active control group [ACG]). The primary outcome assessed was changes in hemoglobin A1c (HbA1c), while secondary outcomes included variations in glucose levels, achievement of glycemic targets, adverse events (AEs), and hypoglycemic events.
Results
From 332 initially screened articles, data from 16 RCTs involving 8,804 subjects were analyzed. Omarigliptin demonstrated superiority over placebo in reducing HbA1c levels (mean difference, –0.58%; 95% confidence interval, –0.75 to –0.40; P<0.00001; I2=91%). Additionally, omarigliptin outperformed placebo in lowering fasting plasma glucose, 2-hour postprandial glucose, and in the percentage of participants achieving HbA1c levels below 7.0% and 6.5%. The glycemic efficacy of omarigliptin was similar to that of the ACG across all measures. Although the omarigliptin group experienced a higher incidence of hypoglycemic events compared to the PCG, the overall AEs, serious AEs, hypoglycemia, and severe hypoglycemia were comparable between the omarigliptin and control groups (PCG and ACG).
Conclusion
Omarigliptin has a favorable glycemic efficacy and safety profile for managing T2DM.

Review Article
- Adrenal gland
- The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin
- Eline C. Nijenhuis-Noort, Kirsten A. Berk, Sebastian J. C. M. M. Neggers, Aart J. van der Lely
- Endocrinol Metab. 2024;39(1):83-89. Published online January 9, 2024
- DOI: https://doi.org/10.3803/EnM.2024.101
- 1,487 View
- 111 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
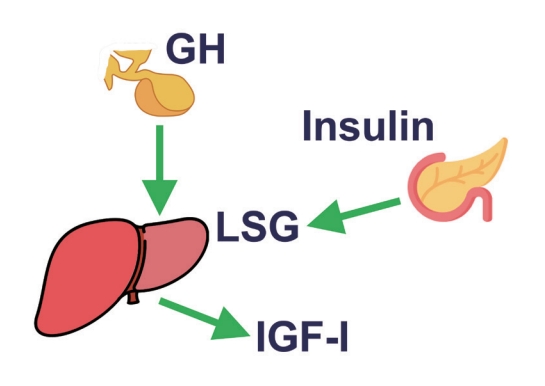
ePub - This review intends to provide the reader with a practical overview of several (patho)physiological conditions in which knowledge of the interplay between growth hormone (GH), insulin-like growth factor-1 (IGF-1), and insulin is important. This might help treating physicians in making the right decisions on how to intervene and improve metabolism for the benefit of patients, and to understand why and how metabolism responds in their specific cases. We will specifically address the interplay between GH, IGF-1, and insulin in type 1 and 2 diabetes mellitus, liver cirrhosis, and acromegaly as examples in which this knowledge is truly necessary.
-
Citations
Citations to this article as recorded by- IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review
Justyna Szydlowska-Gladysz, Adrianna Edyta Gorecka, Julia Stepien, Izabela Rysz, Iwona Ben-Skowronek
International Journal of Molecular Sciences.2024; 25(7): 3966. CrossRef
- IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review

Original Articles
- Effectiveness of a Social Networking Site Based Automatic Mobile Message Providing System on Glycemic Control in Patients with Type 2 Diabetes Mellitus
- Kyuho Kim, Jae-Seung Yun, Joonyub Lee, Yeoree Yang, Minhan Lee, Yu-Bae Ahn, Jae Hyoung Cho, Seung-Hyun Ko
- Received October 30, 2023 Accepted December 21, 2023 Published online December 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1871 [Epub ahead of print]
- 481 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the effectiveness of a social networking site (SNS)-based automatic mobile message providing system on glycemic control in patients with type 2 diabetes mellitus (T2DM).
Methods
A 3-month, randomized, open-label, controlled, parallel-group trial was conducted. One hundred and ten participants with T2DM were randomized to a mobile message system (MMS) (n=55) or control group (n=55). The MMS group received protocolbased automated messages two times per day for 10 weeks regarding diabetes self-management through KakaoTalk SNS messenger. The primary outcome was the difference in the change in glycated hemoglobin (HbA1c) levels (%) from baseline to week 12.
Results
HbA1c levels were more markedly decreased in the MMS group (8.4%±0.7% to 8.0%±1.1%) than in the control group (8.5%±0.8% to 8.4%±0.8%), resulting in a significant between-group difference (P=0.027). No differences were observed in changes in fasting glucose levels, lipid profiles, and the number of participants who experienced hypoglycemia, or in changes in lifestyle behavior between groups. However, the self-monitoring of blood glucose frequency was significantly increased in the MMS group compared to the control group (P=0.003). In addition, sleep duration was increased in the MMS group, but was not changed in the control group.
Conclusion
An SNS-based automatic mobile message providing system was effective in improving glycemic control in patients in T2DM. Studies which based on a more individualized protocol, and investigate longer beneficial effect and sustainability will be required in the future.

- Calcium & bone metabolism
- Association between Smoking Status and the Risk of Hip Fracture in Patients with Type 2 Diabetes: A Nationwide Population-Based Study
- Se-Won Lee, Jun-Young Heu, Ju-Yeong Kim, Jinyoung Kim, Kyungdo Han, Hyuk-Sang Kwon
- Endocrinol Metab. 2023;38(6):679-689. Published online December 6, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1760

- 1,134 View
- 63 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Limited longitudinal evidence exists regarding the potential association between smoking status and hip fracture among individuals with type 2 diabetes. We investigated this association using large-scale, nationwide cohort data for the Korean population.
Methods
This nationwide cohort study included 1,414,635 adults aged 40 and older who received Korean National Health Insurance Service health examinations between 2009 and 2012. Subjects with type 2 diabetes were categorized according to their smoking status, amount smoked (pack-years), number of cigarettes smoked per day, and duration of smoking. The results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between smoking status parameters and risk of hip fracture in multivariable Cox proportional hazard regression analysis.
Results
Compared with never-smokers, an increased adjusted HR (aHR) for hip fracture was observed in current smokers (1.681; 95% CI, 1.578 to 1.791), and a comparable aHR for hip fracture was found in former smokers (1.065; 95% CI, 0.999 to 1.136). For former smokers who had smoked 20 pack-years or more, the risk was slightly higher than that for never-smokers (aHR, 1.107; 95% CI, 1.024 to 1.196). The hip fracture risk of female former smokers was similar to that of female current smokers, but the hip fracture risk in male former smokers was similar to that of male never-smokers.
Conclusion
Smoking is associated with an increased risk of hip fracture in patients with type 2 diabetes. Current smokers with diabetes should be encouraged to quit smoking because the risk of hip fracture is greatly reduced in former smokers.

