Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Human Tissue-Engineered Skeletal Muscle: A Tool for Metabolic Research
- Ji-Hoon Kim, Seung-Min Yu, Jang Won Son
- Endocrinol Metab. 2022;37(3):408-414. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.302
- 3,957 View
- 162 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
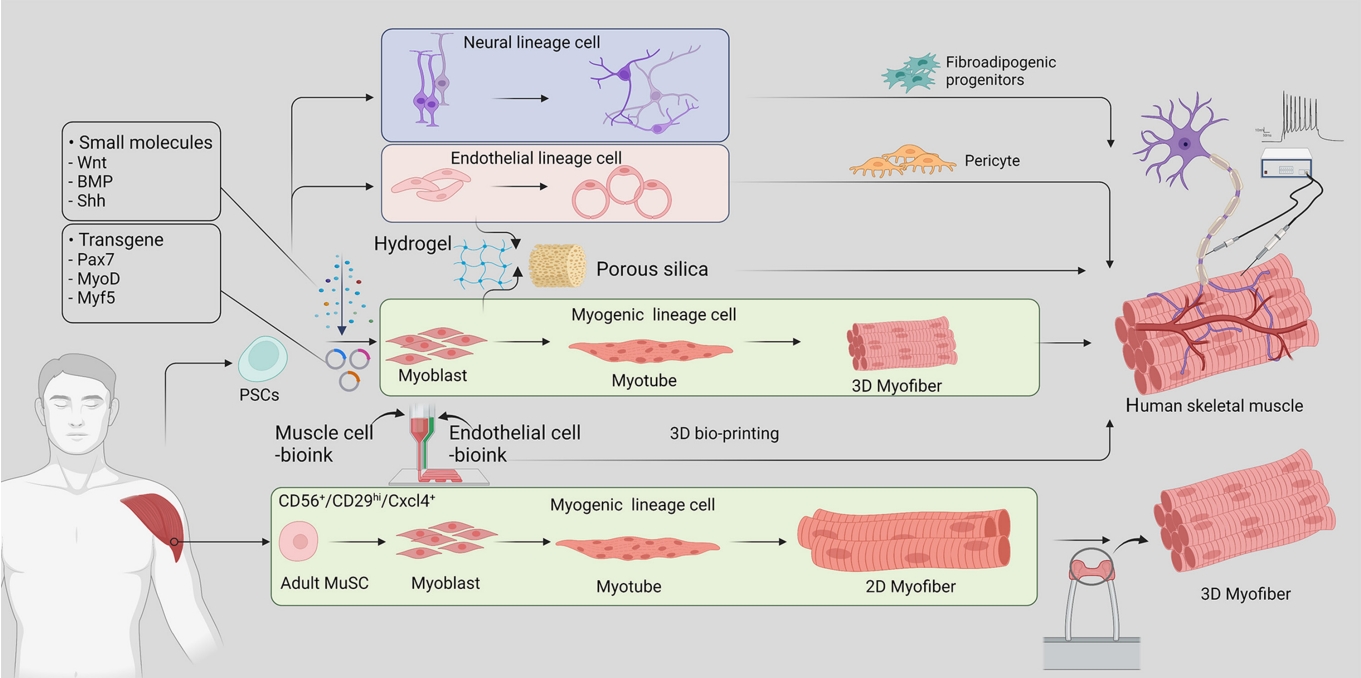
ePub - Skeletal muscle is now regarded as an endocrine organ based on its secretion of myokines and exerkines, which, in response to metabolic stimuli, regulate the crosstalk between the skeletal muscle and other metabolic organs in terms of systemic energy homeostasis. This conceptual basis of skeletal muscle as a metabolically active organ has provided insights into the potential role of physical inactivity and conditions altering muscle quality and quantity in the development of multiple metabolic disorders, including insulin resistance, obesity, and diabetes. Therefore, it is important to understand human muscle physiology more deeply in relation to the pathophysiology of metabolic diseases. Since monolayer cell lines or animal models used in conventional research differ from the pathophysiological features of the human body, there is increasing need for more physiologically relevant in vitro models of human skeletal muscle. Here, we introduce recent studies on in vitro models of human skeletal muscle generated from adult myogenic progenitors or pluripotent stem cells and summarize recent progress in the development of three-dimensional (3D) bioartificial muscle, which mimics the physiological complexity of native skeletal muscle tissue in terms of maturation and functionality. We then discuss the future of skeletal muscle 3D-organoid culture technology in the field of metabolic research for studying pathological mechanisms and developing personalized therapeutic strategies.
-
Citations
Citations to this article as recorded by- Human‐based new approach methodologies to accelerate advances in nutrition research
Manuela Cassotta, Danila Cianciosi, Maria Elexpuru‐Zabaleta, Inaki Elio Pascual, Sandra Sumallo Cano, Francesca Giampieri, Maurizio Battino
Food Frontiers.2024;[Epub] CrossRef - Key indicators of beef safety and quality as important aspects of conservation
S. V. Furman, I. M. Sokulskyi, D. V. Lisohurska, O. V. Lisohurska, B. V. Gutyj
Ukrainian Journal of Veterinary and Agricultural Sciences.2024; 7(1): 68. CrossRef
- Human‐based new approach methodologies to accelerate advances in nutrition research

- Miscellaneous
- Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health
- Hong-Kyu Kim, Chul-Hee Kim
- Endocrinol Metab. 2021;36(6):1161-1174. Published online December 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1348

- 6,378 View
- 283 Download
- 25 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Although age-related changes in skeletal muscles are closely associated with decreases in muscle strength and functional decline, their associations with cardiometabolic diseases in the literature are inconsistent. Such inconsistency could be explained by the fact that muscle quality—which is closely associated with fatty infiltration of the muscle (i.e., myosteatosis)—is as important as muscle quantity in cardiometabolic health. However, muscle quality has been less explored compared with muscle mass. Moreover, the standard definition of myosteatosis and its assessment methods have not been established yet. Recently, some techniques using single axial computed tomography (CT) images have been introduced and utilized in many studies, as the mass and quality of abdominal muscles could be measured opportunistically on abdominal CT scans obtained during routine clinical care. Yet, the mechanisms by which myosteatosis affect metabolic and cardiovascular health remain largely unknown. In this review, we explore the recent advances in the assessment of myosteatosis and its changes associated with aging. We also review the recent literature on the clinical implication of myosteatosis by focusing on metabolic and cardiovascular diseases. Finally, we discuss the challenges and unanswered questions that need addressing to set myosteatosis as a therapeutic target for the prevention or treatment of cardiometabolic diseases.
