Search
- Page Path
- HOME > Search
- Thyroid
- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
- Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
- Endocrinol Metab. 2023;38(3):338-346. Published online June 9, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1664
- 1,684 View
- 100 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
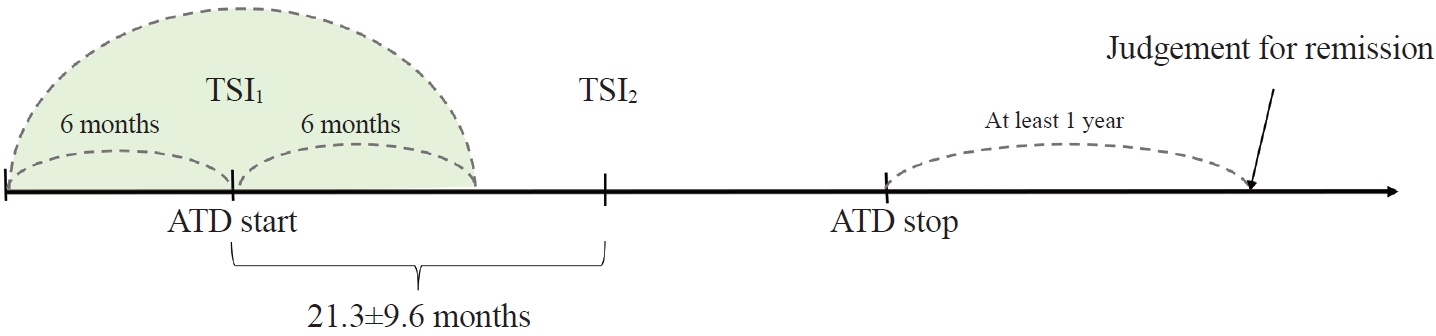
To determine whether baseline thyroid-stimulating immunoglobulin (TSI) bioassay or its early response upon treatment with an anti-thyroid drug (ATD) can predict prognosis of Graves’ disease (GD) in real-world practice.
Methods
This retrospective study enrolled GD patients who had previous ATD treatment with TSI bioassay checked at baseline and at follow-up from April 2010 to November 2019 in one referral hospital. The study population were divided into two groups: patients who experienced relapse or continued ATD (relapse/persistence), and patients who experienced no relapse after ATD discontinuation (remission). The slope and area under the curve at 1st year (AUC1yr) of thyroid-stimulating hormone receptor antibodies including TSI bioassay and thyrotropin-binding inhibitory immunoglobulin (TBII) were calculated as differences between baseline and second values divided by time duration (year).
Results
Among enrolled 156 study subjects, 74 (47.4%) had relapse/persistence. Baseline TSI bioassay values did not show significant differences between the two groups. However, the relapse/persistence group showed less decremental TSI bioassay in response to ATD than the remission group (–84.7 [TSI slope, –198.2 to 8.2] vs. –120.1 [TSI slope, –204.4 to –45.9], P=0.026), whereas the TBII slope was not significantly different between the two groups. The relapse/persistence group showed higher AUC1yr of TSI bioassay and TBII in the 1st year during ATD treatment than the remission group (AUC1yr for TSI bioassay, P=0.0125; AUC1yr for TBII,P =0.001).
Conclusion
Early changes in TSI bioassay can better predict prognosis of GD than TBII. Measurement of TSI bioassay at beginning and follow-up could help predict GD prognosis. -
Citations
Citations to this article as recorded by- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Long-term effect of thyrotropin-binding inhibitor immunoglobulin on atrial fibrillation in euthyroid patients
Jung-Chi Hsu, Kang-Chih Fan, Ting-Chuan Wang, Shu-Lin Chuang, Ying-Ting Chao, Ting-Tse Lin, Kuan-Chih Huang, Lian-Yu Lin, Lung-Chun Lin
Endocrine Practice.2024;[Epub] CrossRef
- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study

- Thyroid
- Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
- Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
- Endocrinol Metab. 2022;37(6):891-900. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1590
- 2,460 View
- 253 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
An excess of thyroid hormones in Graves’ disease (GD) has profound effects on systemic energy metabolism that are currently partially understood. In this study, we aimed to provide a comprehensive understanding of the metabolite changes that occur when patients with GD transition from hyperthyroidism to euthyroidism with methimazole treatment.
Methods
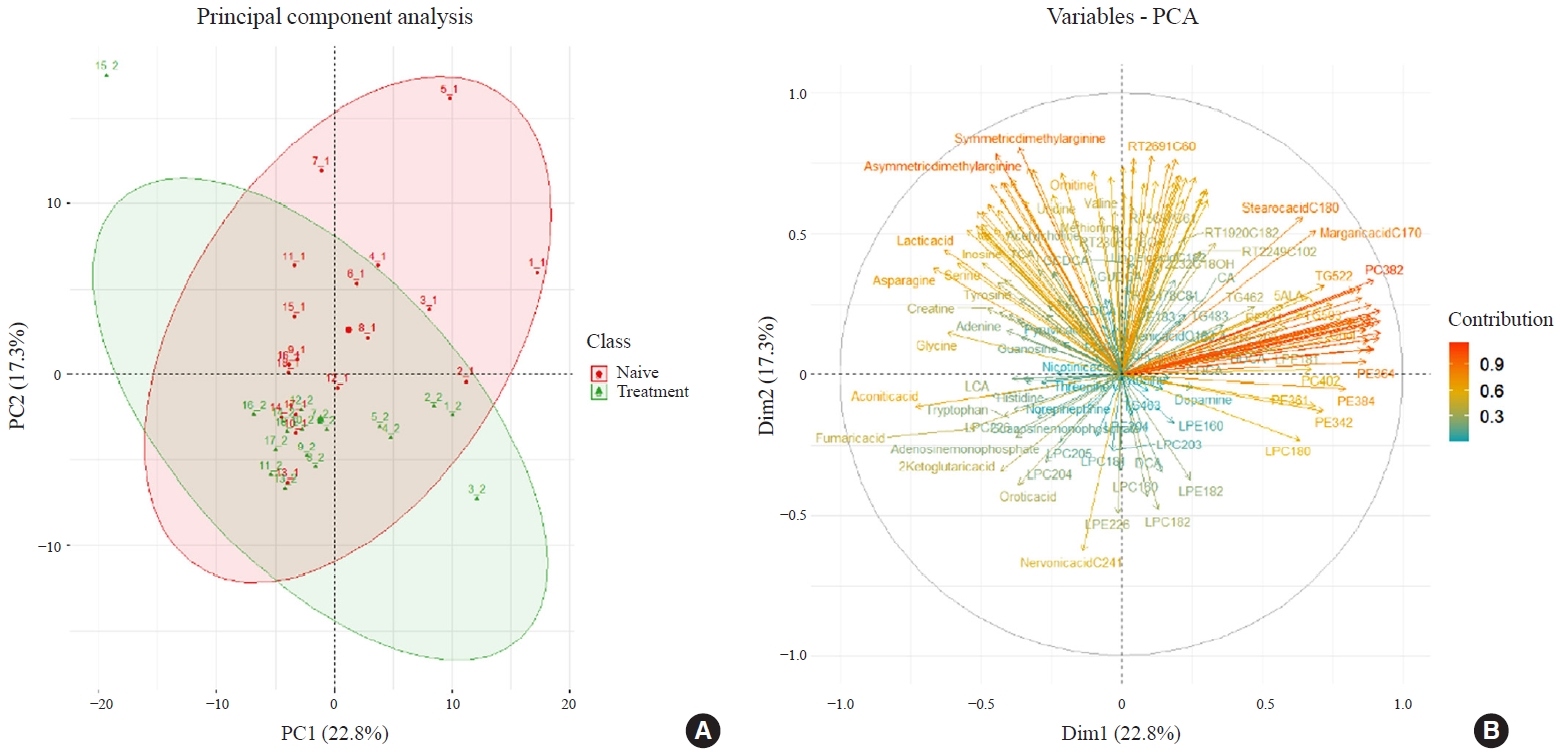
Eighteen patients (mean age, 38.6±14.7 years; 66.7% female) with newly diagnosed or relapsed GD attending the endocrinology outpatient clinics in a single institution were recruited between January 2019 and July 2020. All subjects were treated with methimazole to achieve euthyroidism. We explored metabolomics by performing liquid chromatography-mass spectrometry analysis of plasma samples of these patients and then performed multivariate statistical analysis of the metabolomics data.
Results
Two hundred metabolites were measured before and after 12 weeks of methimazole treatment in patients with GD. The levels of 61 metabolites, including palmitic acid (C16:0) and oleic acid (C18:1), were elevated in methimazole-naïve patients with GD, and these levels were decreased by methimazole treatment. The levels of another 15 metabolites, including glycine and creatinine, were increased after recovery of euthyroidism upon methimazole treatment in patients with GD. Pathway analysis of metabolomics data showed that hyperthyroidism was closely related to aminoacyl-transfer ribonucleic acid biosynthesis and branched-chain amino acid biosynthesis pathways.
Conclusion
In this study, significant variations of plasma metabolomic patterns that occur during the transition from hyperthyroidism to euthyroidism were detected in patients with GD via untargeted metabolomics analysis. -
Citations
Citations to this article as recorded by- Associations of serum keratin 1 with thyroid function and immunity in Graves’ disease
Chao-Wen Cheng, Wen-Fang Fang, Jiunn-Diann Lin, Appuwawadu Mestri Nipun Lakshitha de Silva
PLOS ONE.2023; 18(11): e0289345. CrossRef
- Associations of serum keratin 1 with thyroid function and immunity in Graves’ disease

