Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Extra-Glycemic Effects of Anti-Diabetic Medications: Two Birds with One Stone?
- Eun-Jung Rhee
- Endocrinol Metab. 2022;37(3):415-429. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.304
- 4,473 View
- 261 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
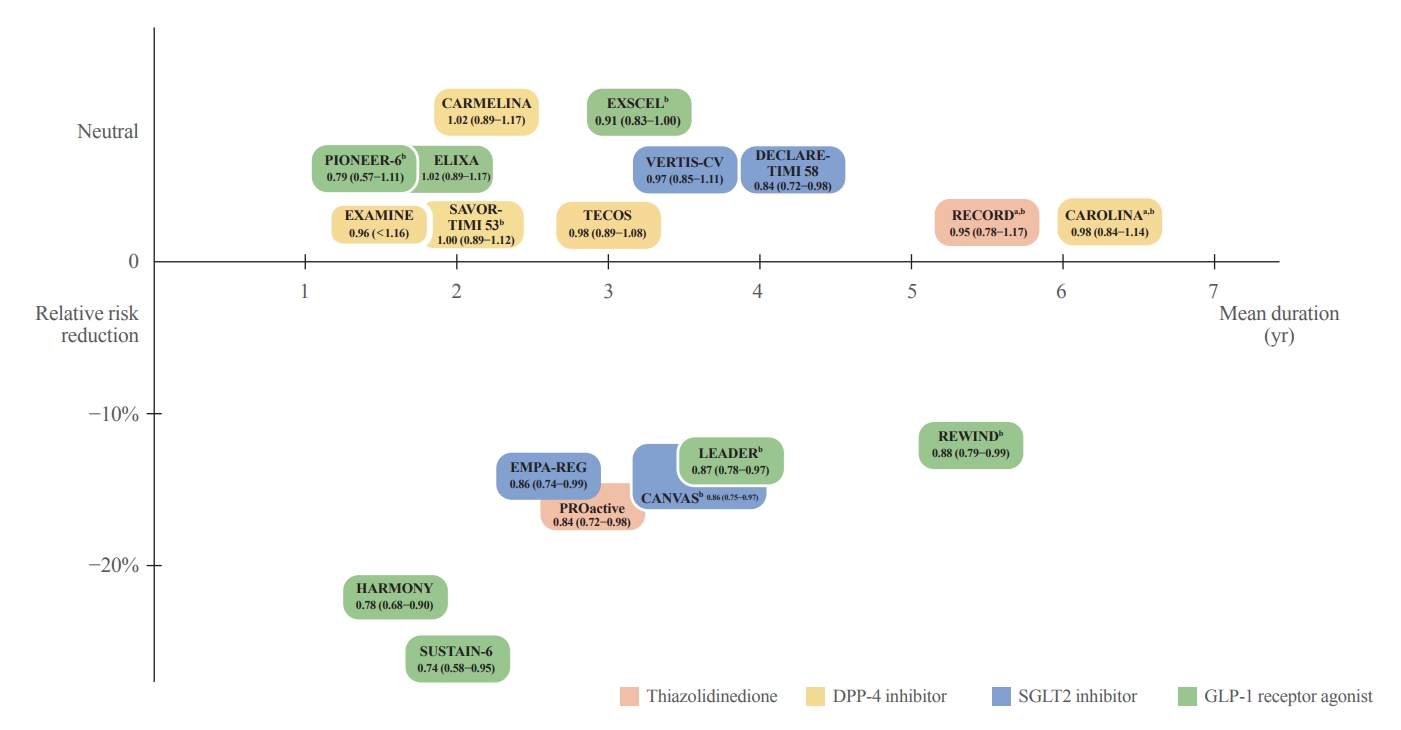
ePub - The world is suffering from a rapid increase in the number of people with diabetes due to the increased prevalence of obesity and lengthened life span. Since the development of insulin thanks to the efforts of Prof. Banting and Dr. Best in 1922, for which they won the Nobel Prize, remarkable developments in anti-diabetic medications have dramatically lengthened the lifespan of patients with diabetes. However, the control rate of hyperglycemia in patients with diabetes remains unsatisfactory, since glycemic control requires both medication and lifestyle modifications to slow the deterioration of pancreatic beta-cell function and prevent diabetic complications. From the initial “triumvirate” to the “ominous octet,” and now the “egregious eleven,” the number of organs recognized as being involved in hyperglycemia and diabetes has increased with the development of anti-diabetic medications. Recent unexpected results from outcome trials of anti-diabetic medications have enabled anti-diabetic medications to be indicated for the prevention of chronic kidney disease and heart failure, even in patients without diabetes. In this review, I would like to summarize the extra-glycemic effects of anti-diabetic medications.
-
Citations
Citations to this article as recorded by- Association between underweight and risk of heart failure in diabetes patients
Tae Kyung Yoo, Kyung‐Do Han, Eun‐Jung Rhee, Won‐Young Lee
Journal of Cachexia, Sarcopenia and Muscle.2024; 15(2): 671. CrossRef - Glucagon-Like Peptide Receptor Agonist Inhibits Angiotensin II-Induced Proliferation and Migration in Vascular Smooth Muscle Cells and Ameliorates Phosphate-Induced Vascular Smooth Muscle Cells Calcification
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Diabetes & Metabolism Journal.2024; 48(1): 83. CrossRef - To do one and to get more: Part I. Diabetes and bone
Wen-Ling Lee, Peng-Hui Wang, Szu-Ting Yang, Chia-Hao Liu, Wen-Hsun Chang, Fa-Kung Lee
Journal of the Chinese Medical Association.2022; 85(10): 965. CrossRef
- Association between underweight and risk of heart failure in diabetes patients

- Diabetes
- Cardiorenal Protection in Diabetic Kidney Disease
- Jason F. Lee, Ecaterina Berzan, Vikas S. Sridhar, Ayodele Odutayo, David Z.I. Cherney
- Endocrinol Metab. 2021;36(2):256-269. Published online April 19, 2021
- DOI: https://doi.org/10.3803/EnM.2021.987

- 5,688 View
- 299 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Over the last 5 years there have been many new developments in the management of diabetic kidney disease. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter-2 (SGLT2) inhibitors were initially used for glycemic control, but more recent studies have now shown that their benefits extend to cardiovascular and kidney outcomes. The recent addition of data on the novel mineralocorticoid receptor antagonist (MRA) gives us another approach to further decrease the residual risk of diabetic kidney disease progression. In this review we describe the mechanism of action, key studies, and possible adverse effects related to these three classes of medications. The management of type 2 diabetes now includes an increasing number of medications for the management of comorbidities in a patient population at significant risk of cardiovascular disease and progression of chronic kidney disease. It is from this perspective that we seek to outline the rationale for the sequential and/or combined use of SGLT2 inhibitors, GLP-1 RA and MRAs in patients with type 2 diabetes for heart and kidney protection.
-
Citations
Citations to this article as recorded by- Relative and Absolute Risks of Adverse Events with Real-World Use of SGLT2 Inhibitors in CKD
Ayodele Odutayo, Adeera Levin
Clinical Journal of the American Society of Nephrology.2023; 18(5): 557. CrossRef - Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease
Dong-Lim Kim, Seung-Eun Lee, Nan Hee Kim
Endocrinology and Metabolism.2023; 38(1): 43. CrossRef - Intrarenal Mechanisms of Sodium-Glucose Cotransporter-2 Inhibitors on Tubuloglomerular Feedback and Natriuresis
Eun Sil Koh, Gheun-Ho Kim, Sungjin Chung
Endocrinology and Metabolism.2023; 38(4): 359. CrossRef - SGLT2 and DPP4 inhibitors improve Alzheimer’s disease–like pathology and cognitive function through distinct mechanisms in a T2D–AD mouse model
A Young Sim, Da Hyun Choi, Jong Youl Kim, Eun Ran Kim, A-ra Goh, Yong-ho Lee, Jong Eun Lee
Biomedicine & Pharmacotherapy.2023; 168: 115755. CrossRef - Narrative review investigating the nephroprotective mechanisms of sodium glucose cotransporter type 2 inhibitors in diabetic and nondiabetic patients with chronic kidney disease
Emma S. Speedtsberg, Martin Tepel
Frontiers in Endocrinology.2023;[Epub] CrossRef - Management of CKD
Nimrit Goraya, Jennifer D. Moran
Nephrology Self-Assessment Program.2022; 21(2): 146. CrossRef - Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders − New perspectives for combination therapy
Peter Kolkhof, Amer Joseph, Ulrich Kintscher
Pharmacological Research.2021; 172: 105859. CrossRef - Sodium‐Glucose Cotransporter 2 Inhibitors, All‐Cause Mortality, and Cardiovascular Outcomes in Adults with Type 2 Diabetes: A Bayesian Meta‐Analysis and Meta‐Regression
Ayodele Odutayo, Bruno R. da Costa, Tiago V. Pereira, Vinay Garg, Samir Iskander, Fatimah Roble, Rahim Lalji, Cesar A. Hincapié, Aquila Akingbade, Myanca Rodrigues, Arnav Agarwal, Bishoy Lawendy, Pakeezah Saadat, Jacob A. Udell, Francesco Cosentino, Peter