-
Citations
Citations to this article as recorded by- A CT-based Deep Learning Model for Predicting Subsequent Fracture Risk in Patients with Hip Fracture
Yisak Kim, Young-Gon Kim, Jung-Wee Park, Byung Woo Kim, Youmin Shin, Sung Hye Kong, Jung Hee Kim, Young-Kyun Lee, Sang Wan Kim, Chan Soo Shin
Radiology.2024;[Epub] CrossRef - Myosteatosis is associated with poor survival after kidney transplantation: a large retrospective cohort validation
Jie Chen, Yue Li, Chengjie Li, Turun Song
Abdominal Radiology.2024; 49(4): 1210. CrossRef - Fatty infiltration of gastrocnemius–soleus muscle complex: Considerations for myosteatosis rehabilitation
Catherine Hatzantonis, Lalith Satkunam, Karyne N. Rabey, Jennifer C. Hocking, Anne M. R. Agur
Journal of Anatomy.2024;[Epub] CrossRef - Muscle attenuation, not skeletal muscle index, is an independent prognostic factor for survival in gastric cancer patients with overweight and obesity
Cheng-Le Zhuang, Hao-Fan Wu, Hao-Jie Jiang, Feng-Min Zhang, Han-Ping Shi, Zhen Yu, Xian Shen, Xiao-Lei Chen, Su-Lin Wang
Nutrition.2024; 122: 112391. CrossRef - Myosteatosis is associated with coronary artery calcification in patients with type 2 diabetes
Fu-Peng Liu, Mu-Jie Guo, Qing Yang, Yan-Ying Li, Yan-Gang Wang, Mei Zhang
World Journal of Diabetes.2024; 15(3): 429. CrossRef - Unlocking liver health: Can tackling myosteatosis spark remission in metabolic dysfunction‐associated steatotic liver disease?
Guillaume Henin, Audrey Loumaye, Louise Deldicque, Isabelle A. Leclercq, Nicolas Lanthier
Liver International.2024;[Epub] CrossRef - Association of serum gamma-glutamyl transferase with myosteatosis assessed by muscle quality mapping using abdominal computed tomography
Han Na Jung, Yun Kyung Cho, Hwi Seung Kim, Eun Hee Kim, Min Jung Lee, Joong-Yeol Park, Woo Je Lee, Hong-Kyu Kim, Chang Hee Jung
Clinical Imaging.2023; 93: 4. CrossRef - Increased visceral fat area to skeletal muscle mass ratio is positively associated with the risk of cardiometabolic diseases in a Chinese natural population: A cross‐sectional study
Shi Zhang, Yaping Huang, Jing Li, Xincheng Wang, Xiaohe Wang, Minying Zhang, Yanju Zhang, Meiyang Du, Jingna Lin, Chunjun Li
Diabetes/Metabolism Research and Reviews.2023;[Epub] CrossRef - Association between hypertension and myosteatosis evaluated by abdominal computed tomography
Han Na Jung, Yun Kyung Cho, Hwi Seung Kim, Eun Hee Kim, Min Jung Lee, Woo Je Lee, Hong-Kyu Kim, Chang Hee Jung
Hypertension Research.2023; 46(4): 845. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef - Muscle fat infiltration in chronic kidney disease: a marker related to muscle quality, muscle strength and sarcopenia
Carla Maria Avesani, Aline Miroski de Abreu, Heitor S. Ribeiro, Torkel B. Brismar, Peter Stenvinkel, Alice Sabatino, Bengt Lindholm
Journal of Nephrology.2023; 36(3): 895. CrossRef - IDF2022-1139 Association Between Dyslipidemia And Myosteatosis Using Visual Muscular Quality Map In Computed Tomography
H.S. Kim, H.N. Jung, Y.K. Cho, E.H. Kim, M.J. Lee, W.J. Lee, J.Y. Park, H.K. Kim, C.H. Jung
Diabetes Research and Clinical Practice.2023; 197: 110467. CrossRef - The role of skeletal muscle mass on cardiovascular disease risk: an emerging role on modulating lipid profile
Evangelia Damigou, Matina Kouvari, Demosthenes Panagiotakos
Current Opinion in Cardiology.2023; 38(4): 352. CrossRef - Reference values for low muscle mass and myosteatosis using tomographic muscle measurements in living kidney donors
Lisa B. Westenberg, Marcel Zorgdrager, Tim D. A. Swaab, Marco van Londen, Stephan J. L. Bakker, Henri G. D. Leuvenink, Alain R. Viddeleer, Robert A. Pol
Scientific Reports.2023;[Epub] CrossRef - Association between sarcopenic obesity and poor muscle quality based on muscle quality map and abdominal computed tomography
Yun Kyung Cho, Han Na Jung, Eun Hee Kim, Min Jung Lee, Joong‐Yeol Park, Woo Je Lee, Hong‐Kyu Kim, Chang Hee Jung
Obesity.2023; 31(6): 1547. CrossRef - Increase in skeletal muscular adiposity and cognitive decline in a biracial cohort of older men and women
Caterina Rosano, Anne Newman, Adam Santanasto, Xiaonan Zhu, Bret Goodpaster, Iva Miljkovic
Journal of the American Geriatrics Society.2023; 71(9): 2759. CrossRef - Evaluation of Paraspinal Muscle Degeneration on Pain Relief after Percutaneous Epidural Adhesiolysis in Patients with Degenerative Lumbar Spinal Disease
Misun Kang, Shin Hyung Kim, Minju Jo, Hyun Eom Jung, Jungbin Bae, Hee Jung Kim
Medicina.2023; 59(6): 1118. CrossRef - Sarcopenic obesity and its relation with muscle quality and mortality in patients on chronic hemodialysis
Alice Sabatino, Carla Maria Avesani, Giuseppe Regolisti, Marianna Adinolfi, Giuseppe Benigno, Marco Delsante, Enrico Fiaccadori, Ilaria Gandolfini