- Thyroid
- Clinical Outcomes of Repeated Radioactive Iodine Therapy for Graves’ Disease
- Min Joo Kim, Sun Wook Cho, Ye An Kim, Hoon Sung Choi, Young Joo Park, Do Joon Park, Bo Youn Cho
- Endocrinol Metab. 2022;37(3):524-532. Published online June 16, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1418
- 4,757 View
- 225 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
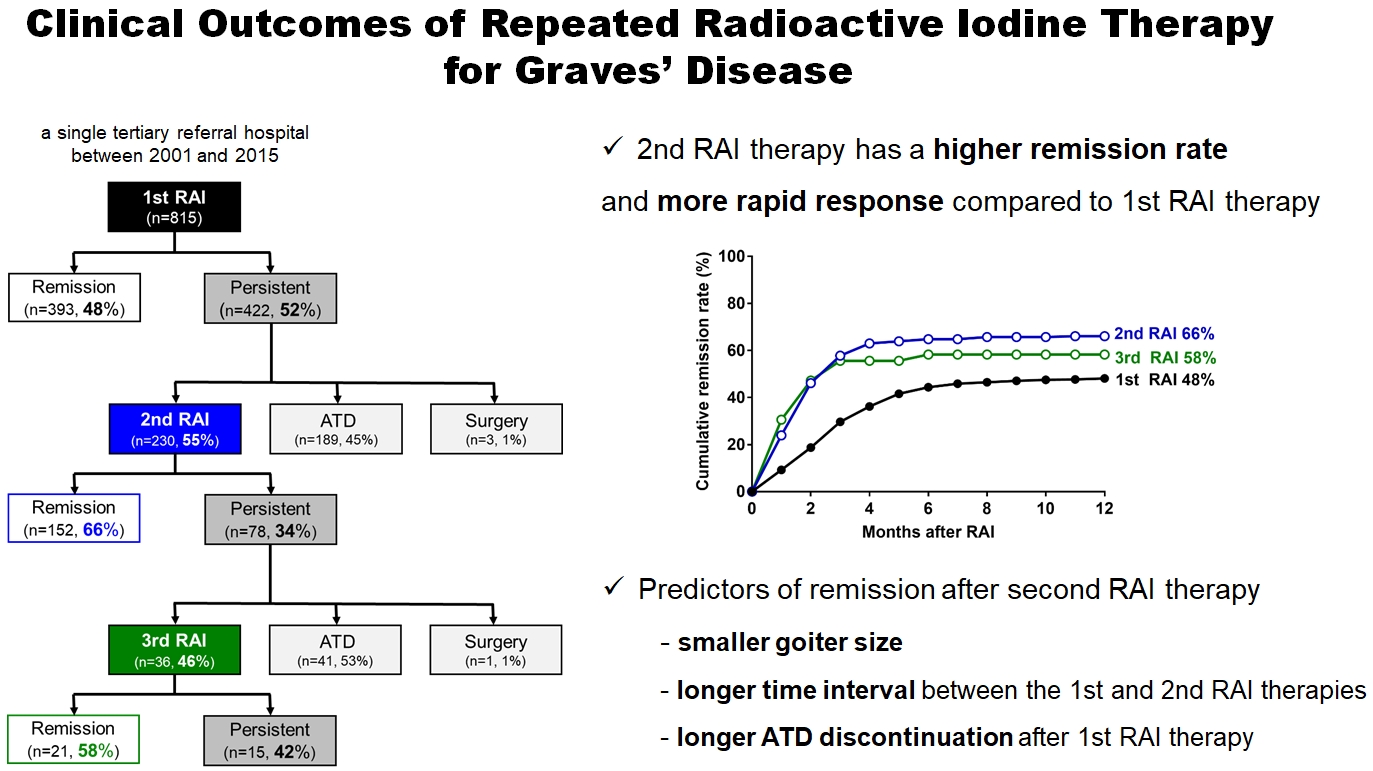
Radioactive iodine (RAI) therapy is a successful therapeutic modality for Graves’ disease. However, RAI therapy can fail, and RAI therapy after antithyroid drugs (ATDs) has a lower remission rate. Therefore, many patients require repeated RAI therapy. This study investigated the clinical outcomes of repeated RAI therapy for Graves’ disease.
Methods
Patients who underwent RAI therapy as second-line therapy after failure of ATD treatment between 2001 and 2015 were reviewed. Remission was defined as hypothyroid or euthyroid status without ATD, and with or without levothyroxine at 12 months after RAI therapy.
Results
The 1-year remission rate after 2nd RAI therapy (66%, 152/230) is significantly higher than that after 1st RAI therapy (48%, 393/815) or long-term ATD treatment after 1st RAI therapy failure (42%). The clinical response to 2nd RAI therapy was more rapid. The median time intervals from the 2nd RAI therapy to ATD discontinuation (1.3 months) and to the start of levothyroxine replacement (2.5 months) were significantly shorter than those for the 1st RAI therapy. A smaller goiter size, a longer time interval between the 1st and 2nd RAI therapies, and a longer ATD discontinuation period predicted remission after the 2nd RAI therapy. Finally, in 78 patients who failed the 2nd RAI therapy, the mean ATD dosage significantly reduced 5.1 mg over 12 months.
Conclusion
Repeated RAI therapy can be a good therapeutic option, especially in patients with smaller goiters and those who are more responsive to the 1st RAI therapy. -
Citations
Citations to this article as recorded by- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Effect of liver dysfunction on outcome of radioactive iodine therapy for Graves’ disease
Yuyang Ze, Fei Shao, Xuefeng Feng, Shanmei Shen, Yan Bi, Dalong Zhu, Xiaowen Zhang
BMC Endocrine Disorders.2022;[Epub] CrossRef
- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease

- Thyroid
- Immunoglobulin G4-Related Thyroid Disease: A Single-Center Experience and Literature Review
- Meihua Jin, Bictdeun Kim, Ahreum Jang, Min Ji Jeon, Young Jun Choi, Yu-Mi Lee, Dong Eun Song, Won Gu Kim
- Endocrinol Metab. 2022;37(2):312-322. Published online April 25, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1318
- 3,961 View
- 176 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
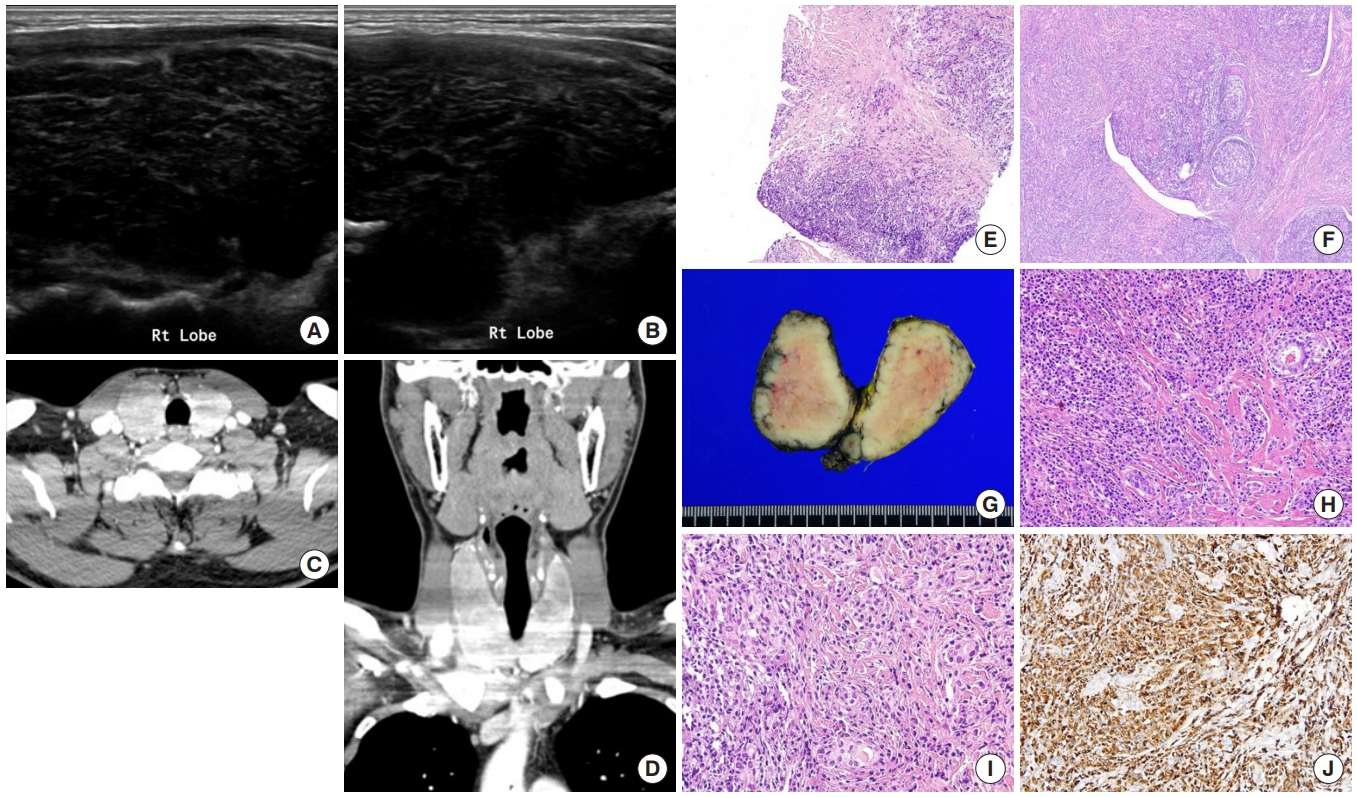
Immunoglobulin G4 (IgG4)-related disease is an entity that can involve the thyroid gland. The spectrum of IgG4-related thyroid disease (IgG4-RTD) includes Hashimoto thyroiditis (HT) and its fibrotic variant, Riedel thyroiditis, as well as Graves’ disease. The early diagnosis of IgG4-RTD is important because it is a medically treatable disease, and a delay in the diagnosis might result in unnecessary surgery. We present a case series of IgG4-RTD with a review of the literature.
Methods
We retrospectively reviewed the clinical presentation and the radiological and pathological findings of patients diagnosed with IgG4-RTD between 2017 and 2021 at a tertiary medical center in Korea. We also conducted a literature review of IgG4-RTD.
Results
Five patients were diagnosed with IgG4-RTD during the study period. The patients’ age ranged from 31 to 76 years, and three patients were men. Most patients visited the clinic for a neck mass, and hypoechogenic nodular lesions were observed on neck ultrasonography. Three patients had IgG4 HT, and two patients had IgG4 Riedel thyroiditis. All patients developed hypothyroidism that necessitated L-thyroxine replacement. The diagnosis of IgG4-RTD was confirmed after a pathological examination of the surgical specimen in the first two cases. However, the early diagnosis was possible after a core needle biopsy in three clinically suspected patients.
Conclusion
The diagnosis of IgG4-RTD requires clinical suspicion combined with serology and histological analyses using IgG4 immunostaining. The early diagnosis of IgG4-RTD is difficult; thus, biopsy with IgG4 immunostaining and serum IgG4 measurements will help diagnose patients suspected of having IgG4-RTD. -
Citations
Citations to this article as recorded by- Are sonographic characteristics of Hashimoto’s thyroiditis related with immunologic parameters? A cross-sectional study
K. Kenarlı, A. B. Bahçecioğlu, Ö. B. Aksu, S. Güllü
Journal of Endocrinological Investigation.2024;[Epub] CrossRef - Reshaping the Concept of Riedel’s Thyroiditis into the Larger Frame of IgG4-Related Disease (Spectrum of IgG4-Related Thyroid Disease)
Mara Carsote, Claudiu Nistor
Biomedicines.2023; 11(6): 1691. CrossRef
- Are sonographic characteristics of Hashimoto’s thyroiditis related with immunologic parameters? A cross-sectional study

