Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- The Effects of Irisin on the Interaction between Hepatic Stellate Cell and Macrophage in Liver Fibrosis
- Dinh Vinh Do, So Young Park, Giang Thi Nguyen, Dae Hee Choi, Eun-Hee Cho
- Endocrinol Metab. 2022;37(4):620-629. Published online July 22, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1412

- 4,432 View
- 195 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Hepatic stellate cells (HSCs) are the central players interacting with multiple cell types in liver fibrosis. The crosstalk between HSCs and macrophages has recently become clearer. Irisin, an exercise-responsive myokine, was known to have a potentially protective role in liver and renal fibrosis, especially in connection with stellate cells. This study investigated the effects of irisin on the interaction between HSCs and macrophages.
Methods
Tamm-Horsfall protein-1 (THP-1) human monocytes were differentiated into macrophages, polarized into the inflammatory M1 phenotype with lipopolysaccharide. Lieming Xu-2 (LX-2) cells, human HSCs, were treated with conditioned media (CM) from M1 macrophages, with or without recombinant irisin. HSCs responses to CM from M1 macrophages were evaluated regarding activation, proliferation, wound healing, trans-well migration, contractility, and related signaling pathway.
Results
CM from M1 macrophages significantly promoted HSC proliferation, wound healing, transwell migration, and contractility, but not activation of HSCs. Irisin co-treatment attenuated these responses of HSCs to CM. However, CM and irisin treatment did not induce any changes in HSC activation. Further, irisin co-treatment alleviated CM-induced increase of phopho-protein kinase B (pAKT), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinases-1 (TIMP-1).
Conclusion
These findings suggested that irisin may play a protective role in the pathogenesis of liver fibrosis, especially when working in the crosstalk between HSCs and macrophages. -
Citations
Citations to this article as recorded by- Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis
Liang Shan, Fengling Wang, Dandan Zhai, Xiangyun Meng, Jianjun Liu, Xiongwen Lv
Biomedicine & Pharmacotherapy.2023; 161: 114472. CrossRef - Potential role of irisin in digestive system diseases
Yueming Zhang, Linxian Zhao, Huan Gao, Jinghui Zhai, Yanqing Song
Biomedicine & Pharmacotherapy.2023; 166: 115347. CrossRef - The effect of sarcopenia and serum myokines on prognosis and survival in cirrhotic patients: a multicenter cross-sectional study
Salih Boga, Abdullah Emre Yildirim, Enver Ucbilek, Ali Riza Koksal, Sevil Tokdemir Sisman, Ibrahim Durak, Ilker Sen, Beril Dogu, Erdinc Serin, Ayse Bolat Ucbilek, Makbule Ozge Yildirim, Sukru Mehmet Erturk, Huseyin Alkim, Canan Alkim
European Journal of Gastroenterology & Hepatology.2022; 34(12): 1261. CrossRef
- Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis

- Obesity and Metabolism
- Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress
- Min Jeong Choi, Saet-Byel Jung, Joon Young Chang, Minho Shong
- Endocrinol Metab. 2021;36(1):1-11. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.956

- 5,415 View
- 226 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Paracrine interactions are imperative for the maintenance of adipose tissue intercellular homeostasis, and intracellular organelle dysfunction results in local and systemic alterations in metabolic homeostasis. It is currently accepted that mitochondrial proteotoxic stress activates the mitochondrial unfolded protein response (UPRmt) in vitro and in vivo. The induction of mitochondrial chaperones and proteases during the UPRmt is a key cell-autonomous mechanism of mitochondrial quality control. The UPRmt also affects systemic metabolism through the secretion of cell non-autonomous peptides and cytokines (hereafter, metabokines). Mitochondrial function in adipose tissue plays a pivotal role in whole-body metabolism and human diseases. Despite continuing interest in the role of the UPRmt and quality control pathways of mitochondria in energy metabolism, studies on the roles of the UPRmt and metabokines in white adipose tissue are relatively sparse. Here, we describe the role of the UPRmt in adipose tissue, including adipocytes and resident macrophages, and the interactive roles of cell non-autonomous metabokines, particularly growth differentiation factor 15, in local adipose cellular homeostasis and systemic energy metabolism.