Clinical Nutrition.2023; 42(8): 1359. CrossRef - Association between computed tomography‐assessed sarcopenia and mortality in patients with anti‐neutrophil cytoplasmic antibody‐associated vasculitis
Sung Soo Ahn, Yong‐Beom Park, Sang‐Won Lee
International Journal of Rheumatic Diseases.2023; 26(9): 1704. CrossRef - Association Between Insulin Resistance and Myosteatosis Measured by Abdominal Computed Tomography
Myung Jin Kim, Yun Kyung Cho, Han Na Jung, Eun Hee Kim, Min Jung Lee, Chang Hee Jung, Joong-Yeol Park, Hong-Kyu Kim, Woo Je Lee
The Journal of Clinical Endocrinology & Metabolism.2023; 108(12): 3100. CrossRef - Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity
Hong-Kyu Kim, Sung-Jin Bae, Min Jung Lee, Eun Hee Kim, Hana Park, Hwi Seung Kim, Yun Kyung Cho, Chang Hee Jung, Woo Je Lee, Jaewon Choe
Clinical and Molecular Hepatology.2023; 29(4): 987. CrossRef - Different computed tomography parameters for defining myosteatosis in patients with advanced non-small cell lung cancer
Wenyi Zhang, Jing Tang, Huiyu Tang, Lingling Xie, Jing Wang, Jinhui Wu, Ming Yang
Clinical Nutrition.2023; 42(12): 2414. CrossRef - All you need to know about sarcopenia: a short guide for an internal medicine physician in questions and answers
G. R. Bikbavova, M. A. Livzan, D. V. Tikhonravova
Bulletin of Siberian Medicine.2023; 22(3): 88. CrossRef - Muscle Fat Content Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in Chinese Adults
W. Guo, X. Zhao, D. Cheng, X. Liang, M. Miao, X. Li, J. Lu, N. Xu, Shuang Hu, Qun Zhang
The Journal of nutrition, health and aging.2023; 27(11): 960. CrossRef - Body Composition Evaluation and Clinical Markers of Cardiometabolic Risk in Patients with Phenylketonuria
Luis M. Luengo-Pérez, Mercedes Fernández-Bueso, Ana Ambrojo, Marta Guijarro, Ana Cristina Ferreira, Luís Pereira-da-Silva, André Moreira-Rosário, Ana Faria, Conceição Calhau, Anne Daly, Anita MacDonald, Júlio César Rocha
Nutrients.2023; 15(24): 5133. CrossRef - Assessment of Muscle Quantity, Quality and Function
Bo Kyung Koo
Journal of Obesity & Metabolic Syndrome.2022; 31(1): 9. CrossRef - Influence of cross‐sectional area and fat infiltration of paraspinal muscles on analgesic efficacy of epidural steroid injection in elderly patients
Hee Jung Kim, Miribi Rho, Kyung Bong Yoon, Minju Jo, Dong Woo Lee, Shin Hyung Kim
Pain Practice.2022; 22(7): 621. CrossRef - Sarcopenia, Obesity, Sarcopenic Obesity and Risk of Poor Nutritional Status in Polish Community-Dwelling Older People Aged 60 Years and Over
Marika Murawiak, Roma Krzymińska-Siemaszko, Aleksandra Kaluźniak-Szymanowska, Marta Lewandowicz, Sławomir Tobis, Katarzyna Wieczorowska-Tobis, Ewa Deskur-Śmielecka
Nutrients.2022; 14(14): 2889. CrossRef - Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation
Amedeo Lonardo, Alessandro Mantovani, Salvatore Petta, Amedeo Carraro, Christopher D. Byrne, Giovanni Targher
Nature Reviews Endocrinology.2022; 18(10): 638. CrossRef
- A CT-based Deep Learning Model for Predicting Subsequent Fracture Risk in Patients with Hip Fracture

- Diabetes, Obesity and Metabolism
- Exercise/Resistance Training and Muscle Stem Cells
- So-ichiro Fukada, Ayasa Nakamura
- Endocrinol Metab. 2021;36(4):737-744. Published online August 10, 2021
- DOI: https://doi.org/10.3803/EnM.2021.401
- 5,208 View
- 258 Download
- 6 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
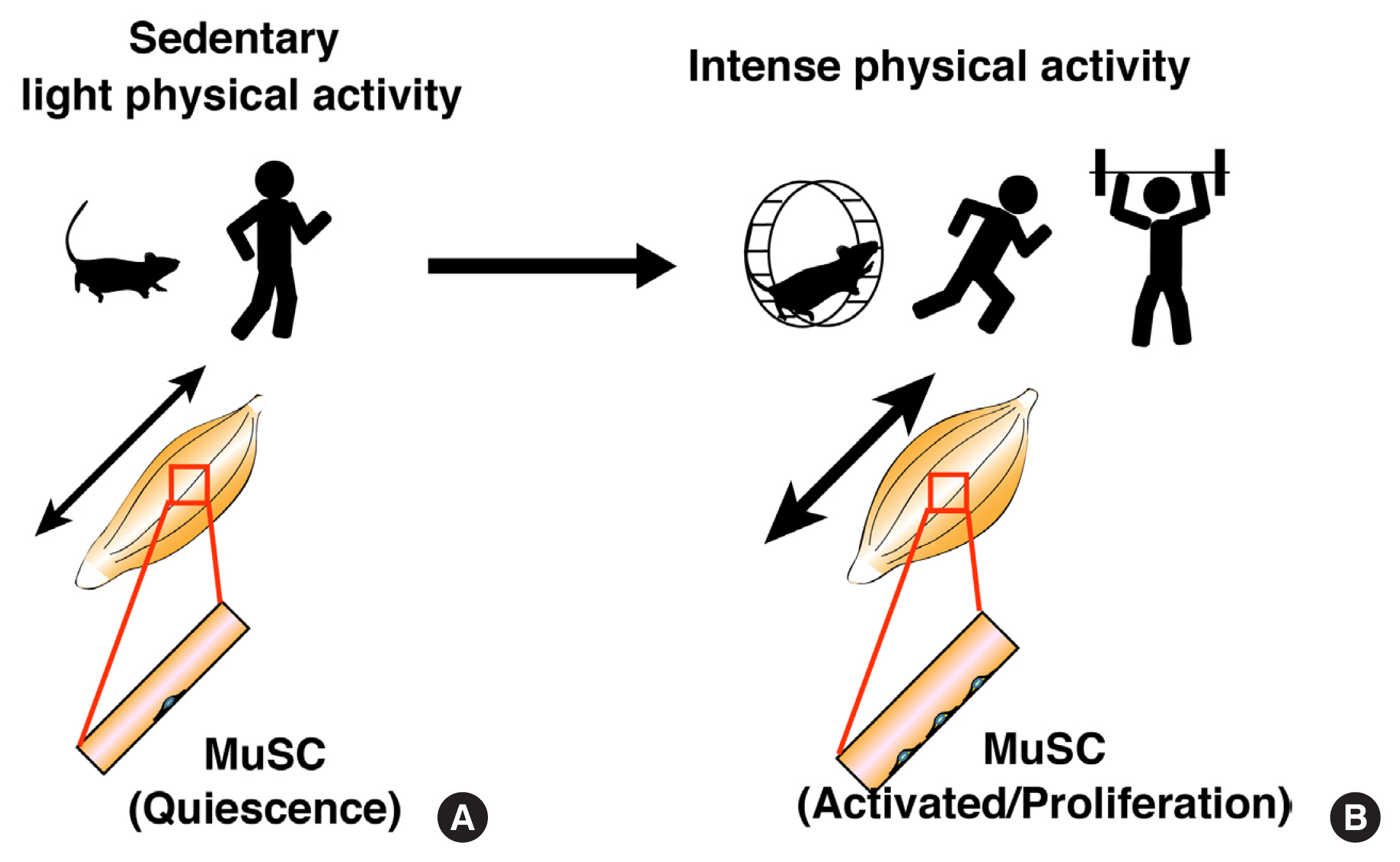
ePub - Skeletal muscle has attracted attention as endocrine organ, because exercise-dependent cytokines called myokines/exerkines are released from skeletal muscle and are involved in systemic functions. While, local mechanical loading to skeletal muscle by exercise or resistance training alters myofiber type and size and myonuclear number. Skeletal muscle-resident stem cells, known as muscle satellite cells (MuSCs), are responsible for the increased number of myonuclei. Under steady conditions, MuSCs are maintained in a mitotically quiescent state but exit from that state and start to proliferate in response to high physical activity. Alterations in MuSC behavior occur when myofibers are damaged, but the lethal damage to myofibers does not seem to evoke mechanical loading-dependent MuSC activation and proliferation. Given that MuSCs proliferate without damage, it is unclear how the different behaviors of MuSCs are controlled by different physical activities. Recent studies demonstrated that myonuclear number reflects the size of myofibers; hence, it is crucial to know the properties of MuSCs and the mechanism of myonuclear accretion by MuSCs. In addition, the elucidation of mechanical load-dependent changes in muscle resident cells, including MuSCs, will be necessary for the discovery of new myokines/exerkines and understating skeletal muscle diseases.