Review Article
- Diabetes, obesity and metabolism
- Initial Combination Therapy in Type 2 Diabetes
- Ji Yoon Kim, Nam Hoon Kim
- Endocrinol Metab. 2024;39(1):23-32. Published online November 30, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1816
- 1,892 View
- 226 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
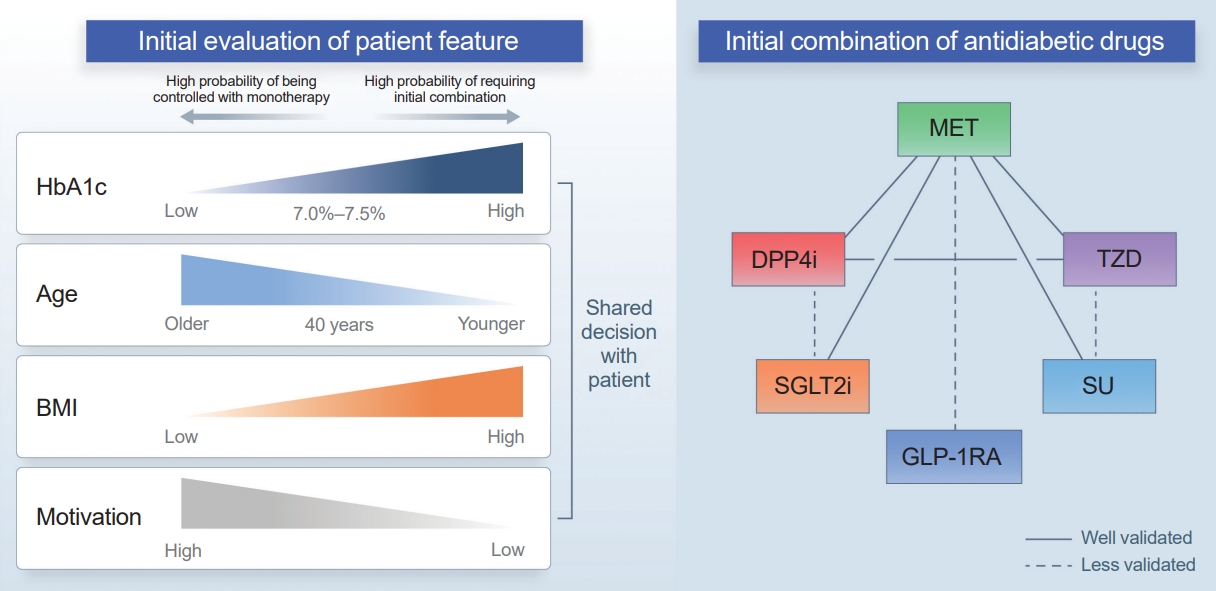
ePub - Type 2 diabetes (T2D) is a progressive disease in which it is challenging to achieve long-term durable glycemic control. However, intensive glycemic control is crucial for preventing diabetes-related complications. Previous studies showed that monotherapy with a stepwise add-on approach was seldom effective for long-term durable glycemic control. Combination therapy, which refers to the use of two or more drugs to control hyperglycemia, has multiple benefits, including the ability to target a variety of pathophysiological processes underlying hyperglycemia. In clinical trials, initial combination therapy showed better glycemic control than monotherapy or a stepwise approach. Emerging evidence indicates that initial combination therapy is associated with preserved β-cell function and fewer complications in T2D. However, cost-effectiveness and adverse events with combination therapy are issues that should be considered. Therefore, initial combination therapy is an important option for patients with T2D that clinicians should consider with a view toward balancing benefits and potential harms. In this review, we summarize the literature addressing initial combination therapy in T2D, and we suggest optimal strategies based on clinical situations and patient characteristics.

Original Article
- Miscellaneous
- Prediction of Cardiovascular Complication in Patients with Newly Diagnosed Type 2 Diabetes Using an XGBoost/GRU-ODE-Bayes-Based Machine-Learning Algorithm
- Joonyub Lee, Yera Choi, Taehoon Ko, Kanghyuck Lee, Juyoung Shin, Hun-Sung Kim
- Endocrinol Metab. 2024;39(1):176-185. Published online November 21, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1739

- 1,170 View
- 53 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Cardiovascular disease is life-threatening yet preventable for patients with type 2 diabetes mellitus (T2DM). Because each patient with T2DM has a different risk of developing cardiovascular complications, the accurate stratification of cardiovascular risk is critical. In this study, we proposed cardiovascular risk engines based on machine-learning algorithms for newly diagnosed T2DM patients in Korea.
Methods
To develop the machine-learning-based cardiovascular disease engines, we retrospectively analyzed 26,166 newly diagnosed T2DM patients who visited Seoul St. Mary’s Hospital between July 2009 and April 2019. To accurately measure diabetes-related cardiovascular events, we designed a buffer (1 year), an observation (1 year), and an outcome period (5 years). The entire dataset was split into training and testing sets in an 8:2 ratio, and this procedure was repeated 100 times. The area under the receiver operating characteristic curve (AUROC) was calculated by 10-fold cross-validation on the training dataset.
Results
The machine-learning-based risk engines (AUROC XGBoost=0.781±0.014 and AUROC gated recurrent unit [GRU]-ordinary differential equation [ODE]-Bayes=0.812±0.016) outperformed the conventional regression-based model (AUROC=0.723± 0.036).
Conclusion
GRU-ODE-Bayes-based cardiovascular risk engine is highly accurate, easily applicable, and can provide valuable information for the individualized treatment of Korean patients with newly diagnosed T2DM.