Journal of the American Heart Association.2021;[Epub] CrossRef - Finerenone: A Potential Treatment for Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus
Luis D’Marco, María Jesús Puchades, Lorena Gandía, Claudia Forquet, Elena Giménez-Civera, Nayara Panizo, Javier Reque, Isabel Juan-García, Valmore Bermúdez, José Luis Gorriz
touchREVIEWS in Endocrinology.2021; 17(2): 84. CrossRef - Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review
Jorge I. Fonseca-Correa, Ricardo Correa-Rotter
Frontiers in Medicine.2021;[Epub] CrossRef
- Relative and Absolute Risks of Adverse Events with Real-World Use of SGLT2 Inhibitors in CKD

- Clinical Study
Big Data Articles (National Health Insurance Service Database) - Effect of Teneligliptin versus Sulfonylurea on Major Adverse Cardiovascular Outcomes in People with Type 2 Diabetes Mellitus: A Real-World Study in Korea
- Da Hea Seo, Kyoung Hwa Ha, So Hun Kim, Dae Jung Kim
- Endocrinol Metab. 2021;36(1):70-80. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.777
- 4,943 View
- 191 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
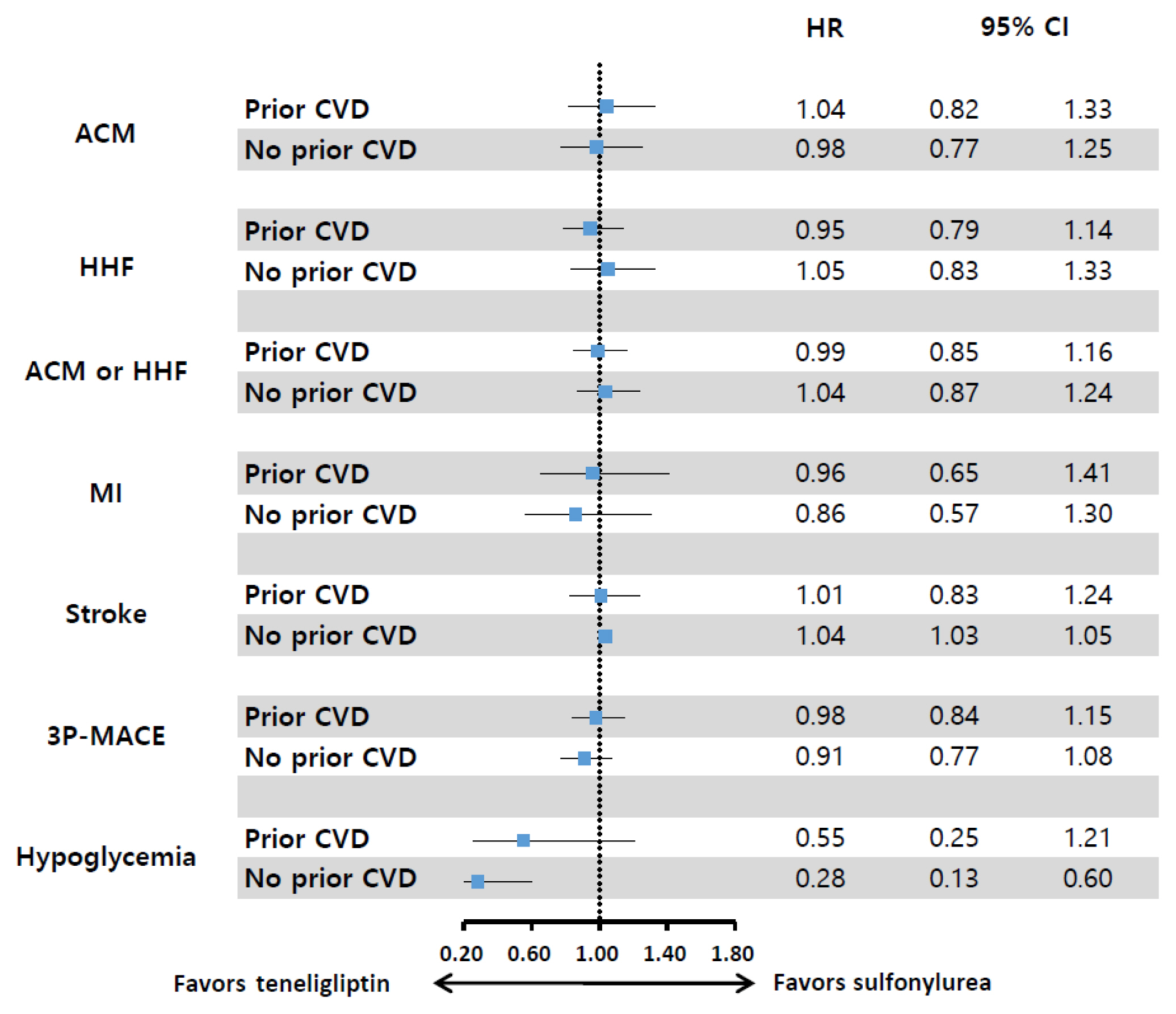
Results regarding the cardiovascular (CV) effects of dipeptidyl peptidase-4 (DPP-4) inhibitors are inconsistent. This study aimed to assess the effects of teneligliptin, a DPP-4 inhibitor, on the risk of major CV outcomes in type 2 diabetes mellitus (T2DM) patients compared to sulfonylurea.
Methods
From January 1, 2015 to December 31, 2017, we conducted a retrospective cohort study using the Korean National Health Insurance Service database. A total of 6,682 T2DM patients who were newly prescribed DPP-4 inhibitors or sulfonylurea were selected and matched in a 1:1 ratio by propensity score. The hazard ratios (HRs) for all-cause mortality, hospitalization for heart failure (HHF), all-cause mortality or HHF, myocardial infarction (MI), stroke, and hypoglycemia were assessed.
Results
During 641 days of follow-up, the use of teneligliptin was not associated with an increased risk of all-cause mortality (HR, 1.00; 95% confidence interval [CI], 0.85 to 1.19), HHF (HR, 0.99; 95% CI, 0.86 to 1.14), all-cause mortality or HHF (HR, 1.02; 95% CI, 0.90 to 1.14), MI (HR, 0.90; 95% CI, 0.68 to 1.20), and stroke (HR, 1.00; 95% CI, 0.86 to 1.17) compared to the use of sulfonylurea. However, it was associated with a significantly lower risk of hypoglycemia (HR, 0.68; 95% CI, 0.49 to 0.94) compared to sulfonylurea therapy.
Conclusion
Among T2DM patients, teneligliptin therapy was not associated with an increased risk of CV events including HHF, but was associated with a lower risk of hypoglycemia compared to sulfonylurea therapy. -
Citations
Citations to this article as recorded by- Association between age at diagnosis of type 2 diabetes and cardiovascular morbidity and mortality risks: A nationwide population-based study
Da Hea Seo, Mina Kim, Young Ju Suh, Yongin Cho, Seong Hee Ahn, Seongbin Hong, So Hun Kim
Diabetes Research and Clinical Practice.2024; 208: 111098. CrossRef - Systematic review and meta-analysis of teneligliptin for treatment of type 2 diabetes
R. Pelluri, S. Kongara, V. R. Nagasubramanian, S. Mahadevan, J. Chimakurthy
Journal of Endocrinological Investigation.2023; 46(5): 855. CrossRef - Finding the most cost-effective option from commonly used Dipeptidyl peptidase-4 inhibitors in India: a systematic study
Harmanjit Singh, Ekta Arora, Seerat Narula, Mandeep Singla, Armaan Otaal, Jatin Sharma
Expert Review of Endocrinology & Metabolism.2023; 18(4): 347. CrossRef - Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients With Diabetes
Yewon Na, Soo Wan Kim, Ie Byung Park, Soo Jung Choi, Seungyoon Nam, Jaehun Jung, Dae Ho Lee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(11): 3022. CrossRef
- Association between age at diagnosis of type 2 diabetes and cardiovascular morbidity and mortality risks: A nationwide population-based study

- Clinical Study
- The Association of Overt and Subclinical Hyperthyroidism with the Risk of Cardiovascular Events and Cardiovascular Mortality: Meta-Analysis and Systematic Review of Cohort Studies
- Seo Young Sohn, Eunyoung Lee, Min Kyung Lee, Jae Hyuk Lee
- Endocrinol Metab. 2020;35(4):786-800. Published online November 25, 2020
- DOI: https://doi.org/10.3803/EnM.2020.728

- 6,008 View
- 288 Download
- 20 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Whether hyperthyroidism is an independent risk factor for cardiovascular events remains controversial. We aimed to evaluate the association of overt and subclinical hyperthyroidism with the risk of ischemic heart disease (IHD), stroke, heart failure, and cardiovascular mortality.
Methods
Studies regarding the association between hyperthyroidism and cardiovascular events were searched on PubMed and Embase databases. The cardiovascular disease (CVD) risk was classified as high and low, based on pre-existing diseases, including history of coronary, cerebral, or peripheral artery disease; heart failure; atrial fibrillation; diabetes mellitus; or chronic kidney disease.
Results
Thirty-seven cohort studies were included in this meta-analysis. The pooled hazard ratio for subjects with overt hyperthyroidism compared with the control group was 1.11 (95% confidence interval [CI], 1.03 to 1.19) for IHD, 1.35 (95% CI, 1.03 to 1.75) for stroke, and 1.20 (95% CI, 1.00 to 1.46) for cardiovascular mortality. For subjects with subclinical hyperthyroidism, the pooled hazard ratio was 1.24 (95% CI, 1.07 to 1.45) for IHD, when compared with the control group. Subgroup analysis by CVD risk showed that the risk of stroke in overt hyperthyroidism was increased in the low CVD risk group; however, these association was not observed in the high CVD risk group. Similarly, the risk of IHD in subjects with subclinical hyperthyroidism was significantly increased in the low CVD risk group.