-
Citations
Citations to this article as recorded by- Control of muscle satellite cell function by specific exercise‐induced cytokines and their applications in muscle maintenance
Qian Guo, Qing Luo, Guanbin Song
Journal of Cachexia, Sarcopenia and Muscle.2024; 15(2): 466. CrossRef - Resistance exercise preconditioning prevents disuse muscle atrophy by inhibiting apoptosis and protein degradation via SESN2 in C57BL/6J mice
Yating Huang, Chenxin Jiang, Xiuru Li, Sujuan Liu, Yanmei Niu, Li Fu
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(4): 167111. CrossRef - Anthropometric, muscle and serum myokine levels effects of physical exercise with an online platform in female patients with obesity
David Primo, Olatz Izaola, Juan Jose Lopez Gomez, Daniel de Luis
Endocrinología, Diabetes y Nutrición.2023; 70(7): 484. CrossRef - Anthropometric, muscle and serum myokine levels effects of physical exercise with an online platform in female patients with obesity
David Primo, Olatz Izaola, Juan Jose Lopez Gomez, Daniel de Luis
Endocrinología, Diabetes y Nutrición (English ed.).2023; 70(7): 484. CrossRef - The muscle stem cell niche at a glance
Margaret Hung, Hsiao-Fan Lo, Grace E. L. Jones, Robert S. Krauss
Journal of Cell Science.2023;[Epub] CrossRef - Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors
Hamed Alizadeh Pahlavani
Frontiers in Endocrinology.2022;[Epub] CrossRef - Molecular mechanisms of exercise contributing to tissue regeneration
Jibao Chen, Ren Zhou, Ye Feng, Lin Cheng
Signal Transduction and Targeted Therapy.2022;[Epub] CrossRef
- Control of muscle satellite cell function by specific exercise‐induced cytokines and their applications in muscle maintenance

- Diabetes, Obesity and Metabolism
- Receptor-Mediated Muscle Homeostasis as a Target for Sarcopenia Therapeutics
- Jong Hyeon Yoon, Ki-Sun Kwon
- Endocrinol Metab. 2021;36(3):478-490. Published online June 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1081

- 8,811 View
- 333 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Sarcopenia is a disease characterized by age-related decline of skeletal muscle mass and function. The molecular mechanisms of the pathophysiology of sarcopenia form a complex network due to the involvement of multiple interconnected signaling pathways. Therefore, signaling receptors are major targets in pharmacological strategies in general. To provide a rationale for pharmacological interventions for sarcopenia, we herein describe several druggable signaling receptors based on their role in skeletal muscle homeostasis and changes in their activity with aging. A brief overview is presented of the efficacy of corresponding drug candidates under clinical trials. Strategies targeting the androgen receptor, vitamin D receptor, Insulin-like growth factor-1 receptor, and ghrelin receptor primarily focus on promoting anabolic action using natural ligands or mimetics. Strategies involving activin receptors and angiotensin receptors focus on inhibiting catabolic action. This review may help to select specific targets or combinations of targets in the future.