Review Article
- Diabetes, obesity and metabolism
- The Benefits Of Continuous Glucose Monitoring In Pregnancy
- Jee Hee Yoo, Jae Hyeon Kim
- Endocrinol Metab. 2023;38(5):472-481. Published online October 11, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1805
- 2,503 View
- 210 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
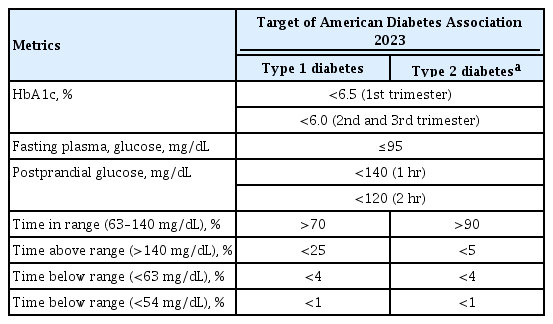
ePub - Previous studies have consistently demonstrated the positive effects of continuous glucose monitoring (CGM) on glycemic outcomes and complications of diabetes in people with type 1 diabetes. Guidelines now consider CGM to be an essential and cost-effective device for managing type 1 diabetes. As a result, insurance coverage for it is available. Evidence supporting CGM continues to grow and expand to broader populations, such as pregnant people with type 1 diabetes, people with type 2 diabetes treated only with basal insulin therapy, and even type 2 diabetes that does not require insulin treatment. However, despite the significant risk of hyperglycemia in pregnancy, which leads to complications in more than half of affected newborns, CGM indications and insurance coverage for those patients are unresolved. In this review article, we discuss the latest evidence for using CGM to offer glycemic control and reduce perinatal complications, along with its cost-effectiveness in pregestational type 1 and type 2 diabetes and gestational diabetes mellitus. In addition, we discuss future prospects for CGM coverage and indications based on this evidence.
-
Citations
Citations to this article as recorded by- Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends
Mohammad Mansour, M. Saeed Darweesh, Ahmed Soltan
Alexandria Engineering Journal.2024; 89: 224. CrossRef
- Wearable devices for glucose monitoring: A review of state-of-the-art technologies and emerging trends

Original Articles
- Diabetes, obesity and metabolism
- Coronary Artery Calcium Score as a Sensitive Indicator of Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus: A Long-Term Cohort Study
- Dae-Jeong Koo, Mi Yeon Lee, Sun Joon Moon, Hyemi Kwon, Sang Min Lee, Se Eun Park, Cheol-Young Park, Won-Young Lee, Ki Won Oh, Sung Rae Cho, Young-Hoon Jeong, Eun-Jung Rhee
- Endocrinol Metab. 2023;38(5):568-577. Published online October 10, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1770

- 1,463 View
- 110 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Coronary artery calcium score (CACS) has become an important tool for evaluating cardiovascular disease (CVD). This study evaluated the significance of CACS for future CVD through more than 10 years of follow-up in asymptomatic Korean populations with type 2 diabetes mellitus (T2DM) known to have a relatively low CACS burden.
Methods
We enrolled 981 asymptomatic T2DM patients without CVD at baseline who underwent CACS evaluation using multidetector computed tomography between January 2008 and December 2014. They were grouped into five predefined CACS categories based on Agatston scores and followed up by August 2020. The primary endpoint was incident CVD events, including coronary, cerebrovascular, and peripheral arterial disease.
Results
The relative risk of CVD was significantly higher in patients with CACS ≥10, and the significance persisted after adjustment for known confounders. A higher CACS category indicated a higher incidence of future CVD: hazard ratio (95% confidence interval) 4.09 (1.79 to 9.36), 12.00 (5.61 to 25.69), and 38.79 (16.43 to 91.59) for 10≤ CACS <100, 100≤ CACS <400, and CACS ≥400, respectively. During the 12-year follow-up period, the difference in event-free survival more than doubled as the category increased. Patients with CACS below 10 had very low CVD incidence throughout the follow-up. The receiver operating characteristic analysis showed better area under curve when the CACS cutoff was 10 than 100.
Conclusion
CACS can be a sensitive marker of CVD risk. Specifically, CACS above 10 is an indicator of CVD high-risk requiring more intensive medical treatment in Koreans with T2DM.