- Thyroid
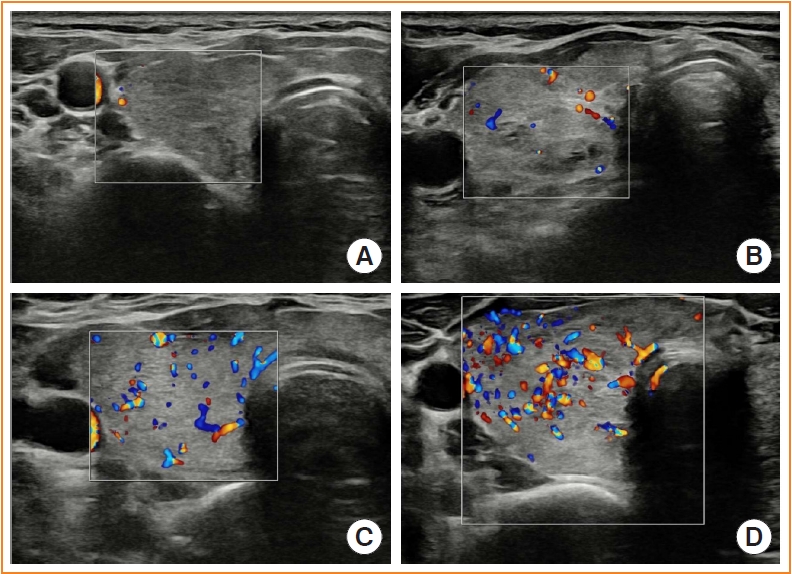
- Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves’ Disease from Destructive Thyroiditis in Thyrotoxic Patients
- Han-Sang Baek, Ji-Yeon Park, Chai-Ho Jeong, Jeonghoon Ha, Moo Il Kang, Dong-Jun Lim
- Endocrinol Metab. 2022;37(2):323-332. Published online April 13, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1413
- 3,635 View
- 142 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Microvascular ultrasonography (MVUS) is a third-generation Doppler technique that was developed to increase sensitivity compared to conventional Doppler. The purpose of this study was to compare MVUS with conventional color Doppler (CD) and power Doppler (PD) imaging to distinguish Graves’ disease (GD) from destructive thyroiditis (DT).
Methods
This prospective study included 101 subjects (46 GDs, 47 DTs, and eight normal controls) from October 2020 to November 2021. All ultrasonography examinations were performed using microvascular flow technology (MV-Flow). The CD, PD, and MVUS images were semi-quantitatively graded according to blood flow patterns. On the MVUS images, vascularity indices (VIs), which were the ratio (%) of color pixels in the total grayscale pixels in a defined region of interest, were obtained automatically. Receiver operating characteristic curve analysis was performed to verify the diagnostic performance of MVUS. The interclass correlation coefficient and Cohen’s kappa analysis were used to analyze the reliability of MVUS (ClinicalTrials.gov:NCT04879173).
Results
The area under the curve (AUC) for CD, PD, MVUS, and MVUS-VI was 0.822, 0.844, 0.808, and 0.852 respectively. The optimal cutoff value of the MVUS-VI was 24.95% for distinguishing GD and DT with 87% sensitivity and 80.9% specificity. We found a significant positive correlation of MVUS-VI with thyrotropin receptor antibody (r=0.554) and with thyroid stimulating immunoglobulin bioassay (r=0.841). MVUS showed high intra- and inter-observer reliability from various statistical method.
Conclusion
In a real time and quantitative manner, MVUS-VI could be helpful to differentiate GD from thyroiditis in thyrotoxic patients, with less inter-observer variability. -
Citations
Citations to this article as recorded by- Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management
Jin Wang, Ke Wan, Xin Chang, Rui-Feng Mao
World Journal of Diabetes.2024; 15(3): 348. CrossRef - The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Duplex Hemodynamic Parameters of Both Superior and Inferior Thyroid Arteries in Evaluation of Thyroid Hyperfunction Disorders
Maha Assem Hussein, Alaa Abdel Hamid, Rasha M Abdel Samie, Elshaymaa Hussein, Shereen Sadik Elsawy
International Journal of General Medicine.2022; Volume 15: 7131. CrossRef - Case 5: A 41-Year-Old Woman With Palpitation
Jiwon Yang, Kabsoo Shin, Jeongmin Lee, Jeonghoon Ha, Dong-Jun Lim, Han-Sang Baek
Journal of Korean Medical Science.2022;[Epub] CrossRef - Microvascular assessment of fascio-cutaneous flaps by ultrasound: A large animal study
Guillaume Goudot, Yanis Berkane, Eloi de Clermont-Tonnerre, Claire Guinier, Irina Filz von Reiterdank, Antonia van Kampen, Korkut Uygun, Curtis L. Cetrulo, Basak E. Uygun, Anahita Dua, Alexandre G. Lellouch
Frontiers in Physiology.2022;[Epub] CrossRef
- Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Graves’ Disease and the Risk of End-Stage Renal Disease: A Korean Population-Based Study
- Yoon Young Cho, Bongseong Kim, Dong Wook Shin, Hye Ryoun Jang, Bo-Yeon Kim, Chan-Hee Jung, Jae Hyeon Kim, Sun Wook Kim, Jae Hoon Chung, Kyungdo Han, Tae Hyuk Kim
- Endocrinol Metab. 2022;37(2):281-289. Published online April 6, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1333

- 3,841 View
- 133 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Hyperthyroidism is associated with an increased glomerular filtration rate (GFR) in the hyperdynamic state, which is reversible after restoring euthyroidism. However, long-term follow-up of renal dysfunction in patients with hyperthyroidism has not been performed.
Methods
This was a retrospective cohort study using the Korean National Health Insurance database and biannual health checkup data. We included 41,778 Graves’ disease (GD) patients and 41,778 healthy controls, matched by age and sex. The incidences of end-stage renal disease (ESRD) were calculated in GD patients and controls. The cumulative dose and duration of antithyroid drugs (ATDs) were calculated for each patient and categorized into the highest, middle, and lowest tertiles.
Results
Among 41,778 GD patients, 55 ESRD cases occurred during 268,552 person-years of follow-up. Relative to the controls, regardless of smoking, drinking, or comorbidities, including chronic kidney disease, GD patients had a 47% lower risk of developing ESRD (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.37 to 0.76). In particular, GD patients with a higher baseline GFR (≥90 mL/min/1.73 m2; HR, 0.33; 95% CI, 0.11 to 0.99), longer treatment duration (>33 months; HR, 0.31; 95% CI, 0.17 to 0.58) or higher cumulative dose (>16,463 mg; HR, 0.29; 95% CI, 0.15 to 0.57) of ATDs had a significantly reduced risk of ESRD.
Conclusion
This was the first epidemiological study on the effect of GD on ESRD, and we demonstrated that GD population had a reduced risk for developing ESRD. -
Citations
Citations to this article as recorded by- Renal function changes in patients with subclinical hyperthyroidism: a novel postulated mechanism
Magdy Mohamed Allam, Hanaa Tarek El-Zawawy, Tarek Hussein El-Zawawy
Endocrine.2023; 82(1): 78. CrossRef - Effect of Hyperthyroidism on Preventing Renal Insufficiency
Tae Yong Kim
Endocrinology and Metabolism.2022; 37(2): 220. CrossRef - Effects and Clinical Value of Peritoneal Dialysis on Water and Water Balance, Adverse Reactions, Quality of Life, and Clinical Prognosis in Patients with Decompensated Chronic Nephropathy: A Systematic Review and Meta-Analysis
Xichao Wang, Miaomiao Zhang, Na Sun, Wenxiu Chang, Gang Chen
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef
- Renal function changes in patients with subclinical hyperthyroidism: a novel postulated mechanism

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Risk of Diabetes in Patients with Long-Standing Graves’ Disease: A Longitudinal Study
- Eyun Song, Min Ji Koo, Eunjin Noh, Soon Young Hwang, Min Jeong Park, Jung A Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Geum Joon Cho, Hye Jin Yoo
- Endocrinol Metab. 2021;36(6):1277-1286. Published online December 16, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1251
- 5,111 View
- 180 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The detrimental effects of excessive thyroid hormone on glucose metabolism have been widely investigated. However, the risk of diabetes in patients with long-standing hyperthyroidism, especially according to treatment modality, remains uncertain, with few longitudinal studies.
Methods
The risk of diabetes in patients with Graves’ disease treated with antithyroid drugs (ATDs) for longer than the conventional duration (≥2 years) was compared with that in age-and sex-matched controls. The risk was further compared according to subsequent treatment modalities after a 24-month course of ATD: continuation of ATD (ATD group) vs. radioactive iodine ablation (RIA) group.
Results
A total of 4,593 patients were included. Diabetes was diagnosed in 751 (16.3%) patients over a follow-up of 7.3 years. The hazard ratio (HR) for diabetes, after adjusting for various known risk factors, was 1.18 (95% confidence interval [CI], 1.10 to 1.28) in patients with hyperthyroidism. Among the treatment modality groups, the RIA group (n=102) had a higher risk of diabetes than the ATD group (n=4,491) with HR of 1.56 (95% CI, 1.01 to 2.42). Further, the risk of diabetes increased with an increase in the ATD treatment duration (P for trend=0.019).
Conclusion
The risk of diabetes was significantly higher in patients with long-standing Graves’ disease than in the general population, especially in patients who underwent RIA and prolonged ATD treatment. Special attention to hyperglycemia during follow-up along with effective control of hyperthyroidism may be necessary to reduce the risk of diabetes in these patients. -
Citations
Citations to this article as recorded by- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience
Nadia Sawicka-Gutaj, Dawid Gruszczyński, Natalia Zawalna, Kacper Nijakowski, Agnieszka Skiba, Mateusz Pochylski, Jerzy Sowiński, Marek Ruchała
Pharmacological Reports.2024; 76(1): 185. CrossRef - Increased risk of diabetes mellitus and hyperlipidemia in patients with differentiated thyroid cancer
Hwa Young Ahn, Jooyoung Lee, Jinmo Kang, Eun Kyung Lee
European Journal of Endocrinology.2024; 190(3): 248. CrossRef - Prevalencia de diabetes en personas con disfunción tiroidea
Juan J. Díez, Pedro Iglesias
Medicina Clínica.2023; 160(8): 333. CrossRef - Control of Thyroid Dysfunction in Spanish Population Registered in
the Primary Care Clinical Database: An Analysis of the Proportion of Patients
with Thyrotropin Values Outside the Reference Range
Juan J. Díez, Pedro Iglesias
Hormone and Metabolic Research.2023; 55(03): 184. CrossRef - Prevalence of thyroid dysfunction and its relationship to income level and employment status: a nationwide population-based study in Spain
Juan J. Díez, Pedro Iglesias
Hormones.2023; 22(2): 243. CrossRef - Prevalence of diabetes in people with thyroid dysfunction
Juan J. Díez, Pedro Iglesias
Medicina Clínica (English Edition).2023; 160(8): 333. CrossRef - Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities
Mihaela Simona Popoviciu, Lorena Paduraru, Raluca Marinela Nutas, Alexandra Maria Ujoc, Galal Yahya, Kamel Metwally, Simona Cavalu
International Journal of Molecular Sciences.2023; 24(16): 12676. CrossRef - Thyroid Eye Disease and Its Association With Diabetes Mellitus: A Major Review
Roshmi Gupta, Pramila Kalra, Lakshmi B. Ramamurthy, Suryasnata Rath
Ophthalmic Plastic & Reconstructive Surgery.2023; 39(6S): S51. CrossRef - Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(6): 891. CrossRef - Diabetes and Hyperthyroidism: Is There a Causal Link?
Sang Yong Kim
Endocrinology and Metabolism.2021; 36(6): 1175. CrossRef
- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience

- Thyroid
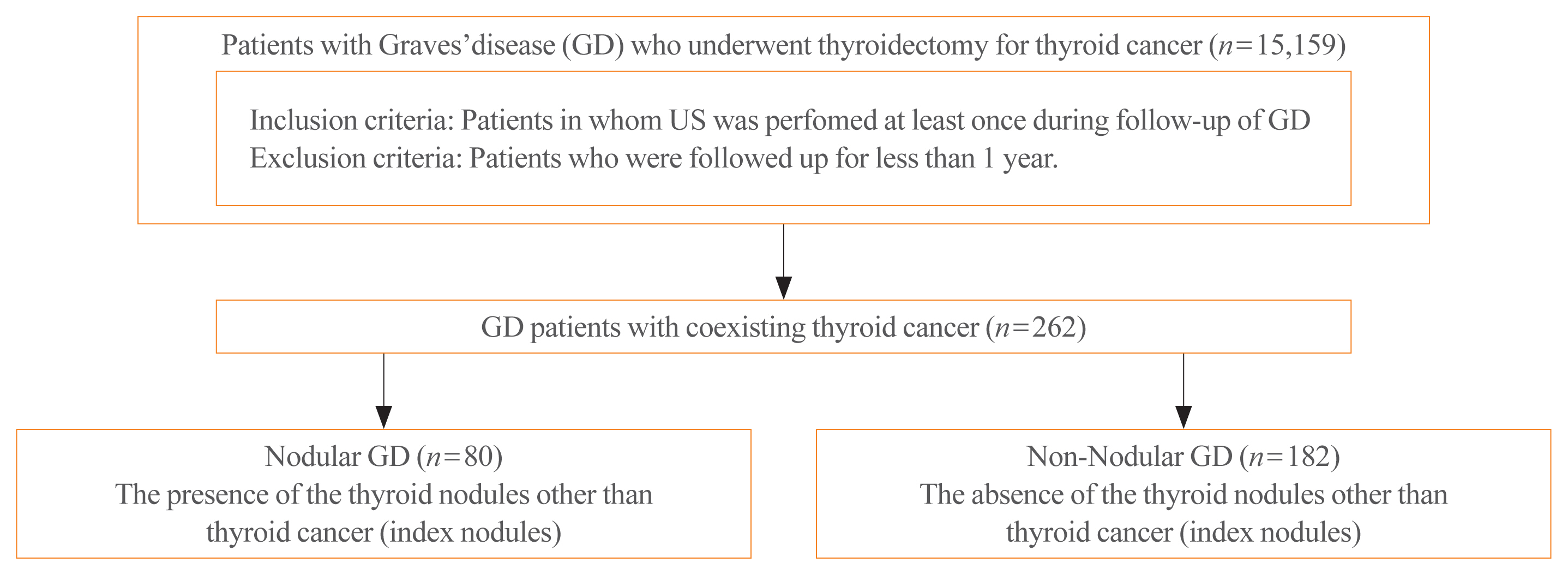
- Clinical Characteristics and Prognosis of Coexisting Thyroid Cancer in Patients with Graves’ Disease: A Retrospective Multicenter Study
- Jee Hee Yoon, Meihua Jin, Mijin Kim, A Ram Hong, Hee Kyung Kim, Bo Hyun Kim, Won Bae Kim, Young Kee Shong, Min Ji Jeon, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(6):1268-1276. Published online November 26, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1227
- 4,774 View
- 184 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The association between Graves’ disease (GD) and co-existing thyroid cancer is still controversial and most of the previously reported data have been based on surgically treated GD patients. This study investigated the clinicopathological findings and prognosis of concomitant thyroid cancer in GD patients in the era of widespread application of ultrasonography.
Methods
Data of GD patients who underwent thyroidectomy for thyroid cancer between 2010 and 2019 in three tertiary hospitals in South Korea (Asan Medical Center, Chonnam National University Hwasun Hospital, and Pusan National University Hospital) were collected and analyzed retrospectively. In the subgroup analysis, aggressiveness and clinical outcomes of thyroid cancer were compared nodular GD and non-nodular GD groups according to the presence or absence of the thyroid nodules other than thyroid cancer (index nodules).
Results
Of the 15,159 GD patients treated at the hospitals during the study period, 262 (1.7%) underwent thyroidectomy for coexisting thyroid cancer. Eleven patients (4.2%) were diagnosed with occult thyroid cancer and 182 patients (69.5%) had microcarcinomas. No differences in thyroid cancer aggressiveness, ultrasonographic findings, or prognosis were observed between the nodular GD and non-nodular GD groups except the cancer subtype. In the multivariate analysis, only lymph node (LN) metastasis was an independent prognostic factor for recurrent/persistent disease of thyroid cancer arising in GD (P=0.020).
Conclusion
The prevalence of concomitant thyroid cancer in GD patients was considerably lower than in previous reports. The clinical outcomes of thyroid cancer in GD patients were also excellent but, more cautious follow-up is necessary for patients with LN metastasis in the same way as for thyroid cancer in non-GD patients. -
Citations
Citations to this article as recorded by- Comparison of Surgical Outcomes of Transoral Versus Open Thyroidectomy for Graves Disease
Suo-Hsien Wang, Wu-Po Chao, Ta-You Lo, Soh-Ching Ng, Yu-Hsien Chen
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2024; 34(2): 150. CrossRef - Outcomes of Surgical Treatment for Graves’ Disease: A Single-Center Experience of 216 Cases
Hanxing Sun, Hui Tong, Xiaohui Shen, Haoji Gao, Jie Kuang, Xi Chen, Qinyu Li, Weihua Qiu, Zhuoran Liu, Jiqi Yan
Journal of Clinical Medicine.2023; 12(4): 1308. CrossRef - Cancer and Mortality Risks of Graves’ Disease in South Korea Based on National Data from 2010 to 2019
Young Ju Choi, Kyungdo Han, Won Kyoung Cho, Min Ho Jung, Byung-Kyu Suh
Clinical Epidemiology.2023; Volume 15: 535. CrossRef - Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review
Marco Palella, Francesca Maria Giustolisi, Adriana Modica Fiascaro, Martina Fichera, Antonella Palmieri, Rossella Cannarella, Aldo E. Calogero, Margherita Ferrante, Maria Fiore
Cancers.2023; 15(10): 2724. CrossRef - Characteristics, staging and outcomes of differentiated thyroid cancer in patients with and without Graves’ disease
Chaitra Gopinath, Hanna Crow, Sujata Panthi, Leonidas Bantis, Kenneth D. Burman, Chitra Choudhary
Journal of Clinical & Translational Endocrinology.2023; 33: 100321. CrossRef - Prevalence, Treatment Status, and Comorbidities of Hyperthyroidism in Korea from 2003 to 2018: A Nationwide Population Study
Hwa Young Ahn, Sun Wook Cho, Mi Young Lee, Young Joo Park, Bon Seok Koo, Hang-Seok Chang, Ka Hee Yi
Endocrinology and Metabolism.2023; 38(4): 436. CrossRef - Hashimoto’s Thyroiditis and Papillary Thyroid Carcinoma: A Follow-Up Study in Patients with Absence of Aggressive Risk Factors at the Surgery of the Primary Tumor
Andrea Marongiu, Susanna Nuvoli, Andrea De Vito, Sonia Vargiu, Angela Spanu, Giuseppe Madeddu
Diagnostics.2023; 13(19): 3068. CrossRef - Table of Contents
Clinical Thyroidology.2022; 34(2): 48. CrossRef - Predisposition to and Prognosis of Thyroid Cancer May Not Be Affected by Graves’ Disease, But Some Questions Still Remain
Yanrui Huang, Haixia Guan
Clinical Thyroidology.2022; 34(2): 59. CrossRef - A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease
Andrea Marongiu, Susanna Nuvoli, Andrea De Vito, Maria Rondini, Angela Spanu, Giuseppe Madeddu
Diagnostics.2022; 12(11): 2801. CrossRef - An unusual case of papillary thyroid carcinoma presenting as Graves’ disease
Pooja Tiwari, Uma Kaimal Saikia, Abhamoni Baro, Ashok Krishna Bhuyan
Thyroid Research and Practice.2022; 19(1): 47. CrossRef
- Comparison of Surgical Outcomes of Transoral Versus Open Thyroidectomy for Graves Disease