-
Citations
Citations to this article as recorded by- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior
Carla Igual Gil, Bethany M Coull, Wenke Jonas, Rachel N Lippert, Susanne Klaus, Mario Ost
Life Science Alliance.2022; 5(11): e202201495. CrossRef - Stress-induced FGF21 and GDF15 in obesity and obesity resistance
Susanne Keipert, Mario Ost
Trends in Endocrinology & Metabolism.2021; 32(11): 904. CrossRef
- Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior

- Clinical Study
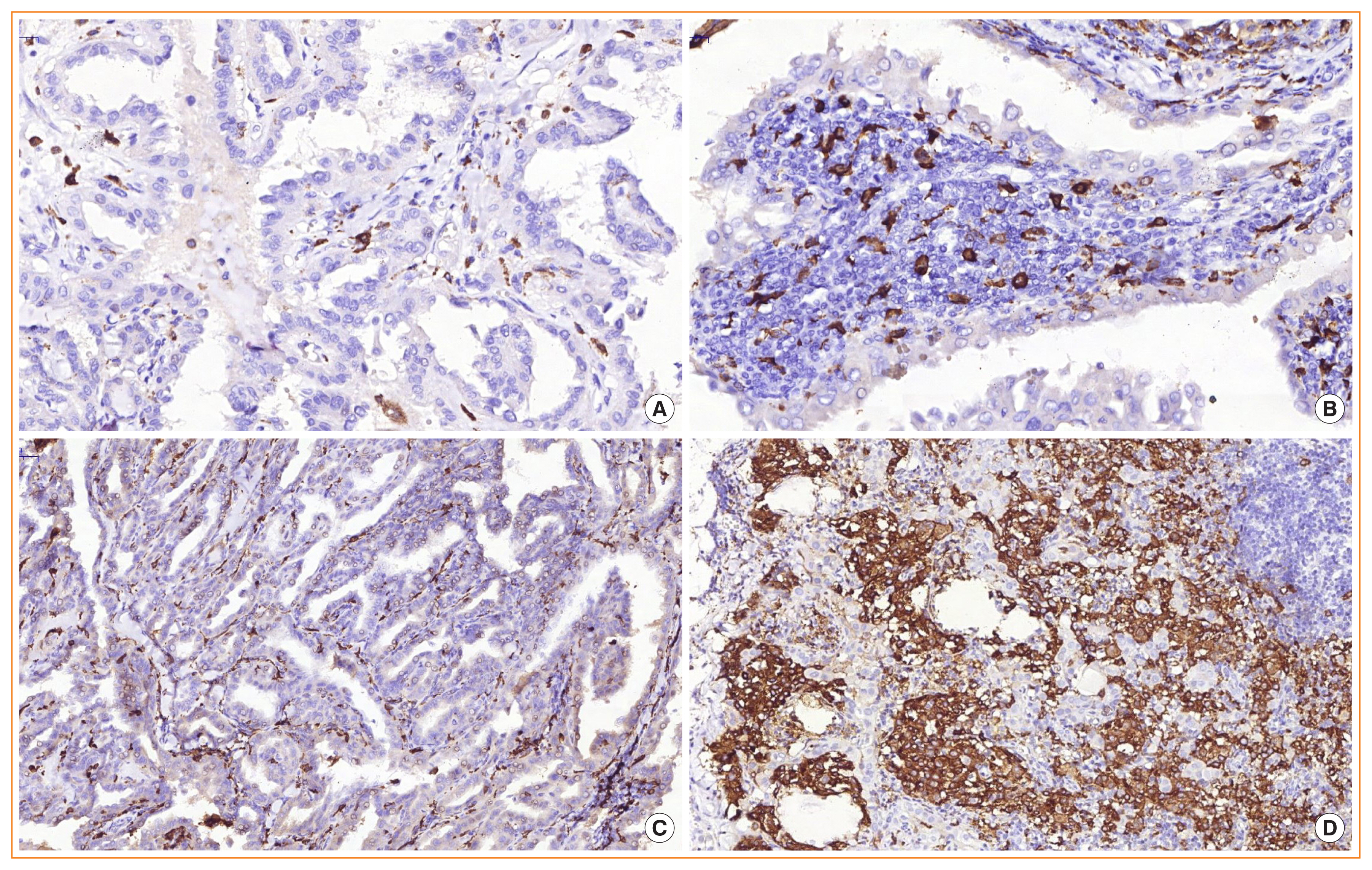
- Mechanisms of the Impact of Hashimoto Thyroiditis on Papillary Thyroid Carcinoma Progression: Relationship with the Tumor Immune Microenvironment
- Oksana Sulaieva, Olena Chernenko, Oleksiy Selesnov, Oleksandr Nechay, Oleksandr Maievskyi, Tetyana Falalyeyeva, Nazarii Kobyliak, Olena Tsyryuk, Yurii Penchuk, Dmytro Shapochka
- Endocrinol Metab. 2020;35(2):443-455. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.443
- 6,501 View
- 167 Download
- 12 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The relationship between Hashimoto thyroiditis (HT) and papillary thyroid carcinoma (PTC) remains uncertain. We assessed the impact of HT on the tumor immune microenvironment (TIME) in PTC.
Methods
Thirty patients with PTC (group 1) and 30 patients with PTC and HT (group 2) were enrolled in this pilot study. The distribution and number of CD8+ lymphocytes, plasma cells (CD138+), regulatory T cells (forkhead box P3 [FOXP3+)], mast cell tryptase (MCT+), and M2 macrophages (CD163+) were evaluated. To test the hypothesis that HT impacts PTC development via signal transducer and activator of transcription 6 (STAT6) activation and M2 macrophage polarization, we investigated STAT6 expression in tumor and stromal cells. We also evaluated vascular endothelial growth factor (VEGF) expression by lymph node metastasis (LNM) status.
Results
TIME showed significant between-group differences. Group 1 patients demonstrated immune desert or immune-excluded immunophenotypes, while an inflamed phenotype with more CD8+ cells (P<0.001) predominated in group 2. Immune-excluded TIME was associated with the highest LNM rate. In PTC, LNM was associated with more numerous CD163+ cells. Moreover, LNM in group 1 was associated with increased numbers of mast cells peritumorally and FOXP3+ cells intratumorally and peritumorally. Group 2 demonstrated higher STAT6 but not higher VEGF expression in tumor cells. High VEGF expression was associated with LNM regardless of HT status.
Conclusion
Concomitant HT impacted PTC signaling via STAT6 and TIME by increasing the number of CD8+ cells. LNM is associated with increases in CD163+ cells and VEGF expression in PTC, whereas HT affected LNM through different mechanisms. -
Citations
Citations to this article as recorded by- Clinical significance and diagnostic value of QPCT, SCEL and TNFRSF12A in papillary thyroid cancer
Tairong Liang, Xiuqian Wu, Lan Wang, Zhengzhong Ni, Ying Fan, Peishan Wu, Hongzhi Wang, Yongdong Niu, Haihua Huang
Pathology - Research and Practice.2023; 245: 154431. CrossRef - Recommend with caution: A meta-analysis investigating papillary thyroid carcinoma tumor progression under active surveillance
Peter P. Issa, Ruhul Munshi, Aaron L. Albuck, Mahmoud Omar, Ruba F. Abu Alhuda, Tyler Metz, Mohammad Hussein, Mohamed Shama, Grace S. Lee, Eman Toraih, Emad Kandil
American Journal of Otolaryngology.2023; 44(6): 103994. CrossRef - Hashimoto's thyroiditis is negatively associated with lymph node metastasis in PTMC
Hui Huang, Siyuan Xu, Song Ni, Wensheng Liu, Shaoyan Liu
Journal of Cancer Research and Clinical Oncology.2023; 149(17): 15525. CrossRef - A nomogram based on the risk factors of cervical lymph node metastasis in papillary thyroid carcinoma coexistent with Hashimoto’s thyroiditis
Huanhuan Miao, Jingwen Zhong, Xuesha Xing, Jiawei Sun, Jiaqi Wu, Chengwei Wu, Yan Yuan, Xianli Zhou, Hongbo Wang
Clinical Hemorheology and Microcirculation.2023; 85(3): 235. CrossRef - The role of vascular endothelial growth factor in the development of papillary thyroid carcinoma in patients with lymphocytic thyroiditis
Nese E. GULCELIK, Safak AKIN, Kadriye AYDIN, Cisel AYDIN MERICOZ, Yesim G. GULER TEZEL, Aydan USMAN
Minerva Endocrinology.2023;[Epub] CrossRef - Identification of Transcriptional Pattern Related to Immune Cell Infiltration With Gene Co-Expression Network in Papillary Thyroid Cancer