- Diabetes, obesity and metabolism
- Intake of Fruit and Glycemic Control in Korean Patients with Diabetes Mellitus Using the Korea National Health and Nutrition Examination Survey
- Eunju Yoon, Ji Cheol Bae, Sunghwan Suh
- Endocrinol Metab. 2023;38(5):538-544. Published online August 8, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1730
- 1,860 View
- 108 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
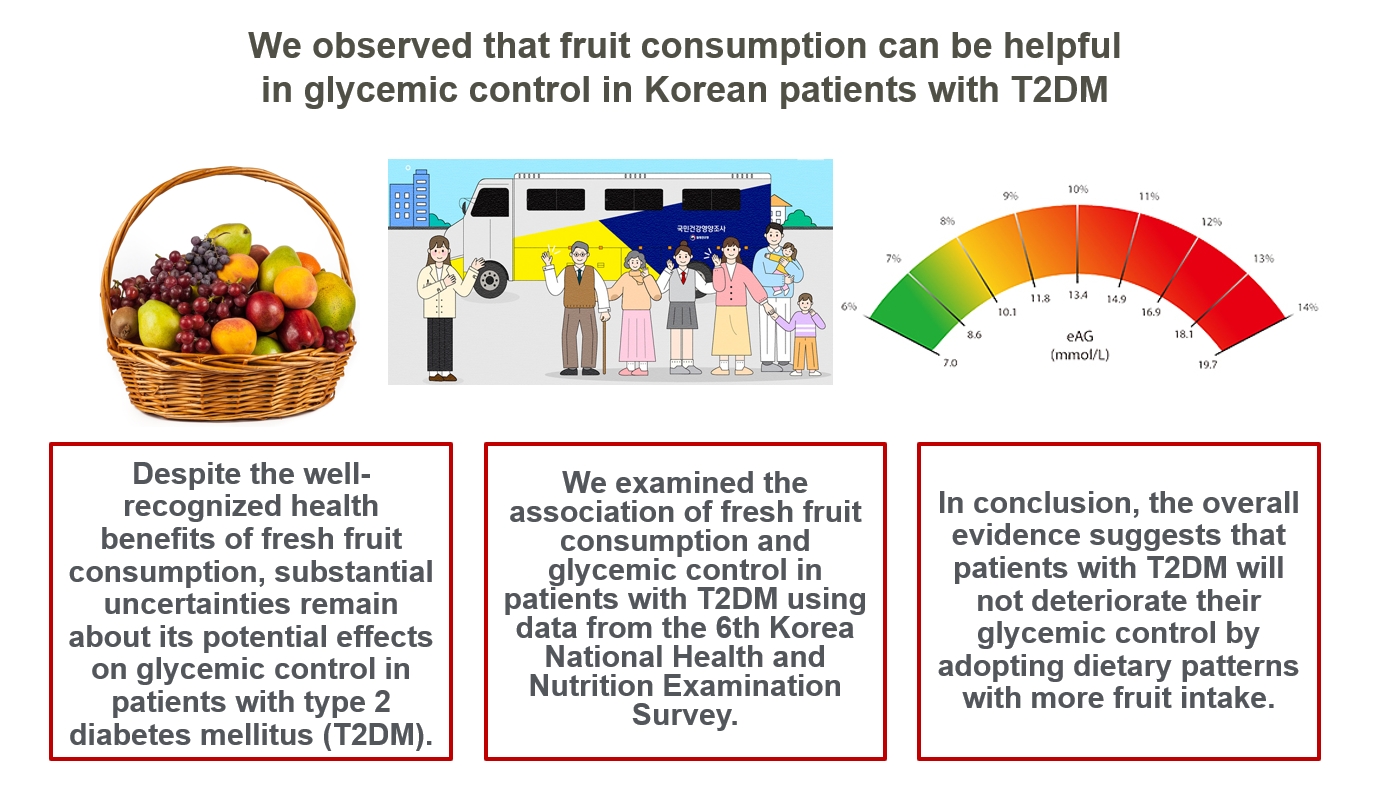
Despite the well-recognized health benefits of fresh fruit consumption, there is still substantial uncertainty about its potential effects on glycemic control in patients with type 2 diabetes mellitus (T2DM).
Methods
We examined the association of fresh fruit consumption and glycemic control in patients with T2DM using data from the 6th Korea National Health and Nutrition Examination Survey. The study sample was divided into three groups based on weekly fruit consumption frequency for the analysis.
Results
Patients with the highest fruit intake were older than those in the other two groups, and women were more likely to consume fruits in general. Being a current smoker and weekly alcohol intake also showed negative correlations according to the fruit intake tertiles. Fruit consumption was positively correlated with better hemoglobin A1c (HbA1c) levels. Moreover, patients in the highest tertile of fruit intake were 3.48 times more likely to be in good glycemic control defined as HbA1c <7%.
Conclusion
We observed that fruit consumption can be helpful in glycemic control in Korean patients with T2DM. -
Citations
Citations to this article as recorded by- The Relationship between Alcohol Consumption and Diabetes in Korean Adults
Gi Tae Kim, Jae Woong Sull
Biomedical Science Letters.2023; 29(3): 159. CrossRef
- The Relationship between Alcohol Consumption and Diabetes in Korean Adults

- Diabetes, obesity and metabolism
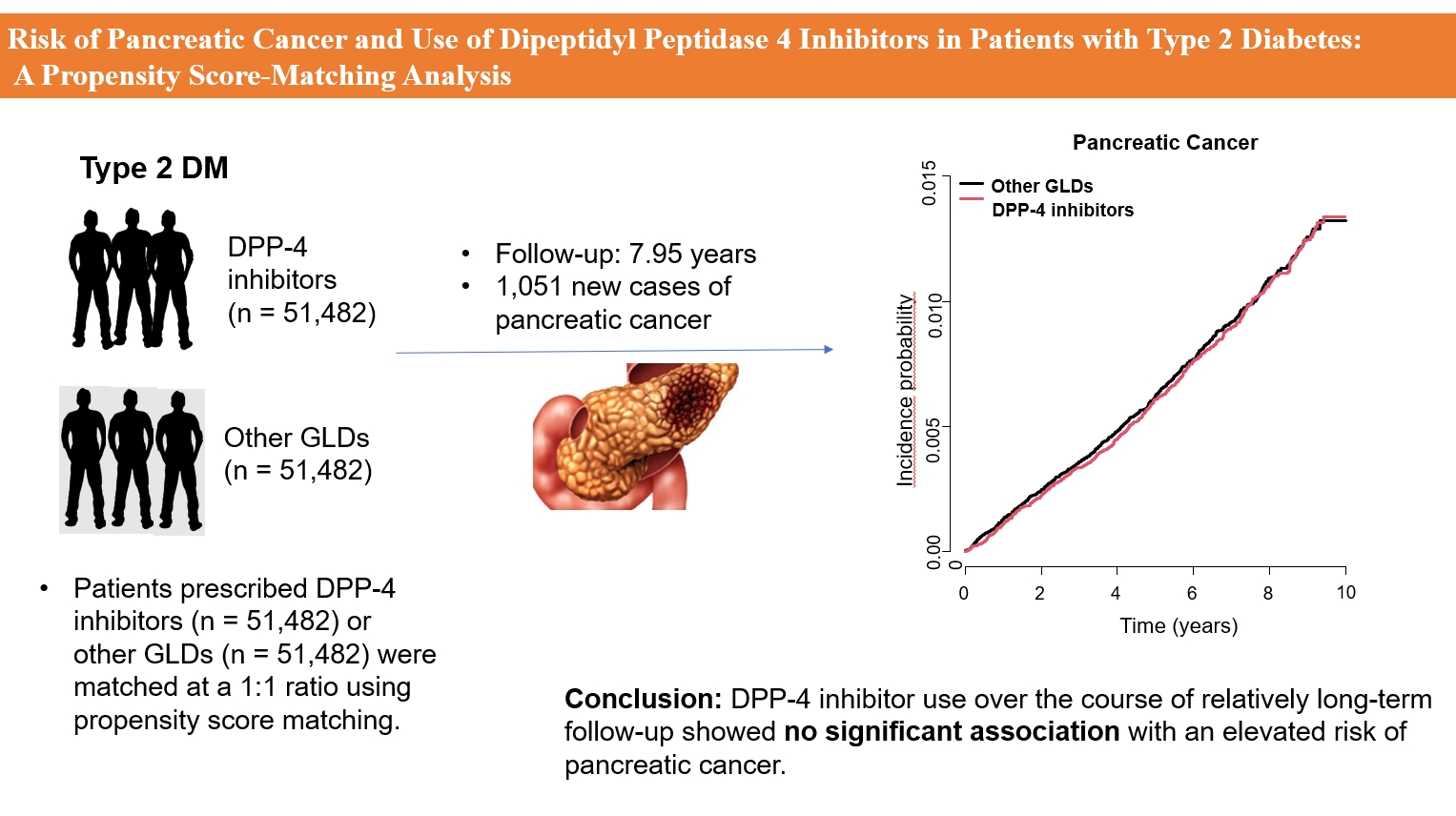
- Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
- Mee Kyoung Kim, Kyungdo Han, Hyuk-Sang Kwon, Soon Jib Yoo
- Endocrinol Metab. 2023;38(4):426-435. Published online July 20, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1737
- 2,054 View
- 132 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The effects of dipeptidyl peptidase 4 (DPP-4) inhibitors over the course of long-term treatment remain unclear, and concerns have been raised regarding the role of DPP-4 inhibitors in carcinogenesis in the pancreas. Earlier studies of pancreatic adverse events have reported conflicting results.
Methods
This study analyzed Korean National Health Insurance Service data from January 2009 to December 2012. Patients who had type 2 diabetes mellitus and took two or more oral glucose-lowering drugs (GLDs) were included. Patients prescribed DPP-4 inhibitors (n=51,482) or other GLDs (n=51,482) were matched at a 1:1 ratio using propensity score matching. The risk of pancreatic cancer was calculated using Kaplan-Meier curves and Cox proportional-hazards regression analysis.
Results
During a median follow-up period of 7.95 years, 1,051 new cases of pancreatic cancer were identified. The adjusted hazard ratio (HR) for DPP-4 inhibitor use was 0.99 (95% confidence interval [CI], 0.88 to 1.12) compared with the other GLD group. In an analysis limited to cases diagnosed with pancreatic cancer during hospitalization, the adjusted HR for the use of DPP-4 inhibitors was 1.00 (95% CI, 0.86 to 1.17) compared with patients who took other GLDs. Using the other GLD group as the reference group, no trend was observed for elevated pancreatic cancer risk with increased DPP-4 inhibitor exposure.
Conclusion
In this population-based cohort study, DPP-4 inhibitor use over the course of relatively long-term follow-up showed no significant association with an elevated risk of pancreatic cancer. -
Citations
Citations to this article as recorded by- Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef
- Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes

- Diabetes, obesity and metabolism
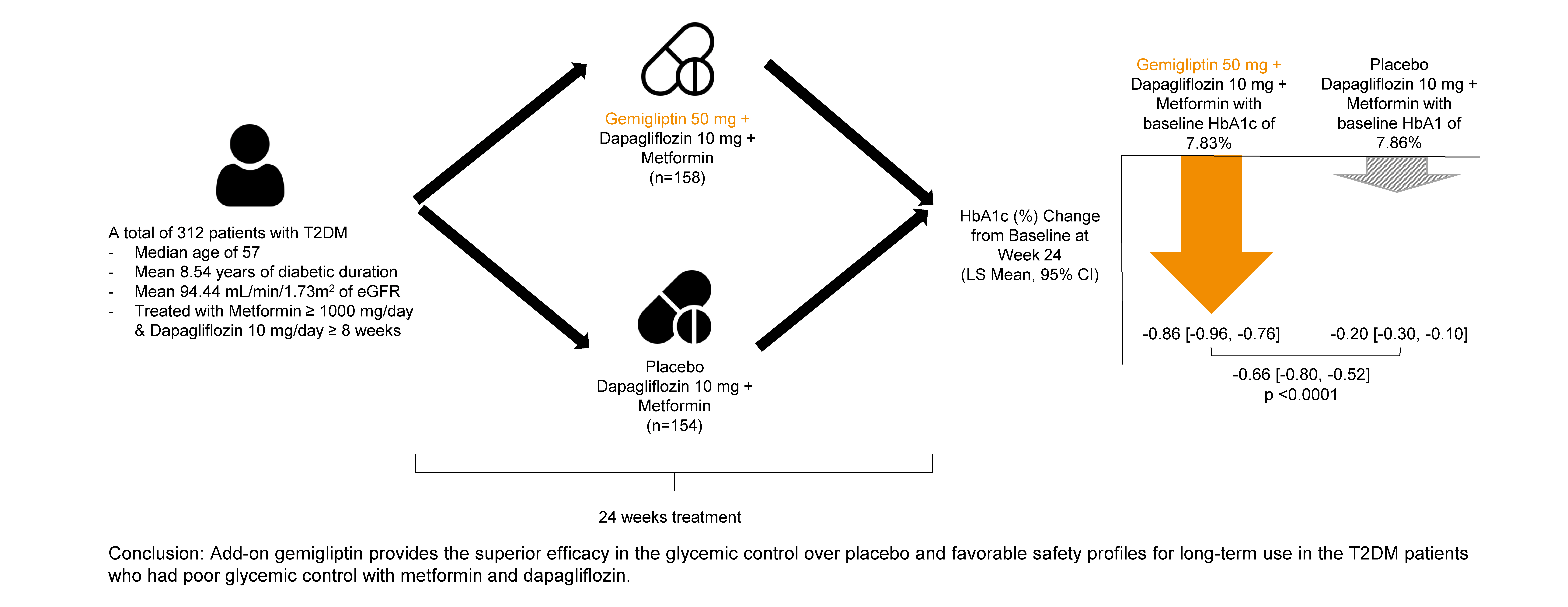
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Byung Wan Lee, KyungWan Min, Eun-Gyoung Hong, Bon Jeong Ku, Jun Goo Kang, Suk Chon, Won-Young Lee, Mi Kyoung Park, Jae Hyeon Kim, Sang Yong Kim, Keeho Song, Soon Jib Yoo
- Endocrinol Metab. 2023;38(3):328-337. Published online June 28, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1688
- 2,341 View
- 253 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study evaluated the efficacy and safety of add-on gemigliptin in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycemic control with metformin and dapagliflozin.
Methods
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study, 315 patients were randomized to receive either gemigliptin 50 mg (n=159) or placebo (n=156) with metformin and dapagliflozin for 24 weeks. After the 24-week treatment, patients who received the placebo were switched to gemigliptin, and all patients were treated with gemigliptin for an additional 28 weeks.
Results
The baseline characteristics were similar between the two groups, except for body mass index. At week 24, the least squares mean difference (standard error) in hemoglobin A1c (HbA1c) changes was –0.66% (0.07) with a 95% confidence interval of –0.80% to –0.52%, demonstrating superior HbA1c reduction in the gemigliptin group. After week 24, the HbA1c level significantly decreased in the placebo group as gemigliptin was administered, whereas the efficacy of HbA1c reduction was maintained up to week 52 in the gemigliptin group. The safety profiles were similar: the incidence rates of treatment-emergent adverse events up to week 24 were 27.67% and 29.22% in the gemigliptin and placebo groups, respectively. The safety profiles after week 24 were similar to those up to week 24 in both groups, and no new safety findings, including hypoglycemia, were noted.
Conclusion
Add-on gemigliptin was well tolerated, providing comparable safety profiles and superior efficacy in glycemic control over placebo for long-term use in patients with T2DM who had poor glycemic control with metformin and dapagliflozin.