-
Citations
Citations to this article as recorded by- The Current Landscape of Pharmacotherapies for Sarcopenia
Gulistan Bahat, Serdar Ozkok
Drugs & Aging.2024; 41(2): 83. CrossRef - Associations of micronutrient dietary patterns with sarcopenia among US adults: a population-based study
Yining Liu, Xiangliang Liu, Linnan Duan, Yixin Zhao, Yuwei He, Wei Li, Jiuwei Cui
Frontiers in Nutrition.2024;[Epub] CrossRef - Impact of Vitamin D Level on Sarcopenia in Elderly People: A Critical Review
Saniya Khan, Sunil Kumar, Sourya Acharya, Anil Wanjari
Journal of Health and Allied Sciences NU.2023; 13(04): 453. CrossRef - Novel Potential Targets for Function-Promoting Therapies: Orphan Nuclear Receptors, Anti-inflammatory Drugs, Troponin Activators, Mas Receptor Agonists, and Urolithin A
Waly Dioh, Vihang Narkar, Anurag Singh, Fady Malik, Luigi Ferrucci, Cendrine Tourette, Jean Mariani, Rob van Maanen, Roger A Fielding, Lewis A Lipsitz
The Journals of Gerontology: Series A.2023; 78(Supplement): 44. CrossRef - Alverine citrate promotes myogenic differentiation and ameliorates muscle atrophy
Jong Hyeon Yoon, Seung-Min Lee, Younglang Lee, Min Ju Kim, Jae Won Yang, Jeong Yi Choi, Ju Yeon Kwak, Kwang-Pyo Lee, Yong Ryoul Yang, Ki-Sun Kwon
Biochemical and Biophysical Research Communications.2022; 586: 157. CrossRef - Adeno-associated virus-mediated expression of an inactive CaMKIIβ mutant enhances muscle mass and strength in mice
Takahiro Eguchi, Yuji Yamanashi
Biochemical and Biophysical Research Communications.2022; 589: 192. CrossRef - Gastric Mobility and Gastrointestinal Hormones in Older Patients with Sarcopenia
Hsien-Hao Huang, Tse-Yao Wang, Shan-Fan Yao, Pei-Ying Lin, Julia Chia-Yu Chang, Li-Ning Peng, Liang-Kung Chen, David Hung-Tsang Yen
Nutrients.2022; 14(9): 1897. CrossRef - Molecular Mechanisms Underlying Intensive Care Unit-Acquired Weakness and Sarcopenia
Marcela Kanova, Pavel Kohout
International Journal of Molecular Sciences.2022; 23(15): 8396. CrossRef
- The Current Landscape of Pharmacotherapies for Sarcopenia

- Diabetes, Obesity and Metabolism
- Reference Values for Skeletal Muscle Mass at the Third Lumbar Vertebral Level Measured by Computed Tomography in a Healthy Korean Population
- Ja Kyung Yoon, Sunyoung Lee, Kyoung Won Kim, Ji Eun Lee, Jeong Ah Hwang, Taeyong Park, Jeongjin Lee
- Endocrinol Metab. 2021;36(3):672-677. Published online June 8, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1041
- 4,225 View
- 156 Download
- 13 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
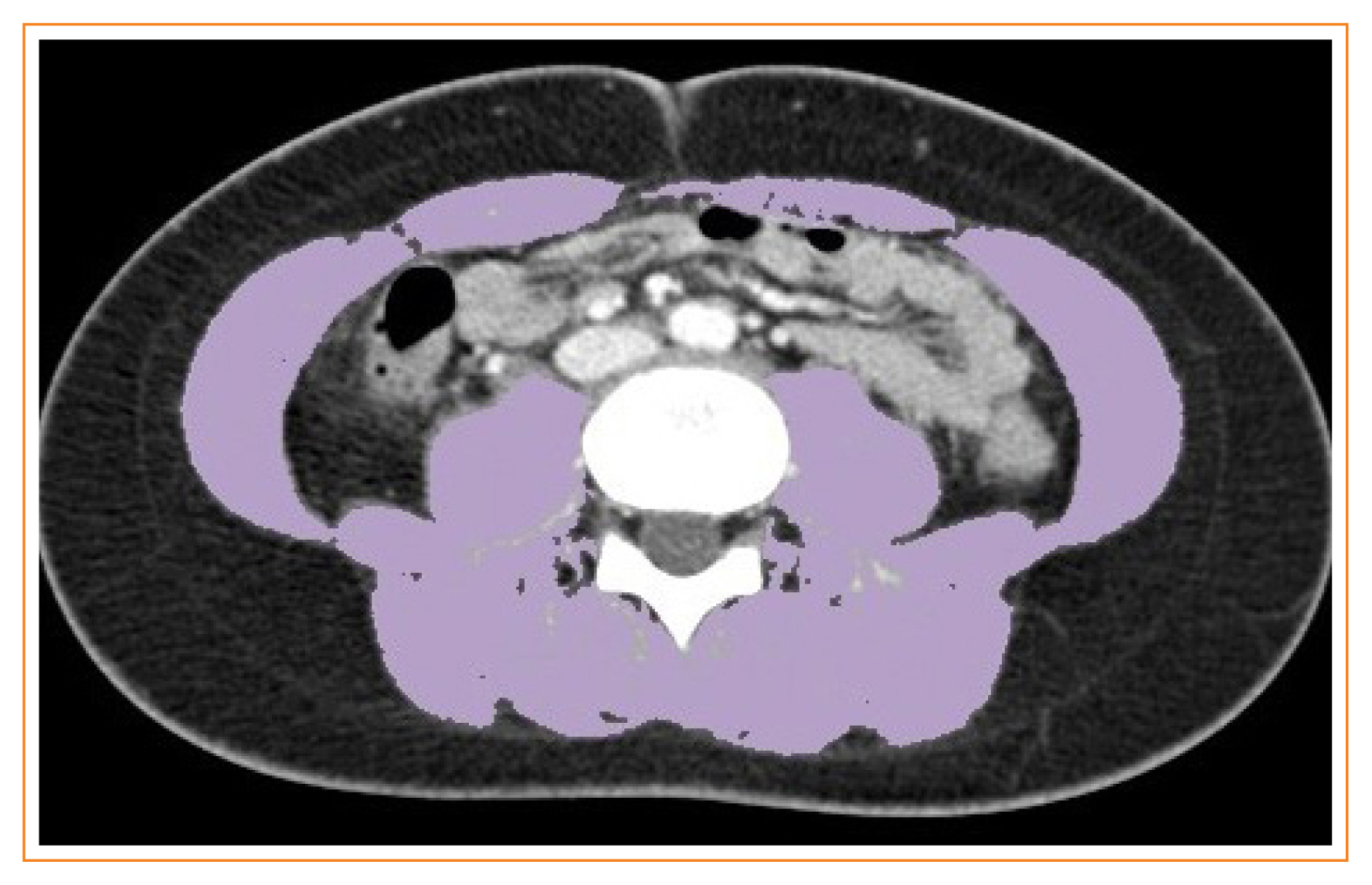
Sarcopenia is defined as the loss of skeletal muscle mass and is associated with negative clinical outcomes. This study aimed to establish sex-specific cutoff values for the skeletal muscle area (SMA) and skeletal muscle index (SMI) at the third lumbar vertebral (L3) level using computed tomography (CT) imaging to identify sarcopenia in healthy Korean liver donors.
Methods
This retrospective study included 659 healthy liver donors (408 men and 251 women) aged 20 to 60 years who had undergone abdominal CT examinations between January 2017 and December 2018. Assessment of body composition was performed with an automated segmentation technique using a deep-learning system. Sex-specific SMA and SMI distributions were assessed, and cutoff values for determining sarcopenia were defined as values at either two standard deviations (SDs) below the mean reference value or below the fifth percentile.
Results
Using the SD definition, cutoff values for SMA and SMI were 117.04 cm2 and 39.33 cm2/m2, respectively, in men and 71.39 cm2 and 27.77 cm2/m2, respectively, in women. Using the fifth percentile definition, cutoff values for SMA and SMI were 126.88 cm2 and 40.96 cm2/m2, respectively, in men and 78.85 cm2 and 30.60 cm2/m2, respectively, in women.