Meiye Li, Jimei Zhang, Zongjing Zhang, Ying Qian, Wei Qu, Zhaoshun Jiang, Baochang Zhao
Frontiers in Endocrinology.2022;[Epub] CrossRef - A Potential Nine-lncRNAs Signature Identification and Nomogram Diagnostic Model Establishment for Papillary Thyroid Cancer
Jin-Ming Yao, Jun-Yu Zhao, Fang-Fang Lv, Xue-Bo Yang, Huan-Jun Wang
Pathology and Oncology Research.2022;[Epub] CrossRef - Hyperhomocysteinemia in the pathogenesis of cardiovascular and endocrine diseases: translational messages
Rostyslav KAMINSKY, Andrii YANCHYSHYN, Natalia BELEMETS, Olena KURYK, Inga SAMBORSKA, Iryna DZEVULSKA, Rinaldo PELLICANO
Minerva Biotechnology and Biomolecular Research.2022;[Epub] CrossRef - Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis
Oleksandr Stakhovskyi, Nazarii Kobyliak, Oleg Voylenko, Eduard Stakhovskyi, Roman Ponomarchuk, Oksana Sulaieva
Cells.2022; 11(11): 1802. CrossRef - Comprehensive analysis of lncRNA-mediated ceRNA regulatory networks and key genes associated with papillary thyroid cancer coexistent with Hashimoto’s thyroiditis
Yuepeng Zhang, Yueli Tian
BMC Endocrine Disorders.2022;[Epub] CrossRef - Research Progress in the Correlation between AID, BRAFV600E and Papillary Thyroid Carcinoma
鑫 焦
Advances in Clinical Medicine.2022; 12(12): 11503. CrossRef - STAT6: A review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology
Georgia Karpathiou, Alexandra Papoudou-Bai, Elise Ferrand, Jean Marc Dumollard, Michel Peoc’h
Pathology - Research and Practice.2021; 223: 153477. CrossRef - Prevalence of Hashimoto Thyroiditis in Adults With Papillary Thyroid Cancer and Its Association With Cancer Recurrence and Outcomes
Siyuan Xu, Hui Huang, Jiaxin Qian, Yang Liu, Ying Huang, Xiaolei Wang, Shaoyan Liu, Zhengang Xu, Jie Liu
JAMA Network Open.2021; 4(7): e2118526. CrossRef - LARINGOPHARINGEAL REFLUX IMPACTS IMMUNE MICROENVIRONMENT OF LARYNGEAL CARCINOMA
D.I. Zabolotnyi, V.V. Kizim, D.D. Zabolotna, Y.V. Kizim, O.N. Sulaieva
Fiziolohichnyĭ zhurnal.2020; 66(4): 12. CrossRef
- Clinical significance and diagnostic value of QPCT, SCEL and TNFRSF12A in papillary thyroid cancer

- The Role of Macrophage Lipophagy in Reverse Cholesterol Transport
- Se-Jin Jeong, Mi-Ni Lee, Goo Taeg Oh
- Endocrinol Metab. 2017;32(1):41-46. Published online March 20, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.41
- 4,376 View
- 72 Download
- 31 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Macrophage cholesterol efflux is a central step in reverse cholesterol transport, which helps to maintain cholesterol homeostasis and to reduce atherosclerosis. Lipophagy has recently been identified as a new step in cholesterol ester hydrolysis that regulates cholesterol efflux, since it mobilizes cholesterol from lipid droplets of macrophages via autophagy and lysosomes. In this review, we briefly discuss recent advances regarding the mechanisms of the cholesterol efflux pathway in macrophage foam cells, and present lipophagy as a therapeutic target in the treatment of atherosclerosis.
-
Citations
Citations to this article as recorded by- Bile salt signaling and bile salt-based therapies in cardiometabolic disease
Claire C.J. Groenen, Thuc-Anh Nguyen, Coen C. Paulusma, Stan F.J. van de Graaf
Clinical Science.2024; 138(1): 1. CrossRef - Huazhuotongmai decoction exerts anti-atherosclerotic effects by modulating the expression of ABCA1/SR-B1/PPAR-γ in vivo and in vitro
Ya-ru Yan, Zi-jun Jia, Ya Wang, Feng-qin Xu, Qing-bing Zhou
Phytomedicine Plus.2023; 3(2): 100436. CrossRef - The SGLT2 Inhibitor Canagliflozin Reduces Atherosclerosis by Enhancing Macrophage Autophagy
Hongping Chen, Da Teng, Bowen Xu, Chunxiao Wang, Hua Wang, Wenjuan Jia, Lei Gong, Haibin Dong, Lin Zhong, Jun Yang
Journal of Cardiovascular Translational Research.2023; 16(5): 999. CrossRef - FURIN suppresses the progression of atherosclerosis by promoting macrophage autophagy
Hongping Chen, Lihui Zhang, Shaohua Mi, Hua Wang, Chunxiao Wang, Wenjuan Jia, Lei Gong, Haibin Dong, Bowen Xu, Yanyan Jing, Peipei Ge, Zhigang Pei, Lin Zhong, Jun Yang
The FASEB Journal.2023;[Epub] CrossRef - Unbalanced Redox With Autophagy in Cardiovascular Disease
Se-Jin Jeong, Goo Taeg Oh
Journal of Lipid and Atherosclerosis.2023; 12(2): 132. CrossRef - Edible Bird’s Nest Effectively Attenuates Atherosclerosis through Modulation of Cholesterol Metabolism via Activation of PPARγ/LXRα Signaling Pathway In Vivo