Review Article
- Calcium & bone metabolism
- Skeletal Senescence with Aging and Type 2 Diabetes
- Joshua Nicholas Farr
- Endocrinol Metab. 2023;38(3):295-301. Published online June 14, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1727

- 2,636 View
- 125 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Osteoporosis and type 2 diabetes (T2D) are common diseases that often coexist. While both of these diseases are associated with poor bone quality and increased fracture risk, their pathogenesis of increased fracture risk differs and is multifactorial. Mounting evidence now indicates that key fundamental mechanisms that are central to both aging and energy metabolism exist. Importantly, these mechanisms represent potentially modifiable therapeutic targets for interventions that could prevent or alleviate multiple complications of osteoporosis and T2D, including poor bone quality. One such mechanism that has gained increasing momentum is senescence, which is a cell fate that contributes to multiple chronic diseases. Accumulating evidence has established that numerous boneresident cell types become susceptible to cellular senescence with old age. Recent work also demonstrates that T2D causes the premature accumulation of senescent osteocytes during young adulthood, at least in mice, although it remains to be seen which other bone-resident cell types become senescent with T2D. Given that therapeutically removing senescent cells can alleviate age-related bone loss and T2D-induced metabolic dysfunction, it will be important in future studies to rigorously test whether interventions that eliminate senescent cells can also alleviate skeletal dysfunction in context of T2D, as it does with aging.
-
Citations
Citations to this article as recorded by- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Cellular Neuroscience.2024;[Epub] CrossRef - The synergistic effect of diabetes mellitus and osteoporosis on the all-cause mortality: a cohort study of an American population
Weihua Li, Siyu Xie, Shengdong Zhong, Liting Lan
Frontiers in Endocrinology.2024;[Epub] CrossRef - Identification of systemic biomarkers and potential drug targets for age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Aging Neuroscience.2024;[Epub] CrossRef
- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration

Original Articles
- Diabetes, obesity and metabolism
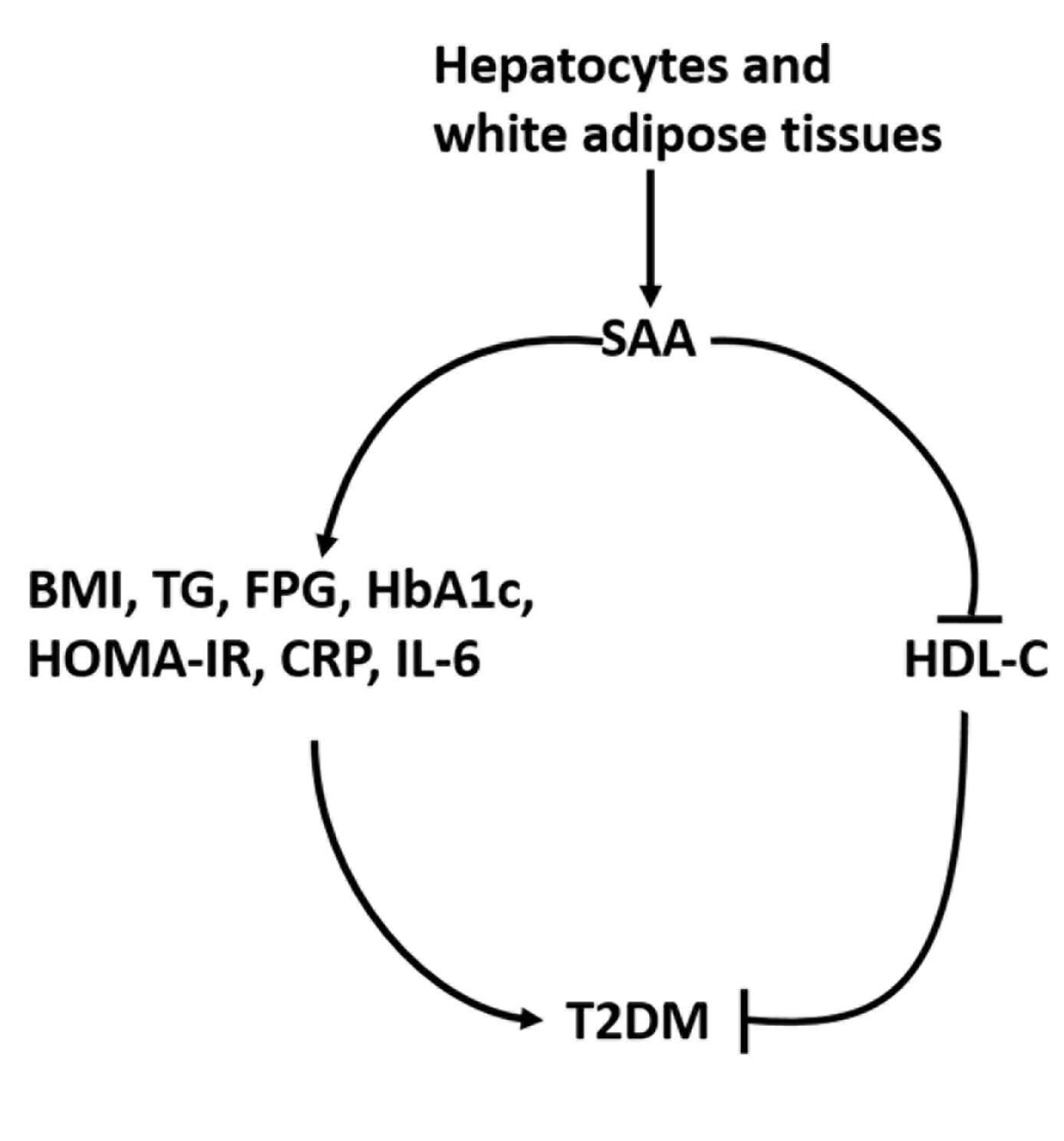
- Association between Serum Amyloid A Levels and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- Ting Liu, Meng Li, Chunying Cui, Jielin Zhou
- Endocrinol Metab. 2023;38(3):315-327. Published online June 7, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1621
- 2,083 View
- 102 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
To date, consistent data have not been reported on the association between serum amyloid A (SAA) levels and type 2 diabetes mellitus (T2DM). The purpose of this study was to systematically summarize their relationship.
Methods
Databases including PubMed, Cochrane Library, Embase, Web of Science, and MEDLINE were searched until August 2021. Cross-sectional and case-control studies were included.
Results
Twenty-one studies with 1,780 cases and 2,070 controls were identified. SAA levels were significantly higher in T2DM patients than in healthy groups (standardized mean difference [SMD], 0.68; 95% confidence interval [CI], 0.39 to 0.98). A subgroup analysis showed that the mean age of participants and the continent that participants were from were related to differences in SAA levels between cases and controls. Furthermore, in T2DM patients, SAA levels were positively associated with body mass index (r=0.34; 95% CI, 0.03 to 0.66), triglycerides (r=0.12; 95% CI, 0.01 to 0.24), fasting plasma glucose (r=0.26; 95% CI, 0.07 to 0.45), hemoglobin A1c (r=0.24; 95% CI, 0.16 to 0.33), homeostasis model assessment for insulin resistance (r=0.22; 95% CI, 0.10 to 0.34), C-reactive protein (r=0.77; 95% CI, 0.62 to 0.91), and interleukin-6 (r=0.42; 95% CI, 0.31 to 0.54), but negatively linked with highdensity lipoprotein cholesterol (r=–0.23; 95% CI, –0.44 to –0.03).
Conclusion
The meta-analysis suggests that high SAA levels may be associated with the presence of T2DM, as well as lipid metabolism homeostasis and the inflammatory response. -
Citations
Citations to this article as recorded by- Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients
Yuanyuan Zhang, Huaizhen Liu
BMC Endocrine Disorders.2024;[Epub] CrossRef - Antioxidant and Anti-Inflammatory Functions of High-Density Lipoprotein in Type 1 and Type 2 Diabetes
Damien Denimal
Antioxidants.2023; 13(1): 57. CrossRef
- Correlation between insulin resistance and the rate of neutrophils-lymphocytes, monocytes-lymphocytes, platelets-lymphocytes in type 2 diabetic patients