Conclusion
Overt hyperthyroidism is associated with increased risk of IHD, stroke, and cardiovascular mortality, and subclinical hyperthyroidism is associated with increased risk of IHD. These associations were particularly observed in the low risk CVD group without underlying CVD. -
Citations
Citations to this article as recorded by- Trends in Prevalence of Thyroid Dysfunction and its Associations With Mortality Among US Participants, 1988-2012
Xiaowen Zhang, Yong Wang, Hongwei Wang, Xinlin Zhang
The Journal of Clinical Endocrinology & Metabolism.2024; 109(2): e657. CrossRef - Adequacy of thyroid hormone replacement for people with hypothyroidism in real‐world settings: A systematic review and meta‐analysis of observational studies
Agathoklis Efthymiadis, Matthew Henry, Dimitrios Spinos, Marianthi Bourlaki, Alexandros Tsikopoulos, Angeliki Bourazana, Anastasios Bastounis, Konstantinos Tsikopoulos
Clinical Endocrinology.2024; 100(5): 488. CrossRef - Thyroid Disorders and Peripheral Arterial Disease
Katica Bajuk Studen, Simona Gaberscek, Katja Zaletel, Ales Blinc, Miso Sabovic, Gerit-Holger Schernthaner, Panagiotis Anagnostis, Pier Luigi Antignani, Mojca Jensterle, Dimitri P Mikhailidis, Pavel Poredos
Current Vascular Pharmacology.2024; 22(1): 36. CrossRef - Higher Risk of Incident Hyperthyroidism in Patients With Atrial Fibrillation
Pang-Shuo Huang, Jen-Fang Cheng, Jien-Jiun Chen, Yi-Chih Wang, Juey-Jen Hwang, Cho-Kai Wu, Chia-Ti Tsai
The Journal of Clinical Endocrinology & Metabolism.2023; 109(1): 92. CrossRef - Eurasian clinical guidelines for the diagnosis and treatment of secondary (symptomatic) forms of arterial hypertension (2022)
I. E. Chazova, N. M. Chikhladze, N. V. Blinova, Zh. E. Belaya, N. M. Danilov, E. M. Elfimova, A. Yu. Litvin, L. Ya. Rozhinskaya, N. Yu. Sviridenko, M. Yu. Shvetsov, V. A. Azizov, E. A. Grigorenko, N. P. Mit’kovskaja, I. I. Mustafaev, A. G. Polupanov, A. S
Eurasian heart journal.2023; (1): 6. CrossRef -
Sympathetic Activation Promotes Cardiomyocyte Apoptosis in a Rabbit Susceptibility Model of Hyperthyroidism-Induced Atrial Fibrillation via the p38 MAPK Signaling Pathway
Jialin Zheng, Shijian Zhao, Qishi Yang, Yantao Wei, Jianmei Li, Tao Guo
Critical Reviews in Eukaryotic Gene Expression.2023; 33(5): 17. CrossRef - Cardiovascular outcomes in subclinical thyroid disease: an update
Matthew D. Ettleson
Current Opinion in Endocrinology, Diabetes & Obesity.2023; 30(5): 218. CrossRef - Lower free triiodothyronine levels are associated with higher all-cause and cardiovascular mortality in people with diabetes-NHANES 2007–2012
Chang Liu, Zhong Xin, Lin Hua
Diabetes Research and Clinical Practice.2023; 202: 110811. CrossRef - Hyperthyroidism
Sun Y. Lee, Elizabeth N. Pearce
JAMA.2023; 330(15): 1472. CrossRef - Is Thyroid Dysfunction Associated with Unruptured Intracranial Aneurysms? A Population-Based, Nested Case–Control Study from Korea
Hyeree Park, Sun Wook Cho, Sung Ho Lee, Kangmin Kim, Hyun-Seung Kang, Jeong Eun Kim, Aesun Shin, Won-Sang Cho
Thyroid®.2023; 33(12): 1483. CrossRef - Risks of suboptimal and excessive thyroid hormone replacement across ages
U. Feldt-Rasmussen, G. Effraimidis, S. Bliddal, M. Klose
Journal of Endocrinological Investigation.2023; 47(5): 1083. CrossRef - Association of Mild Thyroid Dysfunction and Adverse Prognosis Among Chinese Patients With Acute ST Segment Elevation Myocardial Infarction
Mei-Fang Li, Ze-Tao Wei, Shuai Li, Qi-Ming Feng, Jing-Bo Li
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Cardiovascular Effects of Subclinical Hyperthyroidism following Percutaneous Coronary Intervention
Ricardo Correa, Ricardo Villela
Clinical Thyroidology.2022; 34(6): 240. CrossRef - Weight Gain and Body Composition Changes during the Transition of Thyroid Function in Patients with Graves’ Disease Undergoing Radioiodine Treatment
Zhenqin Cai, Qiyu Chen, Yan Ling, Henrik Falhammar
International Journal of Endocrinology.2022; 2022: 1. CrossRef - Minor perturbations of thyroid homeostasis and major cardiovascular endpoints—Physiological mechanisms and clinical evidence
Patrick Müller, Melvin Khee-Shing Leow, Johannes W. Dietrich
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Role of thyroid hormones-induced oxidative stress on cardiovascular physiology
María Laura Barreiro Arcos
Biochimica et Biophysica Acta (BBA) - General Subjects.2022; 1866(12): 130239. CrossRef - Yearly Incidence of Stroke and Bleeding in Atrial Fibrillation with Concomitant Hyperthyroidism: A National Discharge Database Study
Juqian Zhang, Arnaud Bisson, Grégoire Fauchier, Alexandre Bodin, Julien Herbert, Pierre Henri Ducluzeau, Gregory Y. H. Lip, Laurent Fauchier
Journal of Clinical Medicine.2022; 11(5): 1342. CrossRef - Platelet abnormalities in autoimmune thyroid diseases: A systematic review and meta-analysis
Yu-tian Cao, Kai-yu Zhang, Jing Sun, Yan Lou, Tian-su Lv, Xinyi Yang, Wen-hui Zhang, Jiang-yi Yu, Qi-biao Wu, Xi-qiao Zhou
Frontiers in Immunology.2022;[Epub] CrossRef - Subclinical Hyperthyroidism: A Review of the Clinical Literature
Karen Tsai, Angela M. Leung
Endocrine Practice.2021; 27(3): 254. CrossRef - Thyroid and heart, a clinically relevant relationship
G. Corona, L. Croce, C. Sparano, L. Petrone, A. Sforza, M. Maggi, L. Chiovato, M. Rotondi
Journal of Endocrinological Investigation.2021; 44(12): 2535. CrossRef - Antithyroid Drug Treatment in Graves’ Disease
Jae Hoon Chung
Endocrinology and Metabolism.2021; 36(3): 491. CrossRef - Cardiovascular Outcomes in Thyroid Cancer Patients Treated With Thyroidectomy: A Meta-Analysis
Eun Kyung Lee, Hwa Young Ahn, Eu Jeong Ku, Won Sang Yoo, Young Ki Lee, Kee-Hyun Nam, Young Jun Chai, Shinje Moon, Yuh-Seog Jung
The Journal of Clinical Endocrinology & Metabolism.2021;[Epub] CrossRef
- Trends in Prevalence of Thyroid Dysfunction and its Associations With Mortality Among US Participants, 1988-2012

- Obesity and Metabolism
- Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond
- Md Abu Bakkar Siddik, Andrew C. Shin
- Endocrinol Metab. 2019;34(3):234-246. Published online September 26, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.3.234
- 11,669 View
- 253 Download
- 78 Web of Science
- 77 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Branched-chain amino acids (BCAAs) are essential amino acids that are not synthesized in our body; thus, they need to be obtained from food. They have shown to provide many physiological and metabolic benefits such as stimulation of pancreatic insulin secretion, milk production, adipogenesis, and enhanced immune function, among others, mainly mediated by mammalian target of rapamycin (mTOR) signaling pathway. After identified as a reliable marker of obesity and type 2 diabetes in recent years, an increasing number of studies have surfaced implicating BCAAs in the pathophysiology of other diseases such as cancers, cardiovascular diseases, and even neurodegenerative disorders like Alzheimer's disease. Here we discuss the most recent progress and review studies highlighting both correlational and potentially causative role of BCAAs in the development of these disorders. Although we are just beginning to understand the intricate relationships between BCAAs and some of the most prevalent chronic diseases, current findings raise a possibility that they are linked by a similar putative mechanism.
-
Citations
Citations to this article as recorded by- First trimester metabolomics 1H-NMR study of the urinary profile predicts gestational diabetes mellitus development in obese women
Cristina Piras, Isabella Neri, Roberta Pintus, Antonio Noto, Elisabetta Petrella, Francesca Monari, Angelica Dessì, Vassilios Fanos, Luigi Atzori, Fabio Facchinetti
The Journal of Maternal-Fetal & Neonatal Medicine.2024; 35(25): 8275. CrossRef - Branched‐Chain Amino Acid Accumulation Fuels the Senescence‐Associated Secretory Phenotype
Yaosi Liang, Christopher Pan, Tao Yin, Lu Wang, Xia Gao, Ergang Wang, Holly Quang, De Huang, Lianmei Tan, Kun Xiang, Yu Wang, Peter B. Alexander, Qi‐Jing Li, Tso‐Pang Yao, Zhao Zhang, Xiao‐Fan Wang
Advanced Science.2024;[Epub] CrossRef - Deficiency of BCAT2-mediated branched-chain amino acid catabolism promotes colorectal cancer development
Zi-Ran Kang, Shanshan Jiang, Ji-Xuan Han, Yaqi Gao, Yile Xie, Jinxian Chen, Qiang Liu, Jun Yu, Xin Zhao, Jie Hong, Haoyan Chen, Ying-Xuan Chen, Huimin Chen, Jing-Yuan Fang
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(2): 166941. CrossRef - Microbiome metabolite quantification methods enabling insights into human health and disease
Jarrod Roach, Rohit Mital, Jacob J. Haffner, Nathan Colwell, Randy Coats, Horvey M. Palacios, Zongyuan Liu, Joseane L.P. Godinho, Monica Ness, Thilini Peramuna, Laura-Isobel McCall
Methods.2024; 222: 81. CrossRef - Branched-chain amino acids promote occurrence and development of cardiovascular disease dependent on triglyceride metabolism via activation of the mTOR/SREBP-1/betatrophin pathway
Jie Zhang, Ziyu Liu, Yaojun Ni, Yang Yu, Fei Guo, Yanwen Lu, Xiaoqing Wang, Hairong Hao, Shayan Li, Pan Wei, Weinan Yu, Wen Hu
Molecular and Cellular Endocrinology.2024; 584: 112164. CrossRef - Heat stress reduces brown adipose tissue activity by exacerbating mitochondrial damage in type 2 diabetic mice
Penghua Lai, Linlin Zhang, Yan Qiu, Jie Ren, Xue Sun, Ting Zhang, Liuyi Wang, Sijie Cheng, Sijia Liu, Hongli Zhuang, Daiwei Lu, Shaoliang Zhang, Huiqing Liang, Shaodong Chen
Journal of Thermal Biology.2024; 119: 103799. CrossRef - Unraveling Adipose Tissue Dysfunction: Molecular Mechanisms, Novel Biomarkers, and Therapeutic Targets for Liver Fat Deposition
Marta Lopez-Yus, Carlos Hörndler, Sofia Borlan, Vanesa Bernal-Monterde, Jose M. Arbones-Mainar
Cells.2024; 13(5): 380. CrossRef - Microbial production of branched chain amino acids: Advances and perspectives