Nurul Nadiah Mohamad Nasir, Ramlah Mohamad Ibrahim, Rozi Mahmud, Nor Asma Ab Razak, Norsharina Ismail, Kim Wei Chan, Md Zuki Abu Bakar, Akhilesh K. Verma
Journal of Food Biochemistry.2023; 2023: 1. CrossRef - The mitochondrial translocator protein (TSPO, 18 kDa): A key multifunctional molecule in liver diseases
Yuchang Li, Liting Chen, Vassilios Papadopoulos
Biochimie.2023;[Epub] CrossRef - The Differential Metabolomes in Cumulus and Mural Granulosa Cells from Human Preovulatory Follicles
Er-Meng Gao, Bongkoch Turathum, Ling Wang, Di Zhang, Yu-Bing Liu, Rong-Xin Tang, Ri-Cheng Chian
Reproductive Sciences.2022; 29(4): 1343. CrossRef - PHLPP1 promotes neutral lipid accumulation through AMPK/ChREBP-dependent lipid uptake and fatty acid synthesis pathways
Keerthana Balamurugan, Raghavender Medishetti, Jyothi Kotha, Parameshwar Behera, Kanika Chandra, Vijay Aditya Mavuduru, Manjunath B. Joshi, Ramesh Samineni, Madhumohan R. Katika, Writoban Basu Ball, Manjunatha Thondamal, Anil Challa, Kiranam Chatti, Kisho
iScience.2022; 25(2): 103766. CrossRef - Hydrochloride Berberine ameliorates alcohol-induced liver injury by regulating inflammation and lipid metabolism
Xiumei Ke, Ruoyu Zhang, Pan Li, Ling Zuo, Meng Wang, Junxuan Yang, Jianwei Wang
Biochemical and Biophysical Research Communications.2022; 610: 49. CrossRef - Metabolic Regulation of Macrophage Activation
Ourania Kolliniati, Eleftheria Ieronymaki, Eleni Vergadi, Christos Tsatsanis
Journal of Innate Immunity.2022; 14(1): 51. CrossRef - Emerging Roles of Lipophagy in Cancer Metastasis
Haimeng Yin, Ying Shan, Tian Xia, Yan Ji, Ling Yuan, Yiwen You, Bo You
Cancers.2022; 14(18): 4526. CrossRef - Genetic Factors for Coronary Heart Disease and Their Mechanisms: A Meta-Analysis and Comprehensive Review of Common Variants from Genome-Wide Association Studies
Khairul Anwar Zarkasi, Noraidatulakma Abdullah, Nor Azian Abdul Murad, Norfazilah Ahmad, Rahman Jamal
Diagnostics.2022; 12(10): 2561. CrossRef - Caveolin-1 in autophagy: A potential therapeutic target in atherosclerosis
Kai Hou, Shuai Li, Meng Zhang, Xuping Qin
Clinica Chimica Acta.2021; 513: 25. CrossRef - Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy
Jing Zhang, Chuan-Rui Ma, Yun-Qing Hua, Lan Li, Jing-Yu Ni, Yu-Ting Huang, Sophia Esi Duncan, Sheng Li, Shan Gao, Guan-Wei Fan
Life Sciences.2021; 276: 118957. CrossRef - A meta-analysis of HDL cholesterol efflux capacity and concentration in patients with rheumatoid arthritis
Binbin Xie, Jiang He, Yong Liu, Ting Liu, Chaoqun Liu
Lipids in Health and Disease.2021;[Epub] CrossRef - Rosmarinic Acid Increases Macrophage Cholesterol Efflux through Regulation of ABCA1 and ABCG1 in Different Mechanisms
Jean-Baptiste Nyandwi, Young Shin Ko, Hana Jin, Seung Pil Yun, Sang Won Park, Hye Jung Kim
International Journal of Molecular Sciences.2021; 22(16): 8791. CrossRef - FGF21 induces autophagy‐mediated cholesterol efflux to inhibit atherogenesis via RACK1 up‐regulation
Lin Xiaolong, Guo Dongmin, Mihua Liu, Wang Zuo, Hu Huijun, Tan Qiufen, Hu XueMei, Lin Wensheng, Pan Yuping, Lin Jun, Zeng Zhaolin
Journal of Cellular and Molecular Medicine.2020; 24(9): 4992. CrossRef - Lipophagy in atherosclerosis
Qing Liu, Yuan-Mei Wang, Hong-Feng Gu
Clinica Chimica Acta.2020; 511: 208. CrossRef - Lysosomotropic Features and Autophagy Modulators among Medical Drugs: Evaluation of Their Role in Pathologies
Tatiana A. Korolenko, Thomas P. Johnston, Vaclav Vetvicka
Molecules.2020; 25(21): 5052. CrossRef - LncRNA MALAT1 Enhances ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-κB Pathway in Macrophages
Jiaqi Yang, Xuze Lin , Liangshan Wang, Tienan Sun, Qi Zhao, Qian Ma, Yujie Zhou
Current Vascular Pharmacology.2020; 18(6): 652. CrossRef -
CTRP13 inhibits atherosclerosis
via
autophagy‐lysosome‐dependent degradation of CD36
Cheng Wang, Wenjing Xu, Minglu Liang, Dan Huang, Kai Huang
The FASEB Journal.2019; 33(2): 2290. CrossRef - Subclinical atherosclerosis and its progression are modulated by PLIN2 through a feed‐forward loop between LXR and autophagy
P. Saliba‐Gustafsson, M. Pedrelli, K. Gertow, O. Werngren, V. Janas, S. Pourteymour, D. Baldassarre, E. Tremoli, F. Veglia, R. Rauramaa, A.J. Smit, P. Giral, S. Kurl, M. Pirro, U. de Faire, S.E. Humphries, A. Hamsten, I. Gonçalves, M. Orho‐Melander, A. Fr
Journal of Internal Medicine.2019; 286(6): 660. CrossRef - PCSK9: A new participant in lipophagy in regulating atherosclerosis?