- Thyroid
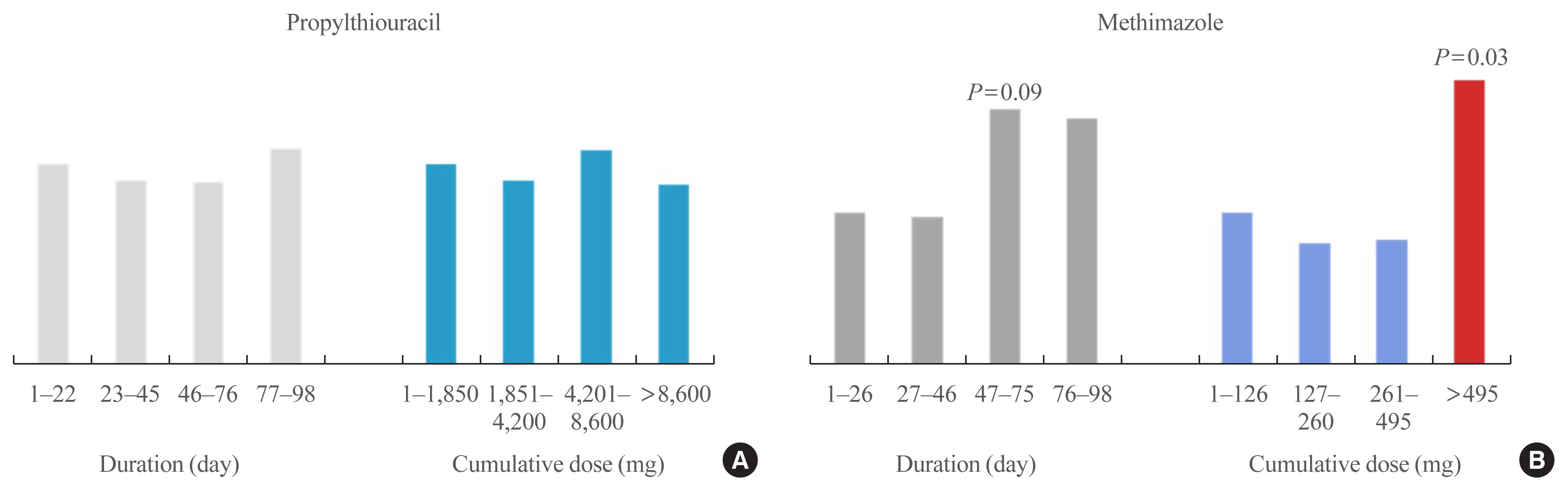
- Antithyroid Drug Treatment in Graves’ Disease
- Jae Hoon Chung
- Endocrinol Metab. 2021;36(3):491-499. Published online June 16, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1070
- 4,930 View
- 333 Download
- 6 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Graves’ disease is associated with thyrotropin (TSH) receptor stimulating antibody, for which there is no therapeutic agent. This disease is currently treated through inhibition of thyroid hormone synthesis or destruction of the thyroid gland. Recurrence after antithyroid drug (ATD) treatment is common. Recent studies have shown that the longer is the duration of use of ATD, the higher is the remission rate. Considering the relationship between clinical outcomes and iodine intake, recurrence of Graves’ disease is more common in iodine-deficient areas than in iodine-sufficient areas. Iodine restriction in an iodine-excessive area does not improve the effectiveness of ATD or increase remission rates. Recently, Danish and Korean nationwide studies noted significantly higher prevalence of birth defects in newborns exposed to ATD during the first trimester compared to that of those who did not have such exposure. The prevalence of birth defects was lowest when propylthiouracil (PTU) was used and decreased by only 0.15% when methimazole was changed to PTU in the first trimester. Therefore, it is best not to use ATD in the first trimester or to change to PTU before pregnancy.
-
Citations
Citations to this article as recorded by- Выраженность окислительного стресса и энзиматическая активность нейтрофилов крови у пациентов с болезнью Грейвса в зависимости от компенсации гипертиреоза
М. А. Дудина, С. А. Догадин, А. А. Савченко, И. И. Гвоздев
Ateroscleroz.2023; 18(4): 411. CrossRef - Application of oral inorganic iodine in the treatment of Graves’ disease
Yixuan Huang, Yihang Xu, Murong Xu, Xiaotong Zhao, Mingwei Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sex-specific risk factors associated with graves’ orbitopathy in Korean patients with newly diagnosed graves’ disease
Jooyoung Lee, Jinmo Kang, Hwa Young Ahn, Jeong Kyu Lee
Eye.2023; 37(16): 3382. CrossRef - Methimazole, an Effective Neutralizing Agent of the Sulfur Mustard Derivative 2-Chloroethyl Ethyl Sulfide
Albert Armoo, Tanner Diemer, Abigail Donkor, Jerrod Fedorchik, Severine Van slambrouck, Rachel Willand-Charnley, Brian A. Logue
ACS Bio & Med Chem Au.2023; 3(5): 448. CrossRef - Increased risk of incident gout in patients with hyperthyroidism: a nationwide retrospective cohort study
Ju-Yeun Lee, So-Yeon Park, Seo Young Sohn
Rheumatology International.2023; 44(3): 451. CrossRef - The influence of thionamides on intra-thyroidal uptake of 131I during radioiodine-131 treatment of Graves’ disease
Christian Happel, Benjamin Bockisch, Britta Leonhäuser, Amir Sabet, Frank Grünwald, Daniel Groener
Scientific Reports.2023;[Epub] CrossRef - Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves’ Disease from Destructive Thyroiditis in Thyrotoxic Patients
Han-Sang Baek, Ji-Yeon Park, Chai-Ho Jeong, Jeonghoon Ha, Moo Il Kang, Dong-Jun Lim
Endocrinology and Metabolism.2022; 37(2): 323. CrossRef - The chemiluminescent and enzymatic activity of blood neutrophils in patients with Graves' disease depending on hyperthyroidism compensation
M. A. Dudina, A. A. Savchenko, S. A. Dogadin, I. I. Gvozdev
Clinical and experimental thyroidology.2022; 18(1): 4. CrossRef - Risk of Diabetes in Patients with Long-Standing Graves’ Disease: A Longitudinal Study
Eyun Song, Min Ji Koo, Eunjin Noh, Soon Young Hwang, Min Jeong Park, Jung A Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Geum Joon Cho, Hye Jin Yoo
Endocrinology and Metabolism.2021; 36(6): 1277. CrossRef
- Выраженность окислительного стресса и энзиматическая активность нейтрофилов крови у пациентов с болезнью Грейвса в зависимости от компенсации гипертиреоза

- Thyroid
- Programmed Cell Death-Ligand 1 (PD-L1) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
- Jee Hee Yoon, Min-ho Shin, Hee Nam Kim, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(3):599-606. Published online June 2, 2021
- DOI: https://doi.org/10.3803/EnM.2021.965
- 3,977 View
- 115 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Programmed cell death-ligand 1 (PD-L1) has an important role in regulating immune reactions by binding to programmed death 1 (PD-1) on immune cells, which could prevent the exacerbation of autoimmune thyroid disease (AITD). The aim of this study was to evaluate the association of PD-L1 polymorphism with AITD, including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT).
Methods
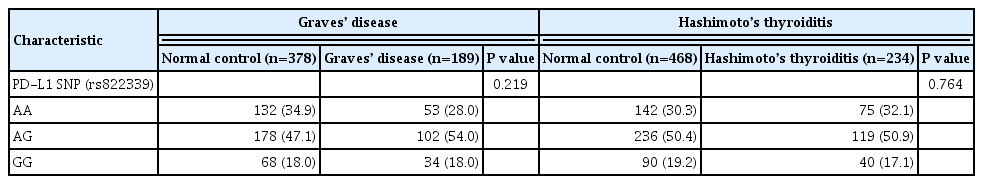
A total of 189 GD patients, 234 HT patients, and 846 healthy age- and sex-matched controls were enrolled in this study. We analyzed PD-L1 single nucleotide polymorphism (SNP) (rs822339) and investigated the associations with clinical disease course and outcome.
Results
Genotype frequency at the PD-L1 marker RS822339 in GD (P=0.219) and HT (P=0.764) patients did not differ from that among healthy controls. In patients with GD, the A/G or G/G genotype group demonstrated higher TBII titer (20.6±20.5 vs. 28.0± 25.8, P=0.044) and longer treatment duration (39.0±40.4 months vs. 62.4±65.0 months, P=0.003) compared to the A/A genotype group. Among patients in whom anti-thyroid peroxidase (TPO) antibody was measured after treatment of GD, post-treatment antiTPO positivity was higher in the A/G or G/G genotype group compared to the A/A genotype group (48.1% vs. 69.9%, P=0.045). Among patients with HT, there was no significant difference of anti-TPO antibody positivity (79.4% vs. 68.6%, P=0.121), anti-thyroglobulin antibody positivity (80.9% vs. 84.7%, P=0.661), or development to overt hypothyroidism (68.0% vs. 71.1%, P=0.632) between the A/A genotype group and the A/G or G/G genotype group.
Conclusion
The genotype frequency of PD-L1 (rs822339) is not different in patients with AITD compared with healthy controls. The intact PD-1/PD-L1 pathway in GD and HT might be important to maintain chronicity of AITD by protecting immune tolerance. However, the PD-L1 SNP could be associated with difficulty in achieving remission in patients with GD, which may be helpful to predict the possibility of longer treatment. Further studies are required to investigate the complex immune tolerance system in patients with AITD. -
Citations
Citations to this article as recorded by- Synergistic effects of BTN3A1, SHP2, CD274, and STAT3 gene polymorphisms on the risk of systemic lupus erythematosus: a multifactorial dimensional reduction analysis
Yang-Yang Tang, Wang-Dong Xu, Lu Fu, Xiao-Yan Liu, An-Fang Huang
Clinical Rheumatology.2024; 43(1): 489. CrossRef - Relationship between CD274 gene polymorphism and systemic lupus erythematosus risk in a Chinese Han population
Lu‐Qi Yang, Zhen Qin, Lu Fu, Wang‐Dong Xu
International Journal of Rheumatic Diseases.2024;[Epub] CrossRef
- Synergistic effects of BTN3A1, SHP2, CD274, and STAT3 gene polymorphisms on the risk of systemic lupus erythematosus: a multifactorial dimensional reduction analysis

- Clinical Characteristics of Graves' Disease Patients with Undetectable Thyrotropin Binding Inhibitor Immunoglubulin (TB2).
- Bo Youn Cho, Won Bae Kim, Hong Gyu Lee, Chang Soon Koh, Seong Yeon Kim, Seok In Lee, Jae Seok Chun, Kyung Soo Park
- J Korean Endocr Soc. 1996;11(1):68-74. Published online November 7, 2019
- 1,602 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Graves disease is an autoimmune disease caused by TSH receptor antibodies. Thyrotropin binding inhibitor immunoglobulins(TBII) are detected in most Graves patients, but some patients have no TBII activities in their sera. It is unknown whether the clinical features of TBII-positive patients are different from those of TBII-negative patients. Methods: To evaluate the prevalence of TBII-negative Graves' patients and its clinical differences from TBII-positive patients, we examined TBII by radioreceptor assay in 686 consecutive untreated Graves patients. We found 84 TBII-negative patients(15 men and 69 women, mean age ±EM: 40.9±.4 years) and compared their clinical characteristics with 87 TBII-positive patients (22 men and 65 women, mean age±EM: 39.9±.5 years) who were selected randomly from the same patients group. Results: In this study, TBII was undetectable in 12.2% of patients with Graves' disease(84 of 686). TBII-negative group had a less weight loss than TBII-positive group. However, there was no significant differences in age, sex ratio, prevalence of ophthalmopathy, duration of illness and positive rate of family history for thyroid diseases between TBII-negative and -positive groups. Serum total T or T levels were not different from each other, but T3-uptake was significantly higher in TBII-positive group than that in TBII-negative group, suggesting that the free hormone levels in TBII-negative group might be lower. The thyroid uptake of 99mTcO4 was significantly higher in TBII positive group than that in TBII-negative group. Thyroid autoantibodies, including antimicrosomal and antithyroglobulin antibodies were detected in almost all patients but there were no differences in titers and positive rate between TBII-negative and -positive groups. Conclusion: Although TBII-negative Graves patients showed less weight loss and low 99mTc04 thyroidal uptake compare to TBII-positive patients, the clinical and immunological characteristics of TBII-negative patients are not different from TBII-positive one.