Conclusion
Our data provide sex-specific cutoff values for the SMA and SMI at the L3 level measured by CT imaging in a healthy Korean population, which may be applicable for identifying sarcopenia in this population. -
Citations
Citations to this article as recorded by- Myosteatosis is associated with poor survival after kidney transplantation: a large retrospective cohort validation
Jie Chen, Yue Li, Chengjie Li, Turun Song
Abdominal Radiology.2024; 49(4): 1210. CrossRef - The effect of biological agent on body composition in patients with Crohn’s disease
Eun Jeong Choi, Dong Hoon Baek, Hong Sub Lee, Geun Am Song, Tae Oh Kim, Yong Eun Park, Chang Min Lee, Jong Hoon Lee
BMC Gastroenterology.2023;[Epub] CrossRef - The Association between the L3 Skeletal Muscle Index Derived from Computed Tomography and Clinical Outcomes in Patients with Urinary Tract Infection in the Emergency Department
Jinjoo An, Seung Pill Choi, Jae Hun Oh, Jong Ho Zhu, Sung Wook Kim, Soo Hyun Kim
Journal of Clinical Medicine.2023; 12(15): 5024. CrossRef - Validity of computed tomography defined body composition as a prognostic factor for functional outcome after kidney transplantation
Tim D. A. Swaab, Evelien E. Quint, Lisa B. Westenberg, Marcel Zorgdrager, Dorry L. Segev, Mara A. McAdams‐DeMarco, Stephan J. L. Bakker, Alain R. Viddeleer, Robert A. Pol
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(6): 2532. CrossRef - Assessment of the Diaphragm Thickness Decrease in Critically Ill COVID-19 Patients: Could Computed Tomography Be of Aid Regarding Diaphragm Muscle Mass?
Oana-Elena Branea, Sanda Maria Copotoiu, Diana Andreea Becica, AnaMaria Romina Budeanu, Razvan Gabriel Budeanu, Mihai Emanuel Becica, Dragos Constantin Cucoranu, Septimiu Voidazan, Monica Chis, Alexandra Elena Lazar
Cureus.2023;[Epub] CrossRef - Clinical implication of thoracic skeletal muscle volume as a predictor of ventilation-weaning failure in brain-injured patients: A retrospective observational study
Jimi Oh, Hyun Lim, Chang Won Jeong, Min Su Kim, Jinseok Lee, Wu Seong Kang, Ui Ri An, Joo Un Park, Youngick Ahn, Youe Ree Kim, Chul Park
Medicine.2023; 102(43): e35847. CrossRef - Estimation of Muscle Mass Using Creatinine/Cystatin C Ratio in Japanese Community-Dwelling Older People
Hiroshi Kusunoki, Yasuharu Tabara, Shotaro Tsuji, Yosuke Wada, Kayoko Tamaki, Koutatsu Nagai, Masako Itoh, Kyoko Sano, Manabu Amano, Hatsuo Maeda, Hideyuki Sugita, Yoko Hasegawa, Hiromitsu Kishimoto, Soji Shimomura, Michiya Igase, Ken Shinmura
Journal of the American Medical Directors Association.2022; 23(5): 902.e21. CrossRef - Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: A multicentre study
Ming Kong, Nan Geng, Ying Zhou, Ning Lin, Wenyan Song, Manman Xu, Shanshan Li, Yuetong Piao, Zuoqing Han, Rong Guo, Chao Yang, Nan Luo, Zhong Wang, Mengyuan Jiang, Lili Wang, Wanchun Qiu, Junfeng Li, Daimeng Shi, Rongkuan Li, Eddie C. Cheung, Yu Chen, Zho
Clinical Nutrition.2022; 41(2): 396. CrossRef - The Value of Artificial Intelligence-Assisted Imaging in Identifying Diagnostic Markers of Sarcopenia in Patients with Cancer
Ying-Tzu Huang, Yi-Shan Tsai, Peng-Chan Lin, Yu-Min Yeh, Ya-Ting Hsu, Pei-Ying Wu, Meng-Ru Shen, Zhongjie Shi
Disease Markers.2022; 2022: 1. CrossRef - Assessment of Muscle Quantity, Quality and Function
Bo Kyung Koo
Journal of Obesity & Metabolic Syndrome.2022; 31(1): 9. CrossRef - Computed Tomography-Derived Skeletal Muscle Radiodensity Is an Early, Sensitive Marker of Age-Related Musculoskeletal Changes in Healthy Adults
Yeon Woo Jung, Namki Hong, Joon Chae Na, Woong Kyu Han, Yumie Rhee
Endocrinology and Metabolism.2021; 36(6): 1201. CrossRef
- Myosteatosis is associated with poor survival after kidney transplantation: a large retrospective cohort validation

- Obesity and Metabolism
- Impact of Skeletal Muscle Mass on Metabolic Health
- Gyuri Kim, Jae Hyeon Kim
- Endocrinol Metab. 2020;35(1):1-6. Published online March 19, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.1.1
- 10,627 View
- 291 Download
- 64 Web of Science
- 68 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Skeletal muscle is regarded as an endocrine and paracrine organ. Muscle-derived secretory proteins, referred to as myokines, mediate interactions between skeletal muscle mass and other organs such as the liver, adipose tissue, pancreas, bone, and the cardiovascular system. As individuals age, reduced levels of physical activity and sarcopenia (loss of skeletal muscle mass and strength) are associated with physical frailty and disability. Recently, several studies have suggested that the loss of skeletal muscle mass may contribute to metabolic disease. Therefore, herein, we focus on the relationships between skeletal muscle mass and metabolic diseases, including metabolic syndrome and non-alcoholic fatty liver disease.
-
Citations
Citations to this article as recorded by- Sex differences in the association between dual‐energy x‐ray absorptiometry‐measured body composition and periodontitis
Peijun Zhu, An Li, Qingqing Cai, Yuntao Chen, Yang Liu, Harriët Jager‐Wittenaar, Geerten‐Has E. Tjakkes, Shulan Xu
Journal of Periodontology.2024; 95(3): 219. CrossRef - Advances in the treatment of functional male hypogonadism
Giovanni Corona, Giulia Rastrelli, Clotilde Sparano, Linda Vignozzi, Alessandra Sforza, Mario Maggi
Expert Review of Endocrinology & Metabolism.2024; 19(2): 163. CrossRef - Heterogeneously elevated branched-chain/aromatic amino acids among new-onset type-2 diabetes mellitus patients are potentially skewed diabetes predictors
Min Wang, Yang Ou, Xiang-Lian Yuan, Xiu-Fang Zhu, Ben Niu, Zhuang Kang, Bing Zhang, Anwar Ahmed, Guo-Qiang Xing, Heng Su
World Journal of Diabetes.2024; 15(1): 53. CrossRef - The Vicious Cycle of Type 2 Diabetes Mellitus and Skeletal Muscle Atrophy: Clinical, Biochemical, and Nutritional Bases
Jose M. Lopez-Pedrosa, Maria Camprubi-Robles, German Guzman-Rolo, Andres Lopez-Gonzalez, Jose Manuel Garcia-Almeida, Alejandro Sanz-Paris, Ricardo Rueda
Nutrients.2024; 16(1): 172. CrossRef - FGF21 Induces Skeletal Muscle Atrophy and Increases Amino Acids in Female Mice: A Potential Role for Glucocorticoids
Karlton R Larson, Devi Jayakrishnan, Karla A Soto Sauza, Michael L Goodson, Aki T Chaffin, Arik Davidyan, Suraj Pathak, Yanbin Fang, Diego Gonzalez Magaña, Benjamin F Miller, Karen K Ryan
Endocrinology.2024;[Epub] CrossRef - Mortality risk relationship using standard categorized BMI or knee-height based BMI – does the overweight/lower mortality paradox hold true?