Yanan Hao, Xuewei Pan, Jiajia You, Guomin Li, Meijuan Xu, Zhiming Rao
Bioresource Technology.2024; 397: 130502. CrossRef - Maintenance of the branched-chain amino acid transporter LAT1 counteracts myotube atrophy following chemotherapy
Stephen Mora, Olasunkanmi A. J. Adegoke
American Journal of Physiology-Cell Physiology.2024; 326(3): C866. CrossRef - Metabolomics of Mice with Type 2 Diabetes and Nonalcoholic Fatty Liver Treated by Acupuncture
Yihui Guo, Liying Zhang, Mengyuan Li, Linan Lin, Fuyu Xue, Wanning Gao, Xiaoru Xu, Haipeng Huang, Abdelilah Arredouani
International Journal of Endocrinology.2024; 2024: 1. CrossRef - Metabolic Signatures Elucidate the Effect of Body Mass Index on Type 2 Diabetes
Qiuling Dong, Sidra Sidra, Christian Gieger, Rui Wang-Sattler, Wolfgang Rathmann, Cornelia Prehn, Jerzy Adamski, Wolfgang Koenig, Annette Peters, Harald Grallert, Sapna Sharma
Metabolites.2023; 13(2): 227. CrossRef - The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice
Klara J. Lohkamp, Anita M. van den Hoek, Gemma Solé-Guardia, Maria Lisovets, Talissa Alves Hoffmann, Konstantina Velanaki, Bram Geenen, Vivienne Verweij, Martine C. Morrison, Robert Kleemann, Maximilian Wiesmann, Amanda J. Kiliaan
Nutrients.2023; 15(7): 1716. CrossRef - The Crosstalk between Gut Microbiota and White Adipose Tissue Mitochondria in Obesity
Luca Colangeli, David Israel Escobar Marcillo, Valeria Simonelli, Egidio Iorio, Tommaso Rinaldi, Paolo Sbraccia, Paola Fortini, Valeria Guglielmi
Nutrients.2023; 15(7): 1723. CrossRef - Prebiotic and Probiotic Modulation of the Microbiota–Gut–Brain Axis in Depression
Daniel E. Radford-Smith, Daniel C. Anthony
Nutrients.2023; 15(8): 1880. CrossRef - Machine learning model to predict obesity using gut metabolite and brain microstructure data
Vadim Osadchiy, Roshan Bal, Emeran A. Mayer, Rama Kunapuli, Tien Dong, Priten Vora, Danny Petrasek, Cathy Liu, Jean Stains, Arpana Gupta
Scientific Reports.2023;[Epub] CrossRef - Insulin Resistance and Impaired Branched-Chain Amino Acid Metabolism in Alzheimer’s Disease
Rui Liu, Lei Zhang, Hao You
Journal of Alzheimer's Disease.2023; 93(3): 847. CrossRef - Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments
Rexiati Ruze, Tiantong Liu, Xi Zou, Jianlu Song, Yuan Chen, Ruiyuan Xu, Xinpeng Yin, Qiang Xu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between arginine, glutamine, and the branched chain amino acid metabolism in the tumor microenvironment
Tanner J. Wetzel, Sheila C. Erfan, Lucas D. Figueroa, Leighton M. Wheeler, Elitsa A. Ananieva
Frontiers in Oncology.2023;[Epub] CrossRef - Stachydrine, N‐acetylornithine and trimethylamine N‐oxide levels as candidate milk biomarkers of maternal consumption of an obesogenic diet during lactation
Pedro Castillo, Ondrej Kuda, Jan Kopecky, Catalina Amadora Pomar, Andreu Palou, Mariona Palou, Catalina Picó
BioFactors.2023; 49(5): 1022. CrossRef - Biofunctionalization of natural extracts, trends in biological activity and kinetic release
Abraham Osiris Martínez-Olivo, Víctor Manuel Zamora-Gasga, Luis Medina-Torres, Alejandro Pérez-Larios, Sonia Guadalupe Sáyago-Ayerdi, Jorge Alberto Sánchez-Burgos
Advances in Colloid and Interface Science.2023; 318: 102938. CrossRef - Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study
Joshua P. Sutherland, Ang Zhou, Elina Hyppönen
Nutrients.2023; 15(12): 2703. CrossRef - Bioactive Ingredients in Traditional Fermented Food Condiments: Emerging Products for Prevention and Treatment of Obesity and Type 2 Diabetes
Alphonse Laya, Honoré Wangso, Iva Fernandes, Raphaël Djakba, Joana Oliveira, Eugenia Carvalho, Wen yi Kang
Journal of Food Quality.2023; 2023: 1. CrossRef - Sex related differences in muscle health and metabolism in chronic obstructive pulmonary disease
Mariëlle P.K.J. Engelen, Sarah K. Kirschner, Kimberly S. Coyle, David Argyelan, Gabriel Neal, Srinivasan Dasarathy, Nicolaas E.P. Deutz
Clinical Nutrition.2023; 42(9): 1737. CrossRef - Depiction of Branched-Chain Amino Acids (BCAAs) in Diabetes with a Focus on Diabetic Microvascular Complications
Daniela Maria Tanase, Evelina Maria Gosav, Tina Botoc, Mariana Floria, Claudia Cristina Tarniceriu, Minela Aida Maranduca, Anca Haisan, Andrei Ionut Cucu, Ciprian Rezus, Claudia Florida Costea
Journal of Clinical Medicine.2023; 12(18): 6053. CrossRef - Genetic and Lifestyle-Related Factors Influencing Serum Hyper-Propionylcarnitine Concentrations and Their Association with Metabolic Syndrome and Cardiovascular Disease Risk
Yong-Hwa Lee, Sunmin Park
International Journal of Molecular Sciences.2023; 24(21): 15810. CrossRef -
Relationship between Components, Intestinal Microbiota, and Mechanism of Hypoglycemic Effect of the Saggy Ink Cap Medicinal Mushroom (Coprinus Comatus, Agaricomycetes): A Review
Wei Wang, Min Sun, Jinyan Yu, Xumin Ma, Chunchao Han
International Journal of Medicinal Mushrooms.2023; 25(12): 81. CrossRef - High-protein diet with excess leucine prevents inactivity-induced insulin resistance in women
Alessandro Mangogna, Filippo Giorgio Di Girolamo, Nicola Fiotti, Pierandrea Vinci, Matteo Landolfo, Filippo Mearelli, Gianni Biolo
Clinical Nutrition.2023; 42(12): 2578. CrossRef - Exploring the functional roles of small-molecule metabolites in disease research: Recent advancements in metabolomics
Aolei Tan, Xiaoxiao Ma
Chinese Chemical Letters.2023; : 109276. CrossRef - Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
Endocrinology and Metabolism.2023; 38(6): 619. CrossRef - Dietary intake of branched-chain amino acids in relation to general and abdominal obesity
Farzaneh Asoudeh, Asma Salari-Moghaddam, Ammar Hassanzadeh Keshteli, Ahmad Esmaillzadeh, Peyman Adibi
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity.2022; 27(4): 1303. CrossRef - Tea polyphenol – gut microbiota interactions: hints on improving the metabolic syndrome in a multi-element and multi-target manner
Hui Ma, Yaozhong Hu, Bowei Zhang, Zeping Shao, Eugeni Roura, Shuo Wang
Food Science and Human Wellness.2022; 11(1): 11. CrossRef - Exogenous isoleucine can confer browning resistance on fresh-cut potato by suppressing polyphenol oxidase activity and improving the antioxidant capacity
Zan Meng, Tong Wang, Aman Ullah Malik, Qingguo Wang
Postharvest Biology and Technology.2022; 184: 111772. CrossRef - Impact of thermal treatment and fermentation by lactic acid bacteria on sorghum metabolite changes, their antioxidant and antidiabetic activities
Fred Kwame Ofosu, Fazle Elahi, Eric Banan-Mwine Daliri, Sang-Ik Han, Deog-Hwan Oh
Food Bioscience.2022; 45: 101502. CrossRef - Infant intakes of human milk branched chain amino acids are negatively associated with infant growth and influenced by maternal body mass index
Jessica L. Saben, Clark R. Sims, Lindsay Pack, Renny Lan, Elisabet Børsheim, Aline Andres
Pediatric Obesity.2022;[Epub] CrossRef - Roux-En-Y Gastric Bypass (RYGB) Surgery during High Liquid Sucrose Diet Leads to Gut Microbiota-Related Systematic Alterations
Laimdota Zizmare, Christina N. Boyle, Sabrina Buss, Sandrine Louis, Laura Kuebler, Ketki Mulay, Ralf Krüger, Lara Steinhauer, Isabelle Mack, Manuel Rodriguez Gomez, Kristina Herfert, Yvonne Ritze, Christoph Trautwein
International Journal of Molecular Sciences.2022; 23(3): 1126. CrossRef - Omics Analyses of Intestinal Microbiota and Hypothalamus Clock Genes in Circadian Disturbance Model Mice Fed with Green Tea Polyphenols
Yuting Zhang, Lu Cheng, Yanan Liu, Ruilin Zhang, Zufang Wu, Kejun Cheng, Xin Zhang
Journal of Agricultural and Food Chemistry.2022; 70(6): 1890. CrossRef - Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort
Vinicius Verri Hernandes, Nikola Dordevic, Essi Marjatta Hantikainen, Baldur Bragi Sigurdsson, Sigurður Vidir Smárason, Vanessa Garcia-Larsen, Martin Gögele, Giulia Caprioli, Ilaria Bozzolan, Peter P. Pramstaller, Johannes Rainer
Metabolites.2022; 12(3): 205. CrossRef - α-ketoisocaproic acid promotes ER stress through impairment of autophagy, thereby provoking lipid accumulation and insulin resistance in murine preadipocytes
Tae Jun Park, Seung Yeon Park, Hyun Jung Lee, A.M. Abd El-Aty, Ji Hoon Jeong, Tae Woo Jung
Biochemical and Biophysical Research Communications.2022; 603: 109. CrossRef - The associations of serum valine with mild cognitive impairment and Alzheimer’s disease
Yong-lan Xiong, Joseph Therriault, Shu-jiang Ren, Xiao-jun Jing, Hua Zhang
Aging Clinical and Experimental Research.2022; 34(8): 1807. CrossRef - The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology
Aikaterini Dimou, Vasilis Tsimihodimos, Eleni Bairaktari
International Journal of Molecular Sciences.2022; 23(7): 4022. CrossRef - Obesity, exercise training, and BCAA supplementation: All that glitters (may not be) gold
Stephen J. Carter, Emily B. Long, Cydne A. Perry
Obesity.2022; 30(6): 1139. CrossRef - Effects of serum branched-chain amino acids on nonalcoholic fatty liver disease and subsequent cardiovascular disease
Fei Guo, Rui Chen, Linghui Kong, Pan Wei, Ziyu Liu, Xiaoqing Wang, Hairong Hao, Yanwen Lu, Wen Hu
Hepatology International.2022; 16(6): 1424. CrossRef - Advances in multi-omics study of biomarkers of glycolipid metabolism disorder
Xinyi Fang, Runyu Miao, Jiahua Wei, Haoran Wu, Jiaxing Tian
Computational and Structural Biotechnology Journal.2022; 20: 5935. CrossRef - Targeted Metabolomics Revealed a Sex-Dependent Signature for Metabolic Syndrome in the Mexican Population
Berenice Palacios-González, Guadalupe León-Reyes, Berenice Rivera-Paredez, Isabel Ibarra-González, Marcela Vela-Amieva, Yvonne N. Flores, Samuel Canizales-Quinteros, Jorge Salmerón, Rafael Velázquez-Cruz
Nutrients.2022; 14(18): 3678. CrossRef - Case report: NAFLD and maple syrup urine disease: Is there an interplay between branched-chain amino acids and fructose consumption?