- Difference of Thyroid Stimulating Antidody Activities Measured in Chinese Hamster Ovary Cells Stably Transfected with Human TSH Receptor and in FRTL-5 Cells in Graves Disease and Its Clinical Correlations.
- Young Kee Shong Young Kee Shong, Hye Young Park, Bo Youn Cho, Won Bae Kim, Hong Gyu Lee, Chang Soon Koh, Yeon Sang Oh
- J Korean Endocr Soc. 1996;11(1):18-29. Published online November 7, 2019
- 1,106 View
- 21 Download
-
 Abstract
Abstract
 PDF
PDF - Background
: Thyroid stimulating antibodies result in the development of hyperthyroidism and goiter in Graves disease. However, thyroid stimulating antibody activities do not correlate with the clinical features in many patients with Graves disease. The purpose of this study is to address this discrepancy between thyroid stimulating antibody activities and clinical features of Graves patients. Methods: We measured thyroid stimulating antibody activities simultaneously using human TSH receptor transfected Chinese hamster(hTSHR-CHO) cells and rat thyroid(FRTL-5) cells in 57 untreated patients with Graves disease, and compared their activities with clinical features including thyroid hormone levels. Results : The detection rate of thyroid stimulating antibody measured by hTSHR-CHO cells was 90% in 57 untreated Graves patients and it was higher than that measured by FRTL-5 cells. Thyroid stimulating antibody activity by hTSHR-CHO cells was significantly correlated with that by FRTL-5 cells(r=0.5, p<0.001), however, 18 of 57(32%) patients showed marked discrepancy of thyroid stimulating antibody activity between in hTSHR-CHO and FRTL-5 systems. Thyroid stimulating antibody activity measured by hTSHR-CHO cells was significantly correlated with serum total T3, free T4 levels, and goiter size but not 99mTc-thyroid uptake. On the other hand, thyroid stimulating antibody activity measured by FRTL-5 cells was significantly correlated with goiter size and 99mTc-thyroid uptake but not thyroid hormone levels. The difference between function and goiter size with respect to thyroid stimulating antibody measurement in two cells system is, nevertheless, particularly evident in the free T4/goiter ratio in patients with high hTSHR-CHO and low FRTL-5 cell assay values. Conclusion: These findings suggest that thyroid stimulating antibodies in Graves disease are heterogeneous population in terms of responses to different origin of cells. Further, thyroid stimulating antibody activities measured by FRTL-5 cells tend to correlate better with goiter size and Tc-thyroid uptake, whereas thyroid stimulating antibody activities measured by hTSH-CHO cells correlate better with thyroid hormone levels.

- Clinical Study
- Changes in Thyroid Peroxidase and Thyroglobulin Antibodies Might Be Associated with Graves' Disease Relapse after Antithyroid Drug Therapy
- Yun Mi Choi, Mi Kyung Kwak, Sang Mo Hong, Eun-Gyoung Hong
- Endocrinol Metab. 2019;34(3):268-274. Published online September 26, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.3.268
- 6,803 View
- 128 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Graves' disease (GD) is an autoimmune thyroid disorder caused by antibodies stimulating the thyrotropin (TSH) receptor. TSH receptor antibody (TRAb) measurement is useful for predicting GD relapse after antithyroid drug (ATD) treatment. However, the association of other thyroid autoantibodies with GD relapse remains obscure.
Methods This retrospective study enrolled patients with GD who were initially treated with ATD. TRAb, thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TgAb) were measured at the initial diagnosis and at the time of ATD discontinuation.
Results A total of 55 patients were enrolled. The mean age was 49.7 years, and 39 patients (70.9%) were female. Antibody positivity at diagnosis was 90.9%, 69.1%, and 61.9% for TRAb, TPOAb, TgAb, respectively. Median ATD treatment period was 15.1 months. At the time of ATD withdrawal, TRAb titers decreased uniformly overall. Conversely, TPOAb and TgAb showed various changes. After withdrawal of ATD, 19 patients (34.5%) experienced relapse. No clinical features or laboratory results were significantly related to relapse in the overall patient group. However, in the TPOAb positive group at diagnosis, increasing titer of TPOAb or TgAb after ATD treatment was significantly and independently related to relapse free survival (TPOAb: hazard ratio [HR], 17.99; 95% confidence interval [CI], 1.66 to 195.43;
P =0.02) (TgAb: HR, 5.73; 95% CI, 1.21 to 27.26;P =0.03).Conclusion Changes in TPOAb or TgAb titers during treatment might be useful for predicting relapse after ATD treatment in patients with positive TPOAb at diagnosis.
-
Citations
Citations to this article as recorded by- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Clinical significance of thyroglobulin antibodies and thyroid peroxidase antibodies in Graves’ disease: a cross-sectional study
Masahito Katahira, Taku Tsunekawa, Akira Mizoguchi, Mariko Yamaguchi, Kahori Tsuru, Hiromi Takashima, Ryoma Terada
Hormones.2023; 22(2): 253. CrossRef - The Clinical Implications of Anti-thyroid Peroxidase Antibodies in Graves’ Disease in Basrah
Emad S Alhubaish, Nassar T Alibrahim, Abbas A Mansour
Cureus.2023;[Epub] CrossRef - Influence of Thyroid Peroxidase Antibodies Serum Levels in Graves' Disease: A Retrospective Cohort Study
Maria L Guia Lopes, Carlos Tavares Bello, José P Cidade, Clotilde Limbert, Joao Sequeira Duarte
Cureus.2023;[Epub] CrossRef - Interpretation of Thyroid Autoantibodies in Hyperthyroidism
Han-Sang Baek, Dong-Jun Lim
The Korean Journal of Medicine.2023; 98(3): 132. CrossRef - Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves’ Disease from Destructive Thyroiditis in Thyrotoxic Patients
Han-Sang Baek, Ji-Yeon Park, Chai-Ho Jeong, Jeonghoon Ha, Moo Il Kang, Dong-Jun Lim
Endocrinology and Metabolism.2022; 37(2): 323. CrossRef - Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings
Jinyoung Kim, Han-Sang Baek, Jeonghoon Ha, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Dong-Jun Lim, Ki-Hyun Baek
Diagnostics.2022; 12(6): 1468. CrossRef - The relationship between atherosclerotic disease and relapse during ATD treatment
Xinxin Zhu, Yaguang Zhang, Xiaoyu Zhao, Xiaona Zhang, Zixuan Ru, Yanmeizhi Wu, Xu Yang, Boyu Hou, Hong Qiao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Programmed Cell Death-Ligand 1 (PD-L1) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
Jee Hee Yoon, Min-ho Shin, Hee Nam Kim, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
Endocrinology and Metabolism.2021; 36(3): 599. CrossRef - Low frequency of IL-10-producing B cells and high density of ILC2s contribute to the pathological process in Graves’ disease, which may be related to elevated-TRAb levels
Xiaoyun Ji, Jie Wan, Rong Chen, Huixuan Wang, Lan Huang, Shwngjun Wang, Zhaoliang Su, Huaxi Xu
Autoimmunity.2020; 53(2): 78. CrossRef - Implication of VDR rs7975232 and FCGR2A rs1801274 gene polymorphisms in the risk and the prognosis of
autoimmune thyroid diseases in the Tunisian population
S Mestiri, I Zaaber, I Nasr, H Marmouch
Balkan Journal of Medical Genetics.2020; 23(1): 69. CrossRef - Thyroid Peroxidase Antibody Positivity is Associated With Relapse-Free Survival Following Antithyroid Drug Treatment for Graves Disease
Christopher A. Muir, Graham R.D. Jones, Jerry R. Greenfield, Andrew Weissberger, Katherine Samaras
Endocrine Practice.2020; 26(9): 1026. CrossRef - Predicting the Risk of Graves Disease Relapse: Commentary on “Thyroid Peroxidase Antibody Positivity is Associated with Relapse-Free Survival Following Antithyroid Drug Treatment for Graves Disease”
D. Gallo, M.L. Tanda, E. Piantanida
Endocrine Practice.2020; 26(9): 1039. CrossRef
- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study