Jun Xiao, Yi-Min Deng, Xiang-Rui Liu, Jian-Ping Cao, Min Zhou, Ya-Ling Tang, Wen-Hao Xiong, Zhi-Sheng Jiang, Zhi-Han Tang, Lu-Shan Liu
Clinica Chimica Acta.2019; 495: 358. CrossRef - Autophagy-Mediated Cholesterol Trafficking Controls Steroid Production
Michael J. Texada, Alina Malita, Christian F. Christensen, Kathrine B. Dall, Nils J. Faergeman, Stanislav Nagy, Kenneth A. Halberg, Kim Rewitz
Developmental Cell.2019; 48(5): 659. CrossRef - Lipophagy in nonliver tissues and some related diseases: Pathogenic and therapeutic implications
Kebing Zhou, Pingbo Yao, Jun He, Hong Zhao
Journal of Cellular Physiology.2019; 234(6): 7938. CrossRef - Autophagy differentially regulates macrophage lipid handling depending on the lipid substrate (oleic acid vs. acetylated-LDL) and inflammatory activation state
Sapir Hadadi-Bechor, Yulia Haim, Tal Pecht, Roni Gat, Tanya Tarnovscki, Martin Gericke, Assaf Rudich
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(12): 158527. CrossRef - Autophagy and Age-Related Eye Diseases
Xue Yang, Xinan Pan, Xiaorui Zhao, Jin Luo, Mingpu Xu, Daoming Bai, Yan Hu, Xu Liu, Qiongfang Yu, Dian Gao
BioMed Research International.2019; 2019: 1. CrossRef - Foam cell formation and cholesterol trafficking and metabolism disturbances in atherosclerosis
Alexandrina Volobueva, Dongwei Zhang, Andrey V. Grechko, Alexander N. Orekhov
Cor et Vasa.2019; 61(1): 48. CrossRef - Oxidative Stress, Lipid Peroxidation, and Loss of Hyaluronic Acid in the Human Vitreous Affected by Synchysis Scintillans
Loredana Bergandi, Oleksii A Skorokhod, Rosalba La Grotta, Evelin Schwarzer, Raffaele Nuzzi
Journal of Ophthalmology.2019; 2019: 1. CrossRef - Programmed cell death protein 4 deficiency suppresses foam cell formation by activating autophagy in advanced glycation end‐product low‐density lipoprotein–induced macrophages
Shan Li, Guangdong Gao, Fuyun Wu, Dan Liu, Hongyan Zhao, Jing Ke, Ying Liu, Fei Li, Jian Li, Zongyun Chen, Zhiming Tang, Lei Bai, Jinxuan Zhang, Wei Zheng, Xin Chen
Journal of Cellular Biochemistry.2019; 120(5): 7689. CrossRef - Mindin deficiency in macrophages protects against foam cell formation and atherosclerosis by targeting LXR-β
Cheng Zhang, Juan-Juan Qin, Fu-Han Gong, Jing-Jing Tong, Wen-Lin Cheng, Haiping Wang, Yan Zhang, Xueyong Zhu, Zhi-Gang She, Hao Xia, Li-Hua Zhu
Clinical Science.2018; 132(11): 1199. CrossRef - LJ-1888, a selective antagonist for the A3 adenosine receptor, ameliorates the development of atherosclerosis and hypercholesterolemia in apolipoprotein E knock-out mice
Jong-Gil Park, Se-Jin Jeong, Jinha Yu, Gyudong Kim, Lak Shin Jeong, Goo Taeg Oh
BMB Reports.2018; 51(10): 520. CrossRef - Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis
Dmitry Y. Litvinov, Eugeny V. Savushkin, Alexander D. Dergunov
Journal of Lipids.2018; 2018: 1. CrossRef
- Bile salt signaling and bile salt-based therapies in cardiometabolic disease

- Endocrine Research
- Macrophage Densities Correlated with CXC Chemokine Receptor 4 Expression and Related with Poor Survival in Anaplastic Thyroid Cancer
- Dae In Kim, Eunyoung Kim, Young A Kim, Sun Wook Cho, Jung Ah Lim, Young Joo Park
- Endocrinol Metab. 2016;31(3):469-475. Published online August 2, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.3.469
- 4,682 View
- 52 Download
- 22 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Tumor associated macrophages (TAMs) and CXC chemokine receptor 4 (CXCR4) have emerged as potential biomarkers in various human cancers. The aims of this study were to investigate the clinical characteristics of anaplastic thyroid cancer (ATC) patients according to the TAM numbers in the tumor tissue, and to evaluate the associations between CXCR4 expressions and macrophage densities in ATC tumor microenvironment.
Methods Total 14 ATC samples from thyroid tissue microarray were used. Immunohistochemical staining was performed using anti-CD163 and anti-CXCR4 antibodies. According to the immunoreactivity of CD163, all subjects were divided into two groups: low-CD163 (
n =8) and high-CD163 (n =6) groups.Results The mean diagnostic age was 65±7 years and the median tumor size was 4.3 cm, ranging 2.5 to 15 cm. Clinicopathological characteristics were not significantly different between low-CD163 and high-CD163 groups, while age of diagnosis was younger in high-CD163 group than that of low-CD163 group with marginal significance (56.9±5.5 years vs. 67.5±6.8 years,
P =0.09). However, overall survival was significantly reduced in high-CD163 group (5.5 months [range, 1 to 10]) compared with low-CD163 groups (8.8 months [range, 6 to 121); log-rank test,P =0.0443). Moreover, high-CD163 group showed strong CXCR4 expressions in both cancer and stromal compartments, while low-CD163 group showed relatively weak, stromal-dominant CXCR4 expressions. Additionally, CD163 and CXCR4 expressions showed a strong positive correlation (γ2=0.432,P =0.013).Conclusion Increased number of TAMs showed poor overall survival in ATC, suggesting TAMs are potentially a prognostic biomarker for ATC. CXCR4 expression was significantly correlated with CD163-positive TAM densities, which suggest the possible role of CXCR4 in TAM recruitments.