- Diabetes, obesity and metabolism
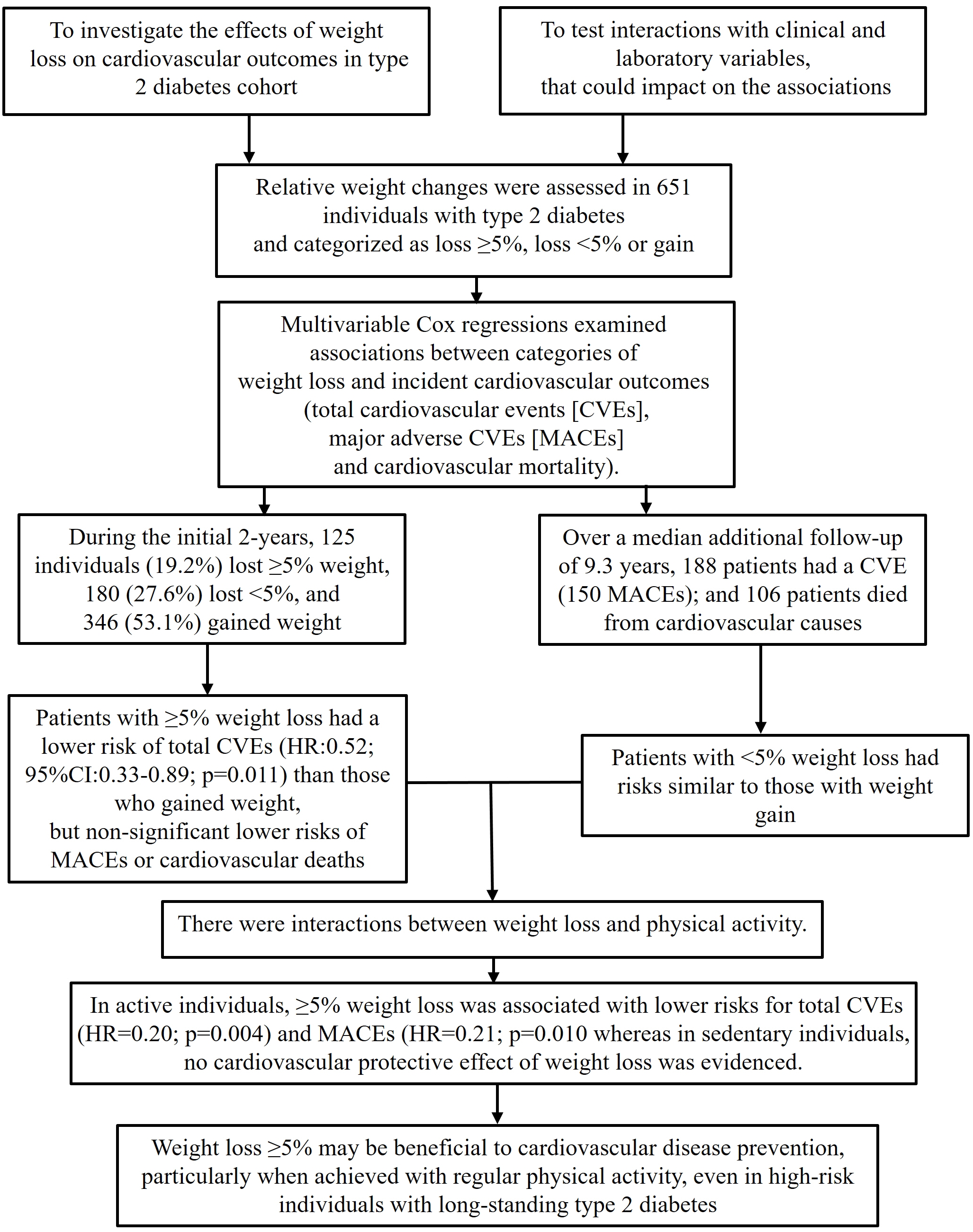
- Effects of Weight Loss and Interaction with Physical Activity on Risks of Cardiovascular Outcomes in Individuals with Type 2 Diabetes
- Claudia R. L. Cardoso, Nathalie C. Leite, Gil F. Salles
- Endocrinol Metab. 2023;38(3):305-314. Published online May 31, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1690
- 2,493 View
- 136 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the effects of weight loss during follow-up on cardiovascular outcomes in a type 2 diabetes cohort and tested interactions with clinical and laboratory variables, particularly physical activity, that could impact the associations.
Methods
Relative weight changes were assessed in 651 individuals with type 2 diabetes and categorized as ≥5% loss, <5% loss, or gain. Associations between weight loss categories and incident cardiovascular outcomes (total cardiovascular events [CVEs], major adverse cardiovascular events [MACEs], and cardiovascular mortality) were assessed using multivariable Cox regression with interaction analyses.
Results
During the initial 2 years, 125 individuals (19.2%) lost ≥5% of their weight, 180 (27.6%) lost <5%, and 346 (53.1%) gained weight. Over a median additional follow-up of 9.3 years, 188 patients had CVEs (150 MACEs) and 106 patients died from cardiovascular causes. Patients with ≥5% weight loss had a significantly lower risk of total CVEs (hazard ratio [HR], 0.52; 95% confidence interval, 0.33 to 0.89; P=0.011) than those who gained weight, but non-significant lower risks of MACEs or cardiovascular deaths. Patients with <5% weight loss had risks similar to those with weight gain. There were interactions between weight loss and physical activity. In active individuals, ≥5% weight loss was associated with significantly lower risks for total CVEs (HR, 0.20; P=0.004) and MACEs (HR, 0.21; P=0.010), whereas in sedentary individuals, no cardiovascular protective effect of weight loss was evidenced.
Conclusion
Weight loss ≥5% may be beneficial for cardiovascular disease prevention, particularly when achieved with regular physical activity, even in high-risk individuals with long-standing type 2 diabetes. -
Citations
Citations to this article as recorded by- Weight change in patients with new‐onset type 2 diabetes mellitus and its association with remission: Comprehensive real‐world data
Jinyoung Kim, Bongseong Kim, Mee Kyoung Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024; 26(2): 567. CrossRef - Cardiovascular Risk Reduction in Type 2 Diabetes: Further Insights into the Power of Weight Loss and Exercise
Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(3): 302. CrossRef - Effects of body weight variability on risks of macro- and microvascular outcomes in individuals with type 2 diabetes: The Rio de Janeiro type 2 diabetes cohort
Claudia R.L. Cardoso, Nathalie C. Leite, Gil F. Salles
Diabetes Research and Clinical Practice.2023; 205: 110992. CrossRef
- Weight change in patients with new‐onset type 2 diabetes mellitus and its association with remission: Comprehensive real‐world data