- Thyroid
- Genome-Wide Association Studies of Autoimmune Thyroid Diseases, Thyroid Function, and Thyroid Cancer
- Yul Hwangbo, Young Joo Park
- Endocrinol Metab. 2018;33(2):175-184. Published online June 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.175
- 7,959 View
- 139 Download
- 58 Web of Science
- 58 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Thyroid diseases, including autoimmune thyroid diseases and thyroid cancer, are known to have high heritability. Family and twin studies have indicated that genetics plays a major role in the development of thyroid diseases. Thyroid function, represented by thyroid stimulating hormone (TSH) and free thyroxine (T4), is also known to be partly genetically determined. Before the era of genome-wide association studies (GWAS), the ability to identify genes responsible for susceptibility to thyroid disease was limited. Over the past decade, GWAS have been used to identify genes involved in many complex diseases, including various phenotypes of the thyroid gland. In GWAS of autoimmune thyroid diseases, many susceptibility loci associated with autoimmunity (human leukocyte antigen [
HLA ], protein tyrosine phosphatase, non-receptor type 22 [PTPN22 ], cytotoxic T-lymphocyte associated protein 4 [CTLA4 ], and interleukin 2 receptor subunit alpha [IL2RA ]) or thyroid-specific genes (thyroid stimulating hormone receptor [TSHR ] and forkhead box E1 [FOXE1 ]) have been identified. Regarding thyroid function, many susceptibility loci for levels of TSH and free T4 have been identified through genome-wide analyses. In GWAS of differentiated thyroid cancer, associations atFOXE1 , MAP3K12 binding inhibitory protein 1 (MBIP )-NK2 homeobox 1 (NKX2-1 ), disrupted in renal carcinoma 3 (DIRC3 ), neuregulin 1 (NRG1 ), and pecanex-like 2 (PCNXL2 ) have been commonly identified in people of European and Korean ancestry, and many other susceptibility loci have been found in specific populations. Through GWAS of various thyroid-related phenotypes, many susceptibility loci have been found, providing insights into the pathogenesis of thyroid diseases and disease co-clustering within families and individuals.-
Citations
Citations to this article as recorded by- A new, all‐encompassing aetiology of type 1 diabetes
Piet C. de Groen
Immunology.2024; 171(1): 77. CrossRef - The role of primary cilia in thyroid diseases
Zijiao Tian, Xinlin Li, Xue Yu, Shuxin Yan, Jingwei Sun, Wenxin Ma, Xiaoyun Zhu, Yang Tang
Frontiers in Endocrinology.2024;[Epub] CrossRef - Investigating the Association of Polygenic Risk Scores With Thyroid Cancer Susceptibility in a Han Chinese Population
Yi-Hao Chen, I Chieh Chen, Chia-Man Chou, Sheng-Yang Huang
Journal of the Endocrine Society.2024;[Epub] CrossRef - Causal relationship between inflammatory cytokines and autoimmune thyroid disease: a bidirectional two-sample Mendelian randomization analysis
Zhiwei Yao, Fengli Guo, Yanlu Tan, Yiyuan Zhang, Yichen Geng, Guang Yang, Song Wang
Frontiers in Immunology.2024;[Epub] CrossRef - Association of autoimmune thyroid disease with type 1 diabetes mellitus and its ultrasonic diagnosis and management
Jin Wang, Ke Wan, Xin Chang, Rui-Feng Mao
World Journal of Diabetes.2024; 15(3): 348. CrossRef - Multidimensional data analysis revealed thyroiditis-associated TCF19 SNP rs2073724 as a highly ranked protective variant in thyroid cancer
Xianhui Ruan, Yu Liu, Shuping Wu, Guiming Fu, Mei Tao, Yue Huang, Dapeng Li, Songfeng Wei, Ming Gao, Shicheng Guo, Junya Ning, Xiangqian Zheng
Aging.2024;[Epub] CrossRef - Exome sequencing to explore the possibility of predicting genetic susceptibility to the joint occurrence of polycystic ovary syndrome and Hashimoto’s thyroiditis
Natalia Zeber-Lubecka, Katarzyna Suchta, Maria Kulecka, Anna Kluska, Magdalena Piątkowska, Michal J. Dabrowski, Katarzyna Jankowska, Monika Grymowicz, Roman Smolarczyk, Ewa E. Hennig
Frontiers in Immunology.2023;[Epub] CrossRef - Molecular Mechanisms in Autoimmune Thyroid Disease
Hernando Vargas-Uricoechea
Cells.2023; 12(6): 918. CrossRef - Identification of multiple novel susceptibility genes associated with autoimmune thyroid disease
Xueying Liu, Yahu Miao, Chao Liu, Wan Lu, Qing Feng, Qiu Zhang
Frontiers in Immunology.2023;[Epub] CrossRef - Novel Susceptibility Genes Drive Familial Non-Medullary Thyroid Cancer in a Large Consanguineous Kindred
Pierre Majdalani, Uri Yoel, Tayseer Nasasra, Merav Fraenkel, Alon Haim, Neta Loewenthal, Raz Zarivach, Eli Hershkovitz, Ruti Parvari
International Journal of Molecular Sciences.2023; 24(9): 8233. CrossRef - An Optimized Flow Cytometric Method to Demonstrate the Differentiation Stage-Dependent Ca2+ Flux Responses of Peripheral Human B Cells
Anna Bajnok, Timea Serény-Litvai, Viktória Temesfői, Jasper Nörenberg, Róbert Herczeg, Ambrus Kaposi, Timea Berki, Emese Mezosi
International Journal of Molecular Sciences.2023; 24(10): 9107. CrossRef - FOXE1 Contributes to the Development of Psoriasis by Regulating WNT5A
Meng Liu, Guanfei Zhang, Ziyang Wang, Xinyi Liu, Ke He, Ruiting Luo, Qiqi Duan, Ruimin Bai, Yuqian Wang, Wenqian Du, Yan Zheng, Yongping Shao
Journal of Investigative Dermatology.2023; 143(12): 2366. CrossRef - Correlation analysis of HHV-6A viral load and anti-TPO antibody levels in patients with Hashimoto's thyroiditis
Noorossadat Seyyedi, Fariba Esfandiyari, Gholamreza Rafiei Dehbidi, Ali Farhadi, Farahnaz Zare, Sepide Namdari, Golrokh Bahmani, Banafsheh Rastegari, Farzaneh Zarghampoor, Abbas Behzad-Behbahani
Future Virology.2023; 18(8): 527. CrossRef - Primary cell cultures for the personalized therapy in aggressive thyroid cancer of follicular origin
Poupak Fallahi, Silvia Martina Ferrari, Giusy Elia, Francesca Ragusa, Armando Patrizio, Sabrina Rosaria Paparo, Gianni Marone, Maria Rosaria Galdiero, Giovanni Guglielmi, Rudy Foddis, Alfonso Cristaudo, Alessandro Antonelli
Seminars in Cancer Biology.2022; 79: 203. CrossRef - The Relationship between PTPN22 R620W Polymorphisms and the Susceptibility to Autoimmune Thyroid Diseases: An Updated Meta-analysis
Huaiyong Wu, Siyuan Wan, Mengying Qu, Bingxuan Ren, Lixiang Liu, Hongmei Shen
Immunological Investigations.2022; 51(2): 438. CrossRef - Identification of Transcriptional Pattern Related to Immune Cell Infiltration With Gene Co-Expression Network in Papillary Thyroid Cancer
Meiye Li, Jimei Zhang, Zongjing Zhang, Ying Qian, Wei Qu, Zhaoshun Jiang, Baochang Zhao
Frontiers in Endocrinology.2022;[Epub] CrossRef - Analysis of rare genetic variation underlying cardiometabolic diseases and traits among 200,000 individuals in the UK Biobank
Sean J. Jurgens, Seung Hoan Choi, Valerie N. Morrill, Mark Chaffin, James P. Pirruccello, Jennifer L. Halford, Lu-Chen Weng, Victor Nauffal, Carolina Roselli, Amelia W. Hall, Matthew T. Oetjens, Braxton Lagerman, David P. vanMaanen, Goncalo Abecasis, Xiao
Nature Genetics.2022; 54(3): 240. CrossRef - Factors affecting recurrence in subacute granulomatous thyroiditis
Çiğdem Tura Bahadir, Merve Yilmaz, Elif Kiliçkan
Archives of Endocrinology and Metabolism.2022;[Epub] CrossRef - Shared etiology of type 1 diabetes and Hashimoto’s thyroiditis: a population-based twin study
Jakob Skov, Ralf Kuja-Halkola, Patrik K E Magnusson, Soffia Gudbjörnsdottir, Olle Kämpe, Sophie Bensing
European Journal of Endocrinology.2022; 186(6): 677. CrossRef - High Prevalence of Common Human Viruses in Thyroid Tissue
Therese Weider, Angelo Genoni, Francesco Broccolo, Trond H. Paulsen, Knut Dahl-Jørgensen, Antonio Toniolo, Sara Salehi Hammerstad
Frontiers in Endocrinology.2022;[Epub] CrossRef - B cells from anti-thyroid antibody positive, infertile women show hyper-reactivity to BCR stimulation
Timea Serény-Litvai, Anna Bajnok, Viktoria Temesfoi, Jasper Nörenberg, Greta Pham-Dobor, Ambrus Kaposi, Akos Varnagy, Kalman Kovacs, Sandor Pentek, Tamas Koszegi, Emese Mezosi, Timea Berki
Frontiers in Immunology.2022;[Epub] CrossRef - What do the structures of GCN5-containing complexes teach us about their function?
Dominique Helmlinger, Gábor Papai, Didier Devys, László Tora
Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms.2021; 1864(2): 194614. CrossRef - Genetic Susceptibility to Joint Occurrence of Polycystic Ovary Syndrome and Hashimoto’s Thyroiditis: How Far Is Our Understanding?
Natalia Zeber-Lubecka, Ewa E. Hennig
Frontiers in Immunology.2021;[Epub] CrossRef - Type 1 Diabetes and Autoimmune Thyroid Disease—The Genetic Link
Lara Frommer, George J. Kahaly
Frontiers in Endocrinology.2021;[Epub] CrossRef - Familial Risk of Hashimoto's Thyroiditis Among First-Degree Relatives: A Population-Based Study in Korea
Hyun Jung Kim, Sayada Zartasha Kazmi, Taeuk Kang, Seo Young Sohn, Dong-Sook Kim, Hoo Jae Hann, Hyeong Sik Ahn
Thyroid.2021; 31(7): 1096. CrossRef - Limited Genetic Overlap Between Overt Hashimoto’s Thyroiditis and Graves’ Disease in Twins: A Population-based Study
Jakob Skov, Jan Calissendorff, Daniel Eriksson, Patrik Magnusson, Olle Kämpe, Sophie Bensing, Ralf Kuja-Halkola
The Journal of Clinical Endocrinology & Metabolism.2021; 106(4): e1101. CrossRef - Endogenous and Synthetic Regulators of the Peripheral Components of the Hypothalamo-Hypophyseal-Gonadal and -Thyroid Axes
A. O. Shpakov
Neuroscience and Behavioral Physiology.2021; 51(3): 332. CrossRef - Programmed Cell Death-Ligand 1 (PD-L1) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
Jee Hee Yoon, Min-ho Shin, Hee Nam Kim, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
Endocrinology and Metabolism.2021; 36(3): 599. CrossRef - Predisposition to Graves’ disease and Graves’ ophthalmopathy by genetic variants of IL2RA
Juan Du, Xin Wang, Guiqin Tan, Wenwen Wei, Fangyu Zhou, Zhongzhi Liang, Hua Li, Hongsong Yu
Journal of Molecular Medicine.2021; 99(10): 1487. CrossRef - Association of IL-1β, NLRP3, and COX-2 Gene Polymorphisms with Autoimmune Thyroid Disease Risk and Clinical Features in the Iranian Population
Zahra Heidari, Saeedeh Salimi, Mohsen Rokni, Mahnaz Rezaei, Neshat Khalafi, Mahdieh Jafari Shahroudi, Azizallah Dehghan, Mohsen Saravani, Rafael S. De Molon
BioMed Research International.2021; 2021: 1. CrossRef - Genetic polymorphisms in the PCNXL2 gene are risk factors for thyroid cancer in the Chinese population
Runmei Hao, Peng Han, Ling Zhang, Ying Bi, Jinfeng Yan, Honghui Li, Yanxia Bai, Chongwen Xu, Baiya Li, Huajing Li
Future Oncology.2021; 17(34): 4677. CrossRef - Genetic Variants Associated with Thyroid Cancer Risk: Comprehensive Research Synopsis, Meta-Analysis, and Cumulative Epidemiological Evidence
Ran Ran, Gang Tu, Hui Li, Hao Wang, Exian Mou, Caiyang Liu, Yuan Seng Wu
Journal of Oncology.2021; 2021: 1. CrossRef - The Identification of Three Key Genes Related to Stemness in Thyroid Carcinoma through Comprehensive Analysis
Tonglong Zhang, Chunhong Yan, Zhengdu Ye, Xingling Yin, Tian-an Jiang
Combinatorial Chemistry & High Throughput Screening.2021; 24(3): 423. CrossRef - Епідеміологія автоімунного тиреоїдиту
V.I. Кravchenko, О.А. Тоvkay, О.V. Rakov, М.D. Тronko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2021; 17(2): 136. CrossRef - Thyroid autoimmune disorders and cancer
Silvia Martina Ferrari, Poupak Fallahi, Giusy Elia, Francesca Ragusa, Ilaria Ruffilli, Sabrina Rosaria Paparo, Alessandro Antonelli
Seminars in Cancer Biology.2020; 64: 135. CrossRef - Association of FCRL3 rs7528684 polymorphism with risk of Hashimoto's thyroiditis in Iranian patients
Kurosh Kalantar, Farzad Ghandehari, Saeed Malek-Hosseini, Hossein Golmoghaddam, Davood Rostamzadeh, Mohamad Hossein Dabbaghmanesh, Zahra Amirghofran
Meta Gene.2020; 24: 100663. CrossRef - Differentiated thyroid cancer and Hashimoto thyroiditis: Utility of the Afirma gene expression classifier
Vardan Papoian, Jennifer E. Rosen, Wen Lee, Leonard Wartofsky, Erin A. Felger
Journal of Surgical Oncology.2020; 121(7): 1053. CrossRef - Familial risks between Graves disease and Hashimoto thyroiditis and other autoimmune diseases in the population of Sweden
Hauke Thomsen, Xinjun Li, Kristina Sundquist, Jan Sundquist, Asta Försti, Kari Hemminki
Journal of Translational Autoimmunity.2020; 3: 100058. CrossRef - Association of rs944289, rs965513, and rs1443434 in TITF1/TITF2 with Risks of Papillary Thyroid Carcinoma and with Nodular Goiter in Northern Chinese Han Populations
Xin Zhang, Yulu Gu, Yong Li, Heran Cui, Xiaoli Liu, Hui Sun, Qiong Yu, Yaqin Yu, Yawen Liu, Siyan Zhan, Yi Cheng
International Journal of Endocrinology.2020; 2020: 1. CrossRef - FLT3 stop mutation increases FLT3 ligand level and risk of autoimmune thyroid disease
Saedis Saevarsdottir, Thorunn A. Olafsdottir, Erna V. Ivarsdottir, Gisli H. Halldorsson, Kristbjorg Gunnarsdottir, Asgeir Sigurdsson, Ari Johannesson, Jon K. Sigurdsson, Thorhildur Juliusdottir, Sigrun H. Lund, Asgeir O. Arnthorsson, Edda L. Styrmisdottir
Nature.2020; 584(7822): 619. CrossRef - Genetic relationship between Hashimoto`s thyroiditis and papillary thyroid carcinoma with coexisting Hashimoto`s thyroiditis
Ohoud Subhi, Hans-Juergen Schulten, Nadia Bagatian, Roa'a Al-Dayini, Sajjad Karim, Sherin Bakhashab, Reem Alotibi, Alaa Al-Ahmadi, Manar Ata, Aisha Elaimi, Saad Al-Muhayawi, Majid Mansouri, Khalid Al-Ghamdi, Osman Abdel Hamour, Awatif Jamal, Jaudah Al-Mag
PLOS ONE.2020; 15(6): e0234566. CrossRef - Thyroid cancer and thyroid autoimmune disease: A review of molecular aspects and clinical outcomes
Natália Medeiros Dias Lopes, Hannah Hamada Mendonça Lens, André Armani, Poliana Camila Marinello, Alessandra Lourenço Cecchini
Pathology - Research and Practice.2020; 216(9): 153098. CrossRef - Immune gene signature delineates a subclass of thyroid cancer with unfavorable clinical outcomes
Jingtai Zhi, Jiaoyu Yi, Mengran Tian, Huijuan Wang, Ning Kang, Xiangqian Zheng, Ming Gao
Aging.2020; 12(7): 5733. CrossRef - VARIETY OF COMBINATIONS OF HASHIMOTO’S THYROIDITIS WITH OTHER BACKGROUND PATHOLOGY OF THYROID PARENCHEMA IN DIFFERENT FORMS OF THYROID CANCER
Yu. I. Karachentsev, V. M. Dubovik, I. V. Gopkalova, V. V. Khaziev, S. S. Sokolova, Y. P. Korchagin, N. G. Filonenko, M. Y. Sazonov, L. V. Gerasimenko
Bulletin of Problems Biology and Medicine.2020; 3(1): 105. CrossRef - Analysis of Polymorphisms rs7093069-IL-2RA, rs7138803-FAIM2, and rs1748033-PADI4 in the Group of Adolescents With Autoimmune Thyroid Diseases
Beata Sawicka, Hanna Borysewicz-Sańczyk, Natalia Wawrusiewicz-Kurylonek, Tommaso Aversa, Domenico Corica, Joanna Gościk, Adam Krętowski, Małgorzata Waśniewska, Artur Bossowski
Frontiers in Endocrinology.2020;[Epub] CrossRef - Familial associations for rheumatoid autoimmune diseases
Hauke Thomsen, Xinjun Li, Kristina Sundquist, Jan Sundquist, Asta Försti, Kari Hemminki
Rheumatology Advances in Practice.2020;[Epub] CrossRef - Familial risks between giant cell arteritis and Takayasu arteritis and other autoimmune diseases in the population of Sweden
Hauke Thomsen, Xinjun Li, Kristina Sundquist, Jan Sundquist, Asta Försti, Kari Hemminki
Scientific Reports.2020;[Epub] CrossRef - The Association of Obesity with Autoimmune Thyroiditis and Thyroid Function-Possible Mechanisms of Bilateral Interaction
Agnieszka Baranowska-Bik, Wojciech Bik, Henrik Falhammar
International Journal of Endocrinology.2020; 2020: 1. CrossRef - The Risk of Recurrence of Subacute Thyroiditis Is HLA-Dependent
Magdalena Stasiak, Bogusław Tymoniuk, Bartłomiej Stasiak, Andrzej Lewiński
International Journal of Molecular Sciences.2019; 20(5): 1089. CrossRef - FOXE1 inhibits cell proliferation, migration and invasion of papillary thyroid cancer by regulating PDGFA
Zheng Ding, Ronghu Ke, Yong Zhang, Youben Fan, Jianxia Fan
Molecular and Cellular Endocrinology.2019; 493: 110420. CrossRef - Current Knowledge of Germline Genetic Risk Factors for the Development of Non-Medullary Thyroid Cancer
Kinga Hińcza, Artur Kowalik, Aldona Kowalska
Genes.2019; 10(7): 482. CrossRef - Thyroid Cancer: The Quest for Genetic Susceptibility Involving DNA Repair Genes
Santos, Gomes, Bastos, Gil, Azevedo, Ferreira, Limbert, Silva, Rueff
Genes.2019; 10(8): 586. CrossRef - Genetic Polymorphisms on 4q21.1 Contributed to the Risk of Hashimoto's Thyroiditis
Dachao Mo, Junjiu Li, Liang Peng, Zhiyuan Liu, Jieyun Wang, Jiru Yuan
Genetic Testing and Molecular Biomarkers.2019; 23(12): 837. CrossRef - A Weighted Genetic Risk Score Using Known Susceptibility Variants to Predict Graves Disease Risk
Yu-Ru Ma, Shuang-Xia Zhao, Lu Li, Feng Sun, Xiao-Ping Ye, Fei-Fei Yuan, Dan Jiang, Zheng Zhou, Qian-Yue Zhang, Yue-Yue Wan, Guang-Ya Zhang, Jing Wu, Rui-Jia Zhang, Ya Fang, Huai-Dong Song
The Journal of Clinical Endocrinology & Metabolism.2019; 104(6): 2121. CrossRef - Architects meets Repairers: The interplay between homeobox genes and DNA repair
Bruno César Feltes
DNA Repair.2019; 73: 34. CrossRef - Development of a prognostic index based on an immunogenomic landscape analysis of papillary thyroid cancer
Peng Lin, Yi-nan Guo, Lin Shi, Xiao-jiao Li, Hong Yang, Yun He, Qing Li, Yi-wu Dang, Kang-lai Wei, Gang Chen
Aging.2019; 11(2): 480. CrossRef - Selenium and Selenoproteins in Immune Mediated Thyroid Disorders
Liliana Santos, Celestino Neves, Miguel Melo, Paula Soares
Diagnostics.2018; 8(4): 70. CrossRef - Sex-specific genetic influence on thyroid-stimulating hormone and free thyroxine levels, and interactions between measurements: KNHANES 2013–2015
Young Ki Lee, Dong Yeob Shin, Hyejung Shin, Eun Jig Lee, Silvia Naitza
PLOS ONE.2018; 13(11): e0207446. CrossRef
- A new, all‐encompassing aetiology of type 1 diabetes