Nivetha Natarajan Gavriilidou, Mats Pihlsgård, Sölve Elmståhl, Henrik Ekström
Aging Clinical and Experimental Research.2024;[Epub] CrossRef - Gromwell (Lithospermum erythrorhizon) Attenuates High-Fat-Induced Skeletal Muscle Wasting by Increasing Protein Synthesis and Mitochondrial Biogenesis
Ji-Sun Kim, Hyunjung Lee, Ahyoung Yoo, Hang Yeon Jeong, Chang Hwa Jung, Jiyun Ahn, Tae-Youl Ha
Journal of Microbiology and Biotechnology.2024; 34(3): 495. CrossRef - L-shaped association between lean body mass to visceral fat mass ratio with hyperuricemia: a cross-sectional study
Longti Li, Ya Shao, Huiqin Zhong, Yu Wang, Rong Zhang, Boxiong Gong, Xiaoxv Yin
Lipids in Health and Disease.2024;[Epub] CrossRef - Prevalence of adiposity-based chronic disease and its association with anthropometric and clinical indices: a cross-sectional study
Luis E González-Salazar, Aurora E Serralde-Zúñiga, Adriana Flores-López, Juan P Díaz-Sánchez, Isabel Medina-Vera, Edgar Pichardo-Ontiveros, Rocío Guizar-Heredia, Karla G Hernández-Gómez, Ana Vigil-Martínez, Liliana Arteaga-Sánchez, Azalia Avila-Nava, Nata
British Journal of Nutrition.2023; 130(1): 93. CrossRef - Skeletal Muscle Myokine Expression in Critical Illness, Association With Outcome and Impact of Therapeutic Interventions
Ilse Vanhorebeek, Jan Gunst, Michaël P Casaer, Inge Derese, Sarah Derde, Lies Pauwels, Johan Segers, Greet Hermans, Rik Gosselink, Greet Van den Berghe
Journal of the Endocrine Society.2023;[Epub] CrossRef - Effect of Circadian Rhythm Disturbance on the Human Musculoskeletal System and the Importance of Nutritional Strategies
Norsham Juliana, Liyana Azmi, Nadia Mohd Effendy, Nur Islami Mohd Fahmi Teng, Izuddin Fahmy Abu, Nur Nabilah Abu Bakar, Sahar Azmani, Noor Anisah Abu Yazit, Suhaini Kadiman, Srijit Das
Nutrients.2023; 15(3): 734. CrossRef - Molecular mechanisms of post‐burn muscle wasting and the therapeutic potential of physical exercise
Dorien Dombrecht, Ulrike Van Daele, Birgit Van Asbroeck, David Schieffelers, Pieter‐Jan Guns, Nick Gebruers, Jill Meirte, Eric van Breda
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(2): 758. CrossRef - Metabolic Impact of Frailty Changes Diabetes Trajectory
Alan J. Sinclair, Ahmed H. Abdelhafiz
Metabolites.2023; 13(2): 295. CrossRef - From Single- to Multi-organ-on-a-Chip System for Studying Metabolic Diseases
Minjeong Jang, Hong Nam Kim
BioChip Journal.2023; 17(2): 133. CrossRef - INFLUENCE OF SARCOPENIA ON THE COURSE AND PROGNOSIS IN PATIENTS WITH CHRONIC HEART FAILURE
Gulyaev N.I., Adamov A.A., Akhmetshin I.M.
"Medical & pharmaceutical journal "Pulse".2023; : 124. CrossRef - Frailty and the Interactions between Skeletal Muscle, Bone, and Adipose Tissue-Impact on Cardiovascular Disease and Possible Therapeutic Measures
María Elena Soto, Israel Pérez-Torres, María Esther Rubio-Ruiz, Agustina Cano-Martínez, Linaloe Manzano-Pech, Verónica Guarner-Lans
International Journal of Molecular Sciences.2023; 24(5): 4534. CrossRef - Sarcoplasmic Reticulum Ca2+ Buffer Proteins: A Focus on the Yet-To-Be-Explored Role of Sarcalumenin in Skeletal Muscle Health and Disease
Elena Conte, Giorgia Dinoi, Paola Imbrici, Annamaria De Luca, Antonella Liantonio
Cells.2023; 12(5): 715. CrossRef - Antarctic krill extracts enhance muscle regeneration and muscle function via mammalian target of rapamycin regulation
Seongmin Lee, Mi-Ock Baek, Sana Abdul Khaliq, Amna Parveen, Sun Yeou Kim, Jin-Hyoung Kim, Il-Chan Kim, Mee-Sup Yoon
Journal of Functional Foods.2023; 103: 105483. CrossRef - Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia
Archana Arjunan, Juhyun Song
Biomedicine & Pharmacotherapy.2023; 163: 114847. CrossRef - PRMT5 links lipid metabolism to contractile function of skeletal muscles
Kun Ho Kim, Zhihao Jia, Madigan Snyder, Jingjuan Chen, Jiamin Qiu, Stephanie N Oprescu, Xiyue Chen, Sabriya A Syed, Feng Yue, Bruno T Roseguini, Anthony N Imbalzano, Changdeng Hu, Shihuan Kuang
EMBO reports.2023;[Epub] CrossRef - “Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice
Jinjie Li, Yi Li, Xiangying Suo, Jiangtao Li, Da Huang, Guangning Kou
Foods.2023; 12(13): 2471. CrossRef - Effects of high-intensity interval training (HIIT) on skeletal muscle atrophy, function, and myokine profile in diabetic myopathy
Yeşim Özçatal, Fırat Akat, Yakup Tatar, Hakan Fıçıcılar, Bilge Serdaroğlu, Ferda Topal Çelikkan, Metin Baştuğ
Cytokine.2023; 169: 156279. CrossRef - Impaired proteostatic mechanisms other than decreased protein synthesis limit old skeletal muscle recovery after disuse atrophy
Jordan D. Fuqua, Marcus M. Lawrence, Zachary R. Hettinger, Agnieszka K. Borowik, Parker L. Brecheen, Marcelina M. Szczygiel, Claire B. Abbott, Frederick F. Peelor, Amy L. Confides, Michael Kinter, Sue C. Bodine, Esther E. Dupont‐Versteegden, Benjamin F. M
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(5): 2076. CrossRef - Body physique rating as a factor to identify at-risk Mexicans for Metabolic Syndrome