- Diabetes, obesity and metabolism
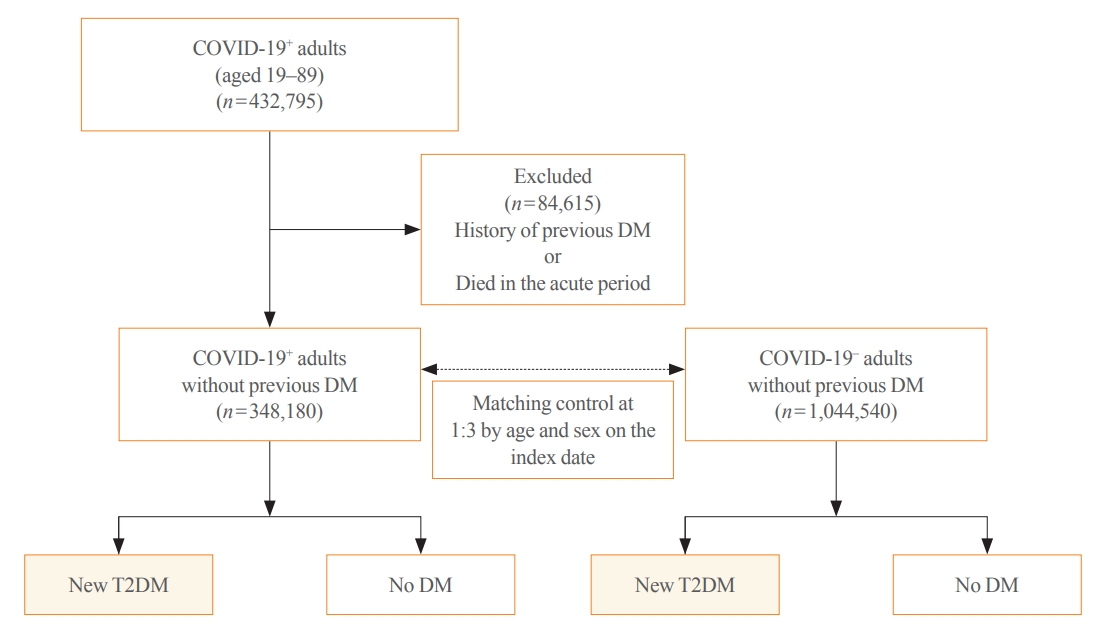
Big Data Articles (National Health Insurance Service Database) - Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
- Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
- Endocrinol Metab. 2023;38(2):245-252. Published online April 5, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1662
- 2,192 View
- 118 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
Methods
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
Conclusion
COVID-19 may increase the risk of developing T2DM beyond the acute period. The higher the severity of COVID-19 in the acute phase, the higher the risk of newly diagnosed T2DM. Therefore, T2DM should be included as a component of managing long-term COVID-19. -
Citations
Citations to this article as recorded by- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review
Anca Pantea Stoian, Ioana-Cristina Bica, Teodor Salmen, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez,
Diabetes Therapy.2024; 15(1): 33. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review


 KES
KES

 First
First Prev
Prev