Helena Moreira-Silva, Sandra Ferreira, Manuela Almeida, Isabel Gonçalves, Maria Augusta Cipriano, J. R. Vizcaíno, Ermelinda Santos-Silva, Esmeralda Gomes-Martins
Frontiers in Pediatrics.2022;[Epub] CrossRef - A self-assembled leucine polymer sensitizes leukemic stem cells to chemotherapy by inhibiting autophagy in acute myeloid leukemia
Xi Xu, Jian Wang, Tong Tong, Wenwen Zhang, Jin Wang, Weiwei Ma, Shunqing Wang, Dunhua Zhou, Jun Wu, Linjia Jiang, Meng Zhao
Haematologica.2022; 107(10): 2344. CrossRef - Effect of dietary protein content shift on aging in elderly rats by comprehensive quantitative score and metabolomics analysis
Wenxuan Zheng, Ruiding Li, Yang Zhou, Fengcui Shi, Yao Song, Yanting Liao, Fan Zhou, Xiaohua Zheng, Jingwen Lv, Quanyang Li
Frontiers in Nutrition.2022;[Epub] CrossRef - Caloric restriction improves glycaemic control without reducing plasma branched-chain amino acids or keto-acids in obese men
M. H. Sayda, M. H. Abdul Aziz, N. Gharahdaghi, D. J. Wilkinson, P. L. Greenhaff, B. E. Phillips, K. Smith, I. Idris, P. J. Atherton
Scientific Reports.2022;[Epub] CrossRef - Dietary Protein and Amino Acid Deficiency Inhibit Pancreatic Digestive Enzyme mRNA Translation by Multiple Mechanisms
Maria Dolors Sans, Stephen J. Crozier, Nancy L. Vogel, Louis G. D’Alecy, John A. Williams
Cellular and Molecular Gastroenterology and Hepatology.2021; 11(1): 99. CrossRef - Isoleucine increases muscle mass through promoting myogenesis and intramyocellular fat deposition
Shuge Liu, Yunmei Sun, Rui Zhao, Yingqian Wang, Wanrong Zhang, Weijun Pang
Food & Function.2021; 12(1): 144. CrossRef - Serum metabolomics study of women with different annual decline rates of anti-Müllerian hormone: an untargeted gas chromatography–mass spectrometry-based study
Nazanin Moslehi, Parvin Mirmiran, Rezvan Marzbani, Hassan Rezadoost, Mehdi Mirzaie, Fereidoun Azizi, Fahimeh Ramezani Tehrani
Human Reproduction.2021; 36(3): 721. CrossRef - Amino acid sensing pathway: A major check point in the pathogenesis of obesity and COVID‐19
Aradhana Mariam Philips, Nooruddin Khan
Obesity Reviews.2021;[Epub] CrossRef - The chemical mechanisms of the enzymes in the branched-chain amino acids biosynthetic pathway and their applications
Yan-Fei Liang, Zi-Xian Long, Ya-Jian Zhang, Cai-Yun Luo, Le-Tian Yan, Wen-Yun Gao, Heng Li
Biochimie.2021; 184: 72. CrossRef - Plasma Metabolomic Profiling in 1391 Subjects with Overweight and Obesity from the SPHERE Study
Gianfranco Frigerio, Chiara Favero, Diego Savino, Rosa Mercadante, Benedetta Albetti, Laura Dioni, Luisella Vigna, Valentina Bollati, Angela Cecilia Pesatori, Silvia Fustinoni
Metabolites.2021; 11(4): 194. CrossRef - Sarcopenic Obesity and Amino Acids: Concord Health and Ageing in Men Project
David G Le Couteur, David J Handelsman, Fiona Stanaway, Louise M Waite, Fiona M Blyth, Vasi Naganathan, Robert G Cumming, Vasant Hirani, Rafael de Cabo
The Journals of Gerontology: Series A.2021; 76(6): 1000. CrossRef - Metabolic signatures of greater body size and their associations with risk of colorectal and endometrial cancers in the European Prospective Investigation into Cancer and Nutrition
Nathalie Kliemann, Vivian Viallon, Neil Murphy, Rebecca J. Beeken, Joseph A. Rothwell, Sabina Rinaldi, Nada Assi, Eline H. van Roekel, Julie A. Schmidt, Kristin Benjaminsen Borch, Claudia Agnoli, Ann H. Rosendahl, Hanna Sartor, José María Huerta, Anne Tjø
BMC Medicine.2021;[Epub] CrossRef - Plasma Amino Acids and Residual Hypertriglyceridemia in Diabetic Patients Under Statins: Two Independent Cross-Sectional Hospital-Based Cohorts
Shuang Wang, Yun-Feng Cao, Xiao-Yu Sun, Mo Hong, Zhong-Ze Fang, Hui-Huan Luo, Huan Sun, Ping Yang
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Identification of Metabolic Phenotypes in Young Adults with Obesity by 1H NMR Metabolomics of Blood Serum
Khin Thandar Htun, Jie Pan, Duanghathai Pasanta, Montree Tungjai, Chatchanok Udomtanakunchai, Sirirat Chancharunee, Siriprapa Kaewjaeng, Hong Joo Kim, Jakrapong Kaewkhao, Suchart Kothan
Life.2021; 11(6): 574. CrossRef - A Metabolic Pattern in Healthy Subjects Given a Single Dose of Metformin: A Metabolomics Approach
Lina A. Dahabiyeh, Muhammad Mujammami, Tawfiq Arafat, Hicham Benabdelkamel, Assim A. Alfadda, Anas M. Abdel Rahman
Frontiers in Pharmacology.2021;[Epub] CrossRef - Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism
Gagandeep Mann, Stephen Mora, Glory Madu, Olasunkanmi A. J. Adegoke
Frontiers in Physiology.2021;[Epub] CrossRef - Identification of TBX15 as an adipose master trans regulator of abdominal obesity genes
David Z. Pan, Zong Miao, Caroline Comenho, Sandhya Rajkumar, Amogha Koka, Seung Hyuk T. Lee, Marcus Alvarez, Dorota Kaminska, Arthur Ko, Janet S. Sinsheimer, Karen L. Mohlke, Nicholas Mancuso, Linda Liliana Muñoz-Hernandez, Miguel Herrera-Hernandez, Maria
Genome Medicine.2021;[Epub] CrossRef - Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts
Soon Yeon Jeong, Eunjin Kim, Ming Zhang, Yun-Seong Lee, Byeongjun Ji, Sun-Hee Lee, Yu Eun Cheong, Soon-Il Yun, Young-Soo Kim, Kyoung Heon Kim, Min Sun Kim, Hyun Soo Chun, Sooah Kim
Metabolites.2021; 11(8): 520. CrossRef - Targeted and Untargeted Mass Spectrometry Reveals the Impact of High-Fat Diet on Peripheral Amino Acid Regulation in a Mouse Model of Alzheimer’s Disease
Amelia L. Taylor, Don E. Davis, Simona G. Codreanu, Fiona E. Harrison, Stacy D. Sherrod, John A. McLean
Journal of Proteome Research.2021; 20(9): 4405. CrossRef - The Association of 9 Amino Acids With Cardiovascular Events in Finnish Men in a 12-Year Follow-up Study
Raimo Jauhiainen, Jagadish Vangipurapu, Annamaria Laakso, Teemu Kuulasmaa, Johanna Kuusisto, Markku Laakso
The Journal of Clinical Endocrinology & Metabolism.2021; 106(12): 3448. CrossRef - Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population
Berenice Palacios-González, Guadalupe León-Reyes, Berenice Rivera-Paredez, Isabel Ibarra-González, Marcela Vela-Amieva, Yvonne N. Flores, Samuel Canizales-Quinteros, Jorge Salmerón, Rafael Velázquez-Cruz
Metabolites.2021; 11(9): 604. CrossRef - Branched chain amino acids—friend or foe in the control of energy substrate turnover and insulin sensitivity?