-
Citations
Citations to this article as recorded by- IL2RA+VSIG4+ tumor-associated macrophage is a key subpopulation of the immunosuppressive microenvironment in anaplastic thyroid cancer
Zongfu Pan, Lisha Bao, Xixuan Lu, Xiaoping Hu, Lu Li, Jinming Chen, Tiefeng Jin, Yiwen Zhang, Zhuo Tan, Ping Huang, Minghua Ge
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2023; 1869(1): 166591. CrossRef - Crosstalk between Thyroid Carcinoma and Tumor-Correlated Immune Cells in the Tumor Microenvironment
Mingyuan Song, Qi Liu, Wei Sun, Hao Zhang
Cancers.2023; 15(10): 2863. CrossRef - Modeling the tumor microenvironment of anaplastic thyroid cancer: an orthotopic tumor model in C57BL/6 mice
Zhen Xu, Hyo Shik Shin, Yoo Hyung Kim, Seong Yun Ha, Jae-Kyung Won, Su-jin Kim, Young Joo Park, Sareh Parangi, Sun Wook Cho, Kyu Eun Lee
Frontiers in Immunology.2023;[Epub] CrossRef - Tumor-associated macrophages as a potential therapeutic target in thyroid cancers
Liya Zhu, Xiu Juan Li, Prakash Gangadaran, Xiuli Jing, Byeong-Cheol Ahn
Cancer Immunology, Immunotherapy.2023; 72(12): 3895. CrossRef - Functional Phenotypes of Peritoneal Macrophages Upon AMD3100 Treatment During Colitis-Associated Tumorigenesis
Shuai Wu, Weiwei Luo, Xing Wu, Zhaohua Shen, Xiaoyan Wang
Frontiers in Medicine.2022;[Epub] CrossRef - Integrated analysis of novel macrophage related signature in anaplastic thyroid cancer
Yi Luo, Yi-Chen Yang, Ben Ma, Wei-Bo Xu, Tian Liao, Yu Wang
Endocrine.2022; 78(3): 517. CrossRef - Secreted Factors by Anaplastic Thyroid Cancer Cells Induce Tumor-Promoting M2-like Macrophage Polarization through a TIM3-Dependent Mechanism
Cinthia Carolina Stempin, Romina Celeste Geysels, Sunmi Park, Luz Maria Palacios, Ximena Volpini, Claudia Cristina Motran, Eva Virginia Acosta Rodríguez, Juan Pablo Nicola, Sheue-yann Cheng, Claudia Gabriela Pellizas, Laura Fozzatti
Cancers.2021; 13(19): 4821. CrossRef - Immunotherapy for anaplastic thyroid carcinoma: the present and future
Xixuan LU, Lisha BAO, Zongfu PAN, Minghua GE
Journal of Zhejiang University (Medical Sciences).2021; 50(6): 675. CrossRef - CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy
Ashraf Mousavi
Immunology Letters.2020; 217: 91. CrossRef - Nobiletin Alone or in Combination with Cisplatin Decreases the Viability of Anaplastic Thyroid Cancer Cell Lines
Diana P. Sousa, Marta Pojo, Ana T. Pinto, Valeriano Leite, Ana Teresa Serra, Branca M. Cavaco
Nutrition and Cancer.2020; 72(2): 352. CrossRef - The Thyroid Tumor Microenvironment: Potential Targets for Therapeutic Intervention and Prognostication
Laura MacDonald, Jonathan Jenkins, Grace Purvis, Joshua Lee, Aime T. Franco
Hormones and Cancer.2020; 11(5-6): 205. CrossRef - Mouse Models as a Tool for Understanding Progression in BrafV600E-Driven Thyroid Cancers
Iñigo Landa, Jeffrey A. Knauf
Endocrinology and Metabolism.2019; 34(1): 11. CrossRef - Low Lymphocyte-to-Monocyte Ratios Are Associated with Poor Overall Survival in Anaplastic Thyroid Carcinoma Patients
Jonghwa Ahn, Eyun Song, Hye-Seon Oh, Dong Eun Song, Won Gu Kim, Tae Yong Kim, Won Bae Kim, Young Kee Shong, Min Ji Jeon
Thyroid.2019; 29(6): 824. CrossRef - Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy
Christian M. Schürch, Matthias A. Roelli, Stefan Forster, Marie-Hélène Wasmer, Frido Brühl, Renaud S. Maire, Sergio Di Pancrazio, Marc-David Ruepp, Roland Giger, Aurel Perren, Anja M. Schmitt, Philippe Krebs, Roch-Philippe Charles, Matthias S. Dettmer
Thyroid.2019; 29(7): 979. CrossRef - Recent advances and emerging therapies in anaplastic thyroid carcinoma
Maria E. Cabanillas, Mark Zafereo, Michelle D. Williams, Renata Ferrarotto, Ramona Dadu, Neil Gross, G. Brandon Gunn, Heath Skinner, Gilbert Cote, Horiana B. Grosu, Priyanka Iyer, Naifa L. Busaidy
F1000Research.2018; 7: 87. CrossRef - Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer
Marika H Tesselaar, Johannes W Smit, James Nagarajah, Romana T Netea-Maier, Theo S Plantinga
Journal of Molecular Endocrinology.2017; 59(4): R141. CrossRef
- IL2RA+VSIG4+ tumor-associated macrophage is a key subpopulation of the immunosuppressive microenvironment in anaplastic thyroid cancer

- Thyroid
- The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
- Seunghwan Kim, Sun Wook Cho, Hye Sook Min, Kang Min Kim, Gye Jeong Yeom, Eun Young Kim, Kyu Eun Lee, Yeo Gyu Yun, Do Joon Park, Young Joo Park
- Endocrinol Metab. 2013;28(3):192-198. Published online September 13, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.3.192
- 4,250 View
- 45 Download
- 40 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Tumor-associated macrophages (TAMs) play a tumorigenic role related to advanced staging and poor prognosis in many human cancers including thyroid cancers. Yet, a functional role of TAMs in papillary thyroid carcinoma (PTC) has not been established. The aim of this study was to investigate TAM expression in human PTC with lymph node (LN) metastasis.
Methods Thirty-six patients who underwent surgery after being diagnosed with PTC with LN metastasis were included. Primary tumor tissues were immunohistochemically stained with an anti-CD68 antibody and clinical characteristics according to TAM density were evaluated.
Results The TAM densities (CD68+ cells) varied from 5% to 70%, in all tumor areas, while few cells were stained in adjacent normal tissues. TAMs were identified as CD68+ cells with thin, elongated cytoplasmic extensions that formed a canopy structure over tumor cells. Comparing clinicopathologic characteristics between tumors with low (<25%) and high (25% to 70%) TAM densities, primary tumors were larger in the high density group than in the low density group (2.0±0.1 vs. 1.5±0.1;
P =0.009).Conclusion TAMs were identified in primary PTC tumors with LN metastasis and higher TAM densities were related to larger tumor sizes, suggesting a tumorigenic role of TAMs in human PTCs.