- Free Thyroxine, Anti-Thyroid Stimulating Hormone Receptor Antibody Titers, and Absence of Goiter Were Associated with Responsiveness to Methimazole in Patients with New Onset Graves' Disease
- Hoon Sung Choi, Won Sang Yoo
- Endocrinol Metab. 2017;32(2):281-287. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.281
- 3,699 View
- 38 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Anti-thyroid drug therapy is considered a treatment of choice for Graves' disease; however, treatment response varies among individuals. Although several studies have reported risk factors for relapse after initial treatment, few have assessed responsiveness during the early treatment period. Our study aimed to identify the clinical characteristics for responsiveness to methimazole.
Methods We included 99 patients diagnosed with Graves' disease for the first time. Drug responsiveness was defined as the correlation coefficients between decreasing rates of free thyroxine level per month and methimazole exposure dose. According to their responsiveness to treatment, the patients were classified into rapid or slow responder groups, and age, sex, free thyroxine level, and thyrotropin binding inhibiting immunoglobulin (TBII) titers were compared between groups.
Results The mean patient age was 44.0±13.5 years and 40 patients were male (40%). The mean TBII titer was 36.6±74.4 IU/L, and the mean free thyroxine concentration was 48.9±21.9 pmol/L. The rapid responder group showed higher TBII titer and free thyroxine level at diagnosis, while age, sex, smoking, and presence of goiter did not differ between the two groups. Logistic regression analyses revealed that high level of serum thyroxine, high titer of TBII, and absence of goiter were significantly associated with a rapid response, while age, sex, and smoking were not significant factors for the prediction of responsiveness.
Conclusion In patients with new onset Graves' disease, high level of free thyroxine, high titer of TBII, and absence of goiter were associated with rapid responsiveness to methimazole treatment.
-
Citations
Citations to this article as recorded by- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study
Xinpan Wang, Tiantian Li, Yue Li, Qiuyi Wang, Yun Cai, Zhixiao Wang, Yun Shi, Tao Yang, Xuqin Zheng
Journal of Translational Medicine.2024;[Epub] CrossRef - Analysis of Related Factors in Refractory Graves’ Disease
鑫 王
Advances in Clinical Medicine.2023; 13(08): 13439. CrossRef - Clinical efficacy of thyroid-stimulating immunoglobulin detection for diagnosing Graves’ disease and predictors of responsiveness to methimazole
KunY Liu, Yu Fu, TianT Li, SunQ Liu, DouD Chen, ChengC Zhao, Yun Shi, Yun Cai, Tao Yang, XuQ Zheng
Clinical Biochemistry.2021; 97: 34. CrossRef - Changes in Thyroid Peroxidase and Thyroglobulin Antibodies Might Be Associated with Graves' Disease Relapse after Antithyroid Drug Therapy
Yun Mi Choi, Mi Kyung Kwak, Sang Mo Hong, Eun-Gyoung Hong
Endocrinology and Metabolism.2019; 34(3): 268. CrossRef - When should antithyroid drug therapy to reduce the relapse rate of hyperthyroidism in Graves’ disease be discontinued?
Suyeon Park, Eyun Song, Hye-Seon Oh, Mijin Kim, Min Ji Jeon, Won Gu Kim, Tae Yong Kim, Young Kee Shong, Doo Man Kim, Won Bae Kim
Endocrine.2019; 65(2): 348. CrossRef
- Enhanced predictive validity of integrative models for refractory hyperthyroidism considering baseline and early therapy characteristics: a prospective cohort study


 KES
KES

 First
First Prev
Prev