Elżbieta Supruniuk, Ewa Żebrowska, Adrian Chabowski
Critical Reviews in Food Science and Nutrition.2021; : 1. CrossRef - Diet Effects on Cerebrospinal Fluid Amino Acids Levels in Adults with Normal Cognition and Mild Cognitive Impairment
Kate J. Russin, K. Sreekumaran Nair, Thomas J. Montine, Laura D. Baker, Suzanne Craft
Journal of Alzheimer's Disease.2021; 84(2): 843. CrossRef - QSHY Granules Promote White Adipose Tissue Browning and Correct BCAAs Metabolic Disorder in NAFLD Mice
Binbin Zhang, Mingzhu Ni, Xiaojing Li, Qiaohong Liu, Yiyang Hu, Yu Zhao
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4241. CrossRef - The Changes in Endogenous Metabolites in Hyperlipidemic Rats Treated with Herbal Mixture Containing Lemon, Apple Cider, Garlic, Ginger, and Honey
Azliana Abu Bakar Sajak, Azrina Azlan, Faridah Abas, Hazilawati Hamzah
Nutrients.2021; 13(10): 3573. CrossRef - Integration of Transcriptome and Metabolome Provides Unique Insights to Pathways Associated With Obese Breast Cancer Patients
Mohammed A. Hassan, Kaltoom Al-Sakkaf, Mohammed Razeeth Shait Mohammed, Ashraf Dallol, Jaudah Al-Maghrabi, Alia Aldahlawi, Sawsan Ashoor, Mabrouka Maamra, Jiannis Ragoussis, Wei Wu, Mohammad Imran Khan, Abdulrahman L. Al-Malki, Hani Choudhry
Frontiers in Oncology.2020;[Epub] CrossRef - Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention
Krasimira Aleksandrova, Caue Egea Rodrigues, Anna Floegel, Wolfgang Ahrens
Current Obesity Reports.2020; 9(3): 219. CrossRef - Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes?
Milan Holeček
Nutrients.2020; 12(10): 3087. CrossRef - Differential gene signature in adipose tissue depots of growth hormone transgenic mice
Silvana Duran‐Ortiz, Jonathan A. Young, Adam Jara, Elizabeth A. Jensen, Reetobrata Basu, Edward O. List, Yanrong Qian, John J. Kopchick, Darlene E. Berryman
Journal of Neuroendocrinology.2020;[Epub] CrossRef - Prognostic Role of Serum Amino Acids in Head and Neck Cancer
Gabriella Cadoni, Luca Giraldi, Carlo Chiarla, Jacopo Gervasoni, Silvia Persichilli, Aniello Primiano, Stefano Settimi, Jacopo Galli, Gaetano Paludetti, Dario Arzani, Stefania Boccia, Ivo Giovannini, Giovanni Almadori, Leigh A. Madden
Disease Markers.2020; 2020: 1. CrossRef - Lipid changes in the metabolome of a single case study with maple syrup urine disease (MSUD) after five days of improved diet adherence of controlled branched-chain amino acids (BCAA)
Teresa D. Douglas, L. Kristin Newby, Julie Eckstrand, Douglas Wixted, Rani H. Singh
Molecular Genetics and Metabolism Reports.2020; 25: 100651. CrossRef - Branched chain amino acids, aging and age-related health
David G. Le Couteur, Samantha M. Solon-Biet, Victoria C. Cogger, Rosilene Ribeiro, Rafael de Cabo, David Raubenheimer, Gregory J. Cooney, Stephen J. Simpson
Ageing Research Reviews.2020; 64: 101198. CrossRef - Branched-chain ketoacid overload inhibits insulin action in the muscle
Dipsikha Biswas, Khoi T. Dao, Angella Mercer, Andrew M. Cowie, Luke Duffley, Yassine El Hiani, Petra C. Kienesberger, Thomas Pulinilkunnil
Journal of Biological Chemistry.2020; 295(46): 15597. CrossRef
- First trimester metabolomics 1H-NMR study of the urinary profile predicts gestational diabetes mellitus development in obese women

- Diabetes
- The Role of Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors in Reducing Cardiovascular Events in Patients with Type 2 Diabetes
- Gwang Sil Kim, Joong Hyun Park, Jong Chul Won
- Endocrinol Metab. 2019;34(2):106-116. Published online May 9, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.2.106
- 5,192 View
- 101 Download
- 15 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The prevalence of type 2 diabetes mellitus (T2DM), which is associated with cardiovascular morbidity and mortality, is increasing worldwide. Although there have been advances in diabetes treatments that reduce microvascular complications (nephropathy, neuropathy, retinopathy), many clinical studies have found that conventional oral hypoglycemic agents and glucose control alone failed to reduce cardiovascular disease. Thus, incretin-based therapies including glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT-2Is) represent a new area of research, and may serve as novel therapeutics for treating hyperglycemia and modifying other cardiovascular risk factors. Recently, it has been confirmed that several drugs in these classes, including canagliflozin, empagliflozin, semaglutide, and liraglutide, are safe and possess cardioprotective effects. We review the most recent cardiovascular outcome trials on GLP-1RAs and SGLT-2Is, and discuss their implications for treating patients with T2DM in terms of protective effects against cardiovascular disease.
-
Citations
Citations to this article as recorded by- Switch to gliflozins and biventricular function improvement in patients with chronic heart failure and diabetes mellitus
Michele Correale, Pietro Mazzeo, Martino Fortunato, Matteo Paradiso, Andrea Furore, Angela I. Fanizzi, Lucia Tricarico, Giuseppe Pastore, Simona Alfieri, Natale D. Brunetti, Olga Lamacchia
Clinical Physiology and Functional Imaging.2024; 44(1): 112. CrossRef - Association between underweight and risk of heart failure in diabetes patients
Tae Kyung Yoo, Kyung‐Do Han, Eun‐Jung Rhee, Won‐Young Lee
Journal of Cachexia, Sarcopenia and Muscle.2024; 15(2): 671. CrossRef - Paradigm Shift in Management of Hyperglycemia in Patients with Type 2 Diabetes: Glucocentric versus Organ Protection
Jong Chul Won
The Journal of Korean Diabetes.2023; 24(2): 59. CrossRef - Posing the rationale for synthetic lipoxin mimetics as an adjuvant treatment to gold standard atherosclerosis therapies
Braden Millar, Monica de Gaetano
Frontiers in Pharmacology.2023;[Epub] CrossRef - Improvement of Left Ventricular Global Longitudinal Strain after 6-Month Therapy with GLP-1RAs Semaglutide and Dulaglutide in Type 2 Diabetes Mellitus: A Pilot Study
Paolo Basile, Andrea Igoren Guaricci, Giuseppina Piazzolla, Sara Volpe, Alfredo Vozza, Marina Benedetto, Maria Cristina Carella, Daniela Santoro, Francesco Monitillo, Andrea Baggiano, Saima Mushtaq, Laura Fusini, Fabio Fazzari, Cinzia Forleo, Nunziata Rib
Journal of Clinical Medicine.2023; 12(4): 1586. CrossRef - Rationale and design of the Biventricular Evaluation of Gliflozins effects In chroNic Heart Failure: BEGIN‐HF study
Michele Correale, Elena‐Laura Antohi, Riccardo M. Inciardi, Pietro Mazzeo, Stefano Coiro, Shiro Ishihara, Renata Petroni, Francesco Monitillo, Marta Leone, Marco Triggiani, Chaudhry M.S. Sarwar, Hans‐Dirk Dungen, Khawaja M. Talha, Natale D. Brunetti, Jave
ESC Heart Failure.2023; 10(3): 2066. CrossRef - Effects of Incretin-Based Treatment on the Diastolic (Dys)Function in Patients with Uncontrolled Type 2 Diabetes Mellitus: A Prospective Study with 1-Year Follow-Up
Elena-Daniela Grigorescu, Cristina-Mihaela Lăcătușu, Mariana Floria, Georgiana-Diana Cazac, Alina Onofriescu, Livia-Amira Sauciuc, Alexandr Ceasovschih, Ioana Crețu, Bogdan-Mircea Mihai, Laurențiu Șorodoc
Diagnostics.2023; 13(17): 2817. CrossRef - Role of moesin in the effect of glucagon-like peptide-1 on advanced glycation end products-induced endothelial barrier dysfunction
Yan Liu, Zhenzhen Chen, Lei Liu, Haitao Tang, Huaqing Zhu, Songtao Tang
Cellular Signalling.2022; 90: 110193. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Effectiveness of liraglutide 3 mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention
Jung Ha Park, Ju Young Kim, Jong Han Choi, Hye Soon Park, Hyun-Young Shin, Jae Min Lee, Jin-Wook Kim, Hae-Jin Ko, Suk Chon, Bu Kyung Kim, Chul Sik Kim, Soo Lim
International Journal of Obesity.2021; 45(4): 776. CrossRef - Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure
Shruti S Joshi, Trisha Singh, David E Newby, Jagdeep Singh
Heart.2021; 107(13): 1032. CrossRef - Effects of antidiabetic drugs on left ventricular function/dysfunction: a systematic review and network meta-analysis
Da-Peng Zhang, Li Xu, Le-Feng Wang, Hong-Jiang Wang, Feng Jiang
Cardiovascular Diabetology.2020;[Epub] CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef - Benefit-Risk Assessment of Alogliptin for the Treatment of Type 2 Diabetes Mellitus
Kohei Kaku, Koichi Kisanuki, Mari Shibata, Takashi Oohira
Drug Safety.2019; 42(11): 1311. CrossRef
- Switch to gliflozins and biventricular function improvement in patients with chronic heart failure and diabetes mellitus

- Diabetes-Related Cardiac Dysfunction
- Lamario J. Williams, Brenna G. Nye, Adam R. Wende
- Endocrinol Metab. 2017;32(2):171-179. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.171
- 12,173 View
- 45 Download
- 37 Web of Science
- 36 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The proposal that diabetes plays a role in the development of heart failure is supported by the increased risk associated with this disease, even after correcting for all other known risk factors. However, the precise mechanisms contributing to the condition referred to as diabetic cardiomyopathy have remained elusive, as does defining the disease itself. Decades of study have defined numerous potential factors that each contribute to disease susceptibility, progression, and severity. Many recent detailed reviews have been published on mechanisms involving insulin resistance, dysregulation of microRNAs, and increased reactive oxygen species, as well as causes including both modifiable and non-modifiable risk factors. As such, the focus of the current review is to highlight aspects of each of these topics and to provide specific examples of recent advances in each area.