-
Citations
Citations to this article as recorded by- Single-cell RNA-seq reveals MIF−(CD74 + CXCR4) dependent inhibition of macrophages in metastatic papillary thyroid carcinoma
Wei Chen, Xinnian Yu, Huixin Li, Shenglong Yuan, Yuqi Fu, Huanhuan Hu, Fangzhou Liu, Yuan Zhang, Shanliang Zhong
Oral Oncology.2024; 148: 106654. CrossRef - H2AFX might be a prognostic biomarker for hepatocellular carcinoma
Hailiang Hu, Tao Zhong, Suwei Jiang
Cancer Reports.2023;[Epub] CrossRef - The Tumor Microenvironment and the Estrogen Loop in Thyroid Cancer
Nerina Denaro, Rebecca Romanò, Salvatore Alfieri, Alessia Dolci, Lisa Licitra, Imperia Nuzzolese, Michele Ghidini, Claudia Bareggi, Valentina Bertaglia, Cinzia Solinas, Ornella Garrone
Cancers.2023; 15(9): 2458. CrossRef - New Insights into Immune Cells and Immunotherapy for Thyroid Cancer
Yujia Tao, Peng Li, Chao Feng, Yuan Cao
Immunological Investigations.2023; 52(8): 1039. CrossRef - Roles and new Insights of Macrophages in the Tumor Microenvironment of Thyroid Cancer
Qi Liu, Wei Sun, Hao Zhang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Construction of a Tumor Immune Microenvironment-Related Prognostic Model in BRAF-Mutated Papillary Thyroid Cancer
Yuxiao Xia, Xue Jiang, Yuan Huang, Qian Liu, Yin Huang, Bo Zhang, Zhanjun Mei, Dongkun Xu, Yuhong Shi, Wenling Tu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Identification and Validation of Prognostic Related Hallmark ATP-Binding Cassette Transporters Associated With Immune Cell Infiltration Patterns in Thyroid Carcinoma
Lidong Wang, Xiaodan Sun, Jingni He, Zhen Liu
Frontiers in Oncology.2022;[Epub] CrossRef - Unraveling the Effects of Carotenoids Accumulation in Human Papillary Thyroid Carcinoma
Alessandra di Masi, Rosario Luigi Sessa, Ylenia Cerrato, Gianni Pastore, Barbara Guantario, Roberto Ambra, Michael Di Gioacchino, Armida Sodo, Martina Verri, Pierfilippo Crucitti, Filippo Longo, Anda Mihaela Naciu, Andrea Palermo, Chiara Taffon, Filippo A
Antioxidants.2022; 11(8): 1463. CrossRef - What is the status of immunotherapy in thyroid neoplasms?
Alejandro Garcia-Alvarez, Jorge Hernando, Ana Carmona-Alonso, Jaume Capdevila
Frontiers in Endocrinology.2022;[Epub] CrossRef - Matrix Metalloproteinase 9 Expression by Immunohistochemistry in Intratumor Macrophages as Tumor-Associated Macrophage Marker of Papillary Thyroid Carcinoma
Ni Wayan Armerinayanti, Samuel Widodo, Desak Putu Oki Lestari
Biomedical and Pharmacology Journal.2022; 15(3): 1671. CrossRef - Cell Component and Function of Tumor Microenvironment in Thyroid Cancer
Eunah Shin, Ja Seung Koo
International Journal of Molecular Sciences.2022; 23(20): 12578. CrossRef - Potential diagnostic of lymph node metastasis and prognostic values of TM4SFs in papillary thyroid carcinoma patients
Kun Wang, Haomin Li, Junyu Zhao, Jinming Yao, Yiran Lu, Jianjun Dong, Jie Bai, Lin Liao
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Thyroid Cancer Stem-Like Cells: From Microenvironmental Niches to Therapeutic Strategies
Elisa Stellaria Grassi, Viola Ghiandai, Luca Persani
Journal of Clinical Medicine.2021; 10(7): 1455. CrossRef - Immune Landscape of Thyroid Cancers: New Insights
Elisa Menicali, Martina Guzzetti, Silvia Morelli, Sonia Moretti, Efisio Puxeddu
Frontiers in Endocrinology.2021;[Epub] CrossRef - FOXE1-Dependent Regulation of Macrophage Chemotaxis by Thyroid Cells In Vitro and In Vivo
Sara Credendino, Marta De Menna, Irene Cantone, Carmen Moccia, Matteo Esposito, Luigi Di Guida, Mario De Felice, Gabriella De Vita
International Journal of Molecular Sciences.2021; 22(14): 7666. CrossRef - Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo
Mara Mazzoni, Giuseppe Mauro, Lucia Minoli, Loredana Cleris, Maria Chiara Anania, Tiziana Di Marco, Emanuela Minna, Sonia Pagliardini, Maria Grazia Rizzetti, Giacomo Manenti, Maria Grazia Borrello, Eugenio Scanziani, Angela Greco
Biology.2021; 10(10): 985. CrossRef - Characterization of the Immune Cell Infiltration Landscape of Thyroid Cancer for Improved Immunotherapy
Jing Gong, Bo Jin, Liang Shang, Ning Liu
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - The Thyroid Tumor Microenvironment: Potential Targets for Therapeutic Intervention and Prognostication
Laura MacDonald, Jonathan Jenkins, Grace Purvis, Joshua Lee, Aime T. Franco
Hormones and Cancer.2020; 11(5-6): 205. CrossRef - Cancer Stem Cells in Thyroid Tumors: From the Origin to Metastasis
Veronica Veschi, Francesco Verona, Melania Lo Iacono, Caterina D'Accardo, Gaetana Porcelli, Alice Turdo, Miriam Gaggianesi, Stefano Forte, Dario Giuffrida, Lorenzo Memeo, Matilde Todaro
Frontiers in Endocrinology.2020;[Epub] CrossRef - The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition
Gilda Varricchi, Stefania Loffredo, Giancarlo Marone, Luca Modestino, Poupak Fallahi, Silvia Martina Ferrari, Amato de Paulis, Alessandro Antonelli, Maria Rosaria Galdiero
International Journal of Molecular Sciences.2019; 20(16): 3934. CrossRef - Impact of tumor‐associated macrophages and BRAFV600E mutation on clinical outcomes in patients with various thyroid cancers