-
Citations
Citations to this article as recorded by-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats
Afolabi C. Akinmoladun, Morenikejimi Bello, Emmanuel Oluwafemi Ibukun
Archives of Physiology and Biochemistry.2023; 129(6): 1219. CrossRef - An Overview of Cardiotonic Medicinal Plants from the Perspective of Iranian Traditional Medicine
Akram Alembagheri, Homa Hajimehdipoor, Rasool Choopani, Somayeh Esmaeili
Jundishapur Journal of Natural Pharmaceutical Products.2023;[Epub] CrossRef - Nanoformulations for the Delivery of Dietary Anthocyanins for the Prevention and Treatment of Diabetes Mellitus and Its Complications
Ana R. Nunes, Elisabete C. Costa, Gilberto Alves, Luís R. Silva
Pharmaceuticals.2023; 16(5): 736. CrossRef - Cyp2e1 knockdown attenuates high glucose-induced apoptosis and oxidative stress of cardiomyocytes by activating PI3K/Akt signaling
Jianying Wang, Han Yang, Chao Wang, Cuie Kan
Acta Diabetologica.2023; 60(9): 1219. CrossRef - Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
Yu-Qi Li, Lei Xin, Yu-Chi Zhao, Shang-Qi Li, Ya-Nuo Li
World Journal of Hepatology.2023; 15(6): 786. CrossRef - Non-Invasive Assessment of the Intraventricular Pressure Using Novel Color M-Mode Echocardiography in Animal Studies: Current Status and Future Perspectives in Veterinary Medicine
Ahmed S. Mandour, Ahmed Farag, Mahmoud A. Y. Helal, Gamal El-Masry, Salim Al-Rejaie, Ken Takahashi, Tomohiko Yoshida, Lina Hamabe, Ryou Tanaka
Animals.2023; 13(15): 2452. CrossRef - Diet‐induced prediabetes: Effects on the activity of the renin–angiotensin–aldosterone system in selected organs
Bongeka Cassandra Mkhize, Palesa Mosili, Phikelelani Sethu Ngubane, Ntethelelo Hopewell Sibiya, Andile Khathi
Journal of Diabetes Investigation.2022; 13(5): 768. CrossRef - Knowledge domain and emerging trends in diabetic cardiomyopathy: A scientometric review based on CiteSpace analysis
Shiyi Tao, Deshuang Yang, Lanxin Zhang, Lintong Yu, Zihan Wang, Lingling Li, Jin Zhang, Ruiqi Yao, Li Huang, Mingjing Shao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Sodium–Glucose Co-transporter-2 Inhibitors
Joshua J Neumiller, Fredrick J Lienhard, Radica Z Alicic, Katherine R Tuttle
European Endocrinology.2022; 18(2): 106. CrossRef - Protective effects of medicinal plant against diabetes induced cardiac disorder: A review
Sadegh Shabab, Zahra Gholamnezhad, Maryam Mahmoudabady
Journal of Ethnopharmacology.2021; 265: 113328. CrossRef - Toward a broader view of mechanisms of drug cardiotoxicity
Polina Mamoshina, Blanca Rodriguez, Alfonso Bueno-Orovio
Cell Reports Medicine.2021; 2(3): 100216. CrossRef - Cardioprotective Effect of Glycyrrhizin on Myocardial Remodeling in Diabetic Rats
Vikram Thakur, Narah Alcoreza, Monica Delgado, Binata Joddar, Munmun Chattopadhyay
Biomolecules.2021; 11(4): 569. CrossRef - Cardioprotective Action of Glycyrrhizin on Diabetic Rats with Myocardial Remodeling
Fuxu Chen, Jie Song, Enas Abdulhay
Journal of Healthcare Engineering.2021; 2021: 1. CrossRef - Management of inflammation in cardiovascular diseases
Sumanta Kumar Goswami, Prabhat Ranjan, Roshan Kumar Dutta, Suresh Kumar Verma
Pharmacological Research.2021; 173: 105912. CrossRef - Diabetic Cardiomyopathy: Clinical and Metabolic Approach
Dragan B. Djordjevic, Goran Koracevic, Aleksandar D. Djordjevic, Dragan B. Lovic
Current Vascular Pharmacology.2021; 19(5): 487. CrossRef - Effect of Acute Chemotherapy on Glucose Levels in Rats
Ahmad H. Alhowail, Gena S. Alfawzan, Maha A. Aldubayan, Lolwah S. Alsalam
International Journal of Pharmacology.2020; 16(3): 276. CrossRef - Transplantation of adipose tissue lacking PAI-1 improves glucose tolerance and attenuates cardiac metabolic abnormalities in high-fat diet-induced obesity
Sijing Liu, Yi Li, Xin Fan, Kai Li, Chunrong Xu, Liping Zhang, Mao Luo, Liqun Wang, Rong Li, Jianbo Wu
Adipocyte.2020; 9(1): 170. CrossRef - Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers
Jeffrey I. Mechanick, Michael E. Farkouh, Jonathan D. Newman, W. Timothy Garvey
Journal of the American College of Cardiology.2020; 75(5): 525. CrossRef - Associated Targets of the Antioxidant Cardioprotection of Ganoderma lucidum in Diabetic Cardiomyopathy by Using Open Targets Platform: A Systematic Review
Fahmi Shaher, Hongbin Qiu, Shuqiu Wang, Yu Hu, Weiqun Wang, Yu Zhang, Yao Wei, Hisham AL-ward, Mahfoudh A. M. Abdulghani, Sattam Khulaif Alenezi, Salem Baldi, Shaobo Zhou
BioMed Research International.2020; 2020: 1. CrossRef - Human trophoblast-derived exosomes attenuate doxorubicin-induced cardiac injury by regulating miR-200b and downstream Zeb1
Jie Ni, Yihai Liu, Lina Kang, Lian Wang, Zhonglin Han, Kun Wang, Biao Xu, Rong Gu
Journal of Nanobiotechnology.2020;[Epub] CrossRef - Clinical Evidence and Proposed Mechanisms for Cardiovascular and Kidney Benefits from Glucagon-like Peptide-1 Receptor Agonists
Emily J Cox, Radica Z Alicic, Joshua J Neumiller, Katherine R Tuttle
US Endocrinology.2020; 16(2): 80. CrossRef - Hyperbaric Oxygen Therapy Dampens Inflammatory Cytokine Production and Does Not Worsen the Cardiac Function and Oxidative State of Diabetic Rats
Rita Benkő, Zsuzsanna Miklós, Viktor Antal Ágoston, Katrine Ihonvien, Csaba Répás, Roland Csépányi-Kömi, Margit Kerék, Nóra Judit Béres, Eszter Mária Horváth
Antioxidants.2019; 8(12): 607. CrossRef - Heart Failure in Type 2 Diabetes Mellitus
Helena C. Kenny, E. Dale Abel
Circulation Research.2019; 124(1): 121. CrossRef - SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice
Damilola D. Adingupu, Sven O. Göpel, Julia Grönros, Margareta Behrendt, Matus Sotak, Tasso Miliotis, Ulrika Dahlqvist, Li-Ming Gan, Ann-Cathrine Jönsson-Rylander
Cardiovascular Diabetology.2019;[Epub] CrossRef - Depressive symptoms in asymptomatic stage B heart failure with Type II diabetic mellitus
Paul J. Mills, Pam R. Taub, Ottar Lunde, Meredith A. Pung, Kathleen Wilson, Christopher Pruitt, Thomas Rutledge, Alan Maisel, Barry H. Greenberg
Clinical Cardiology.2019; 42(6): 637. CrossRef - Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate
Amir M. Al Hroob, Mohammad H. Abukhalil, Omnia E. Hussein, Ayman M. Mahmoud
Biomedicine & Pharmacotherapy.2019; 109: 2155. CrossRef - The Janus face of HMGB1 in heart disease: a necessary update
Angela Raucci, Stefania Di Maggio, Francesco Scavello, Alessandro D’Ambrosio, Marco E. Bianchi, Maurizio C. Capogrossi
Cellular and Molecular Life Sciences.2019; 76(2): 211. CrossRef - Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model
Sabri Sudirman, Ching-Shu Lai, Yi-Ling Yan, Hung-I Yeh, Zwe-Ling Kong
Scientific Reports.2019;[Epub] CrossRef - Microarray profiling analysis identifies the mechanism of miR‐200b‐3p/mRNA‐CD36 affecting diabetic cardiomyopathy via peroxisome proliferator activated receptor‐γ signaling pathway
Liqiong Xu, Wei Chen, Min Ma, Anfang Chen, Chengyue Tang, Chengwei Zhang, Lin Cai
Journal of Cellular Biochemistry.2019; 120(4): 5193. CrossRef - Plasma Low-Density Lipoprotein Cholesterol Correlates With Heart Function in Individuals With Type 2 Diabetes Mellitus: A Cross-Sectional Study
Po-Chung Cheng, Shang-Ren Hsu, Jung-Chi Li, Ching-Pei Chen, Szu-Chi Chien, Shih-Te Tu, Yun-Chung Cheng, Yu-Hsiu Liu, Jeng-Fu Kuo
Frontiers in Endocrinology.2019;[Epub] CrossRef - Impact of diabetes mellitus on the contractile properties of the left and right atrial myofilaments†
Constanze Bening, Khaled Alhussini, Elena-Aura Mazalu, Jonathan Yaqub, Khaled Hamouda, Dejan Radakovic, Christoph Schimmer, Grzegorz Hirnle, Rainer Leyh
European Journal of Cardio-Thoracic Surgery.2018; 54(5): 826. CrossRef - LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling
Lu Gao, Yuan Liu, Sen Guo, Lili Xiao, Leiming Wu, Zheng Wang, Cui Liang, Rui Yao, Yanzhou Zhang
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2018; 1864(10): 3322. CrossRef - Gene expression profiles of rat MMECs with different glucose levels and fgl2 gene silencing
Zhenzhong Zheng, Fan Zhang, Dengpeng Gao, Yujing Wu, Hao Wu
Diabetes/Metabolism Research and Reviews.2018;[Epub] CrossRef - Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes
Kwong-Man Ng, Yee-Man Lau, Vidhu Dhandhania, Zhu-Jun Cai, Yee-Ki Lee, Wing-Hon Lai, Hung-Fat Tse, Chung-Wah Siu
Scientific Reports.2018;[Epub] CrossRef - Apelin‑13 ameliorates metabolic and cardiovascular disorders in a rat model of type 2 diabetes with a high‑fat diet
Meng Li, Huijuan Fang, Jian Hu
Molecular Medicine Reports.2018;[Epub] CrossRef - Adriamycin-induced cardiomyopathy can serve as a model for diabetic cardiomyopathy – a hypothesis
Kaviyarasi Renu, V.G. Abilash, P.B. Tirupathi Pichiah, Thabassum Akthar Syeda, Sankarganesh Arunachalam
Asian Pacific Journal of Tropical Biomedicine.2017; 7(11): 1041. CrossRef
-
Upregulation of PCSK9, rho kinase and cardiac troponin by
Eucalyptus globulus
leaf extract improves fructose-streptozotocin-induced diabetic cardiac dysfunction in rats

- Obesity and Metabolism
- Diabetes Drugs and Cardiovascular Safety
- Ji Cheol Bae
- Endocrinol Metab. 2016;31(2):239-244. Published online June 10, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.239
- 3,604 View
- 38 Download
- 15 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetes is a well-known risk factor of cardiovascular morbidity and mortality, and the beneficial effect of improved glycemic control on cardiovascular complications has been well established. However, the rosiglitazone experience aroused awareness of potential cardiovascular risk associated with diabetes drugs and prompted the U.S. Food and Drug Administration to issue new guidelines about cardiovascular risk. Through postmarketing cardiovascular safety trials, some drugs demonstrated cardiovascular benefits, while some antidiabetic drugs raised concern about a possible increased cardiovascular risk associated with drug use. With the development of new classes of drugs, treatment options became wider and the complexity of glycemic management in type 2 diabetes has increased. When choosing the appropriate treatment strategy for patients with type 2 diabetes at high cardiovascular risk, not only the glucose-lowering effects, but also overall benefits and risks for cardiovascular disease should be taken into consideration.