Jae Won Cho, Won Woong Kim, Yu‐mi Lee, Min Ji Jeon, Won Gu Kim, Dong Eun Song, Yangsoon Park, Ki‐Wook Chung, Suck Joon Hong, Tae‐Yon Sung
Head & Neck.2019; 41(3): 686. CrossRef - Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism
Mara Mazzoni, Giuseppe Mauro, Marco Erreni, Paola Romeo, Emanuela Minna, Maria Grazia Vizioli, Cristina Belgiovine, Maria Grazia Rizzetti, Sonia Pagliardini, Roberta Avigni, Maria Chiara Anania, Paola Allavena, Maria Grazia Borrello, Angela Greco
Journal of Experimental & Clinical Cancer Research.2019;[Epub] CrossRef - Immune and Inflammatory Cells in Thyroid Cancer Microenvironment
Ferrari, Fallahi, Galdiero, Ruffilli, Elia, Ragusa, Paparo, Patrizio, Mazzi, Varricchi, Marone, Antonelli
International Journal of Molecular Sciences.2019; 20(18): 4413. CrossRef - Aberrant Thyroid-Stimulating Hormone Receptor Signaling Increases VEGF-A and CXCL8 Secretion of Thyroid Cancer Cells, Contributing to Angiogenesis and Tumor Growth
Young Shin Song, Min Joo Kim, Hyun Jin Sun, Hwan Hee Kim, Hyo Shik Shin, Young A. Kim, Byung-Chul Oh, Sun Wook Cho, Young Joo Park
Clinical Cancer Research.2019; 25(1): 414. CrossRef - Novel targeted therapies and immunotherapy for advanced thyroid cancers
George E. Naoum, Michael Morkos, Brian Kim, Waleed Arafat
Molecular Cancer.2018;[Epub] CrossRef - Potential involvement of neutrophils in human thyroid cancer
Maria Rosaria Galdiero, Gilda Varricchi, Stefania Loffredo, Claudio Bellevicine, Tiziana Lansione, Anne Lise Ferrara, Raffaella Iannone, Sarah di Somma, Francesco Borriello, Eduardo Clery, Maria Triassi, Giancarlo Troncone, Gianni Marone, Fabrizio Mattei
PLOS ONE.2018; 13(6): e0199740. CrossRef - Bleomycin inhibits proliferation and induces apoptosis in TPC‑1 cells through reversing M2‑macrophages polarization
Hongwei Liu, Huilei Dong, Li Jiang, Zhendong Li, Xiande Ma
Oncology Letters.2018;[Epub] CrossRef - Pathological processes and therapeutic advances in radioiodide refractory thyroid cancer
Marika H Tesselaar, Johannes W Smit, James Nagarajah, Romana T Netea-Maier, Theo S Plantinga
Journal of Molecular Endocrinology.2017; 59(4): R141. CrossRef - The immune network in thyroid cancer
Maria Rosaria Galdiero, Gilda Varricchi, Gianni Marone
OncoImmunology.2016; 5(6): e1168556. CrossRef - Macrophage Densities Correlated with CXC Chemokine Receptor 4 Expression and Related with Poor Survival in Anaplastic Thyroid Cancer
Dae In Kim, Eunyoung Kim, Young A Kim, Sun Wook Cho, Jung Ah Lim, Young Joo Park
Endocrinology and Metabolism.2016; 31(3): 469. CrossRef - CXCL16 signaling mediated macrophage effects on tumor invasion of papillary thyroid carcinoma
Sun Wook Cho, Young A Kim, Hyun Jin Sun, Ye An Kim, Byung-Chul Oh, Ka Hee Yi, Do Joon Park, Young Joo Park
Endocrine-Related Cancer.2016; 23(2): 113. CrossRef - Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer
Xiu Juan Li, Prakash Gangadaran, Senthilkumar Kalimuthu, Ji Min Oh, Liya Zhu, Shin Young Jeong, Sang‐Woo Lee, Jaetae Lee, Byeong‐Cheol Ahn
International Journal of Cancer.2016; 139(11): 2583. CrossRef - The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
Shujun Sun, Xinliang Pan, Limin Zhao, Jianming Zhou, Hongzeng Wang, Yonghong Sun
Clinical and Experimental Otorhinolaryngology.2016; 9(3): 270. CrossRef - Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates
Kyong Yeun Jung, Sun Wook Cho, Young A Kim, Daein Kim, Byung-Chul Oh, Do Joon Park, Young Joo Park
Journal of Pathology and Translational Medicine.2015; 49(4): 318. CrossRef - Association of the Preoperative Neutrophil-to-ymphocyte Count Ratio and Platelet-to-Lymphocyte Count Ratio with Clinicopathological Characteristics in Patients with Papillary Thyroid Cancer
Sang Mi Kim, Eun Heui Kim, Bo Hyun Kim, Jong Ho Kim, Su Bin Park, Yoon Jeong Nam, Kang Hee Ahn, Min Young Oh, Won Jin Kim, Yun Kyung Jeon, Sang Soo Kim, Yong Ki Kim, In Ju Kim
Endocrinology and Metabolism.2015; 30(4): 494. CrossRef - Quantitative Changes in Tumor-Associated M2 Macrophages Characterize Cholangiocarcinoma and their Association with Metastasis
Malinee Thanee, Watcharin Loilome, Anchalee Techasen, Nisana Namwat, Thidarut Boonmars, Chawalit Pairojkul, Puangrat Yongvanit
Asian Pacific Journal of Cancer Prevention.2015; 16(7): 3043. CrossRef - Tumor-Associated Macrophage (TAM) and Angiogenesis in Human Colon Carcinoma
Manal A. Badawi, Dalia M. Abouelfadl, Sonia L. El-Sharkawy, Wafaa E. Abd El-Aal, Naglaa F. Abbas
Open Access Macedonian Journal of Medical Sciences.2015; 3(2): 209. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef - miRNA Expression in Anaplastic Thyroid Carcinomas
Aline Hébrant, Sébastien Floor, Manuel Saiselet, Aline Antoniou, Alice Desbuleux, Bérengère Snyers, Caroline La, Nicolas de Saint Aubain, Emmanuelle Leteurtre, Guy Andry, Carine Maenhaut, Raul M. Luque
PLoS ONE.2014; 9(8): e103871. CrossRef - The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
Bo Hyun Kim
Endocrinology and Metabolism.2013; 28(3): 178. CrossRef
- Single-cell RNA-seq reveals MIF−(CD74 + CXCR4) dependent inhibition of macrophages in metastatic papillary thyroid carcinoma


 KES
KES

 First
First Prev
Prev