-
Citations
Citations to this article as recorded by- Dipeptidyl peptidase-4 inhibitor compared with sulfonylurea in combination with metformin: cardiovascular and renal outcomes in a propensity-matched cohort study
Kyoung Jin Kim, Jimi Choi, Juneyoung Lee, Jae Hyun Bae, Jee Hyun An, Hee Young Kim, Hye Jin Yoo, Ji A. Seo, Nan Hee Kim, Kyung Mook Choi, Sei Hyun Baik, Sin Gon Kim, Nam Hoon Kim
Cardiovascular Diabetology.2019;[Epub] CrossRef - Sodium‐glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter‐organ crosstalk
Jin Hee Kim, Minyoung Lee, Soo Hyun Kim, So Ra Kim, Byung‐Wan Lee, Eun Seok Kang, Bong‐Soo Cha, Jin Won Cho, Yong‐ho Lee
Diabetes, Obesity and Metabolism.2019; 21(4): 801. CrossRef - Glitazones and alpha-glucosidase inhibitors as the second-line oral anti-diabetic agents added to metformin reduce cardiovascular risk in Type 2 diabetes patients: a nationwide cohort observational study
Cheng-Wei Chan, Chu-Leng Yu, Jiunn-Cherng Lin, Yu-Cheng Hsieh, Che-Chen Lin, Chen-Ying Hung, Cheng-Hung Li, Ying-Chieh Liao, Chu-Pin Lo, Jin-Long Huang, Ching-Heng Lin, Tsu-Juey Wu
Cardiovascular Diabetology.2018;[Epub] CrossRef - Normoglucemiantes orales y riesgo cardiovascular
Guillermo Guzmán, Juan Esteban Gómez, Leidy Johanna Plaza, María Claudia Sánchez
Revista Colombiana de Cardiología.2018; 25(5): 333. CrossRef - Dipeptidyl peptidase-4 inhibitor use and risk of diabetic retinopathy: A population-based study
N.H. Kim, J. Choi, N.H. Kim, K.M. Choi, S.H. Baik, J. Lee, S.G. Kim
Diabetes & Metabolism.2018; 44(4): 361. CrossRef - Obesity and Type 2 Diabetes in Our Youth: A Recipe for Cardiovascular Disease
Angela Kaye Wooton, Lynne M. Melchior
The Journal for Nurse Practitioners.2017; 13(3): 222. CrossRef - Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes
Ilaria Campesi, Flavia Franconi, Giuseppe Seghieri, Marco Meloni
Pharmacological Research.2017; 119: 195. CrossRef - The Landscape of Glucose-Lowering Therapy and Cardiovascular Outcomes: From Barren Land to Metropolis
Mona P. Nasrallah, Charbel Abi Khalil, Marwan M. Refaat
BioMed Research International.2017; 2017: 1. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Dipeptidyl peptidase-4 inhibitor compared with sulfonylurea in combination with metformin: cardiovascular and renal outcomes in a propensity-matched cohort study

- Adrenal gland
- Acromegaly with Normal Insulin-Like Growth Factor-1 Levels and Congestive Heart Failure as the First Clinical Manifestation
- Hyae Min Lee, Sun Hee Lee, In-Ho Yang, In Kyoung Hwang, You Cheol Hwang, Kyu Jeung Ahn, Ho Yeon Chung, Hui-Jeong Hwang, In-Kyung Jeong
- Endocrinol Metab. 2015;30(3):395-401. Published online December 9, 2014
- DOI: https://doi.org/10.3803/EnM.2015.30.3.395
- 4,188 View
- 44 Download
- 7 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The leading cause of morbidity and mortality in patients with acromegaly is cardiovascular complications. Myocardial exposure to excessive growth hormone can cause ventricular hypertrophy, hypertension, arrhythmia, and diastolic dysfunction. However, congestive heart failure as a result of systolic dysfunction is observed only rarely in patients with acromegaly. Most cases of acromegaly exhibit high levels of serum insulin-like growth factor-1 (IGF-1). Acromegaly with normal IGF-1 levels is rare and difficult to diagnose. Here, we report a rare case of an acromegalic patient whose first clinical manifestation was severe congestive heart failure, despite normal IGF-1 levels. We diagnosed acromegaly using a glucose-loading growth hormone suppression test. Cardiac function and myocardial hypertrophy improved 6 months after transsphenoidal resection of a pituitary adenoma.
-
Citations
Citations to this article as recorded by- Risk of Neurodegenerative Diseases in Patients With Acromegaly
Sangmo Hong, Kyungdo Han, Kyung-Soo Kim, Cheol-Young Park
Neurology.2022;[Epub] CrossRef - Levels of Serum IGF-1, HCY, and Plasma BNP in Patients with Chronic Congestive Heart Failure and Their Relationship with Cardiac Function and Short-Term Prognosis
Zhengyi Hu, Leifang Mao, Ling Wang, Weiguo Li
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Neurofibromatosis Type 1 with Concurrent Multiple Endocrine Disorders: Adenomatous Goiter, Primary Hyperparathyroidism, and Acromegaly
Shigemitsu Yasuda, Ikuo Inoue, Akira Shimada
Internal Medicine.2021; 60(15): 2451. CrossRef - Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states
Hye Suk Kang, Ho-Chan Cho, Jae-Ho Lee, Goo Taeg Oh, Seung-Hoi Koo, Byung-Hyun Park, In-Kyu Lee, Hueng-Sik Choi, Dae-Kyu Song, Seung-Soon Im
Scientific Reports.2016;[Epub] CrossRef
- Risk of Neurodegenerative Diseases in Patients With Acromegaly

- Reversible Heart Failure and Rhabdomyolysis Caused by Primary Hypoparathyroidism during Lactation.
- Kyongyeun Jung, Jeong Hyun Choi, Hee Jin Kim, Hyun Kyung Chung, Dohee Kim
- Endocrinol Metab. 2011;26(3):268-271. Published online September 1, 2011
- DOI: https://doi.org/10.3803/EnM.2011.26.3.268
- 1,672 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Hypocalcemia can be complicated, on rare occasions, by congestive heart failure and may also be associated with labor and lactation in some cases. Herein, we report a 30-year-old woman with hypocalcemia-induced heart failure secondary to primary idiopathic hypoparathyroidism precipitated by lactation. The patient presented with chest pain and paresthesia in both arms and legs during breast-feeding after her second delivery. She had severe hypocalcemia and low parathyroid hormone levels. Hypocalcemia-induced rhabdomyolysis further aggravated her hypocalcemia symptoms. The echocardiogram showed global hypokinesia with an ejection fraction of 47%. After calcium and vitamin D replacement, her symptoms and ventricular function improved. Hypocalcemia needs to be considered in patients with heart failure, because it is readily reversible. To the best of our knowledge, this is the first report of a patient with heart failure and rhabdomyolysis induced by primary hypoparathyroidism during lactation.

- A Patient with Primary Amyloidosis Misrecognized as Thyrotoxicosis-induced Heart Failure.
- Seok Ju Lee, Seung Hwan Lee, Jung Yeon Chin, Youn Mi Song, Sung Won Lee, Min Hee Kim, Mi Ja Kang, Kang Woo Lee, Hyuk Sang Kwon, Kun Ho Yoon, Ho Young Son, Bong Yun Cha
- J Korean Endocr Soc. 2008;23(5):332-336. Published online October 1, 2008
- DOI: https://doi.org/10.3803/jkes.2008.23.5.332
- 1,703 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - Amyloidosis is caused by deposition of insoluble amyloid protein in the extracellular space of organs and tissues. The causes of amyloidosis are classified as primary, secondary, and hereditary, and symptoms develop according to which organ is involved. Cardiac amyloidosis induces cardiomyopathy and is developed by deposition of amyloid proteins in cardiac tissue. We diagnosed a patient with rhabdomyolysis and thyrotoxicosis with underlying Graves' disease 5 years ago. The patient was readmitted recently complaining of general weakness and mild dyspnea, and was diagnosed as relapsed thyrotoxicosis. An echocardiogram was performed for the evaluation of dyspnea and the findings were compatible with infiltrative cardiomyopathy due to amyloidosis. A biopsy of the abdominal subcutaneous fat and rectal mucosa was performed, and diagnosis was amyloidosis with histologic findings. The cause of heart failure was therefore cardiac amyloidosis rather than thyrotoxicosis. This case indicates the importance of evaluating the cause of heart failure in patients with thyrotoxicosis.


 KES
KES

 First
First Prev
Prev