Oscar Herrera-Fomperosa, Sergio K. Bustamante-Villagomez, Sarahí Vazquez-Álvarez, Gabriela Vázquez-Marroquín, Leonardo M. Porchia, Enrique Torres-Rasgado, Ricardo Pérez-Fuentes, M. Elba Gonzalez-Mejia
Human Nutrition & Metabolism.2023; 33: 200206. CrossRef - Association between Fractional Oxygen Extraction from Resting Quadriceps Muscle and Body Composition in Healthy Men
Rodrigo Yáñez-Sepúlveda, Jorge Olivares-Arancibia, Guillermo Cortés-Roco, Aldo Vasquez-Bonilla, Matías Monsalves-Álvarez, Ildefonso Alvear-Órdenes, Marcelo Tuesta
Journal of Functional Morphology and Kinesiology.2023; 8(4): 149. CrossRef - Correlations between Mental Health, Physical Activity, and Body Composition in American College Students after the COVID-19 Pandemic Lockdown
Luis Torres, Manuela C. Caciula, Alin S. Tomoiaga, Carmen Gugu-Gramatopol
International Journal of Environmental Research and Public Health.2023; 20(22): 7045. CrossRef - Association between total body muscle percentage and prevalence of non-alcoholic fatty liver disease in Korean adults findings from an 18-year follow-up: a prospective cohort study
Byoung Chan Ahn, Chul Yong Park, Jung Hee Hong, Ki Ook Baek
Journal of Yeungnam Medical Science.2023; 40(Suppl): S47. CrossRef - The independent and joint associations among muscle strength, abdominal obesity and cardiometabolic variables among adults
Tiago Rodrigues de Lima, David Alejandro González‐Chica, Xuemei Sui, Diego Augusto Santos Silva
European Journal of Sport Science.2022; 22(7): 1122. CrossRef - Mentale Gesundheit und physische Aktivität
Wolfgang Laube
Manuelle Medizin.2022; 60(1): 13. CrossRef - A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction
Danbi Jo, Gwangho Yoon, Oh Yoen Kim, Juhyun Song
Biomedicine & Pharmacotherapy.2022; 147: 112636. CrossRef - Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle
James W. Daily, Sunmin Park
Cells.2022; 11(3): 338. CrossRef - Leveraging deep phenotyping from health check-up cohort with 10,000 Korean individuals for phenome-wide association study of 136 traits
Eun Kyung Choe, Manu Shivakumar, Anurag Verma, Shefali Setia Verma, Seung Ho Choi, Joo Sung Kim, Dokyoon Kim
Scientific Reports.2022;[Epub] CrossRef - Zika virus disrupts gene expression in human myoblasts and myotubes: Relationship with susceptibility to infection
Ingo Riederer, Daniella Arêas Mendes-da-Cruz, Guilherme Cordenonsi da Fonseca, Mariela Natacha González, Otavio Brustolini, Cássia Rocha, Guilherme Loss, Joseane Biso de Carvalho, Mariane Talon Menezes, Lidiane Menezes Souza Raphael, Alexandra Gerber, Myr
PLOS Neglected Tropical Diseases.2022; 16(2): e0010166. CrossRef - Teil 1: Muskeldysfunktionen – mit Training gegen Schmerz
Wolfgang Laube
Manuelle Medizin.2022; 60(2): 84. CrossRef - Transcription factors KLF15 and PPARδ cooperatively orchestrate genome-wide regulation of lipid metabolism in skeletal muscle
Liyan Fan, David R. Sweet, Erica K. Fan, Domenick A. Prosdocimo, Annmarie Madera, Zhen Jiang, Roshan Padmanabhan, Saptarsi M. Haldar, Vinesh Vinayachandran, Mukesh K. Jain
Journal of Biological Chemistry.2022; 298(6): 101926. CrossRef - An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches
Mizael C. Araújo, Suzany H. S. Soczek, Jaqueline P. Pontes, Leonardo A. C. Marques, Gabriela S. Santos, Gisele Simão, Laryssa R. Bueno, Daniele Maria-Ferreira, Marcelo N. Muscará, Elizabeth S. Fernandes
Cells.2022; 11(8): 1292. CrossRef - Effects of a 10-Week Physical Activity Intervention on Asylum Seekers’ Physiological Health
Matheus Guerra, Danilo Garcia, Maryam Kazemitabar, Erik Lindskär, Erica Schütz, Daniel Berglind
Brain Sciences.2022; 12(7): 822. CrossRef - Low muscle mass and mortality risk later in life: A 10-year follow-up study
Cristina Camargo Pereira, Valéria Pagotto, Cesar de Oliveira, Erika Aparecida Silveira, Kiyoshi Sanada
PLOS ONE.2022; 17(7): e0271579. CrossRef - Independent and joint associations of weightlifting and aerobic activity with all-cause, cardiovascular disease and cancer mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
Jessica Gorzelitz, Britton Trabert, Hormuzd A Katki, Steven C Moore, Eleanor L Watts, Charles E Matthews
British Journal of Sports Medicine.2022; 56(22): 1277. CrossRef - Handgrip Strength Cutoff Value Among Korean Adolescents with Metabolic Syndrome Components: Korean National Health and Nutrition Examination Survey Data 2014–2017
Chang Hoon Lee, Jun Hyeok Lee, Yong Whi Jeong, Hong Koh, Yunkoo Kang
Metabolic Syndrome and Related Disorders.2022; 20(10): 584. CrossRef - Physical activity level, sitting time, and skeletal muscle mass between esports players and non-esports players
Zhi H. SEE, Mohamad S. ABDUL HAMID
Gazzetta Medica Italiana Archivio per le Scienze Mediche.2022;[Epub] CrossRef - Impact of the Nutrition–Inflammation Status on the Functionality of Patients with Chronic Kidney Disease
Ángel Nogueira, Graciela Álvarez, Guillermina Barril
Nutrients.2022; 14(22): 4745. CrossRef - Does Timing Matter? A Narrative Review of Intermittent Fasting Variants and Their Effects on Bodyweight and Body Composition
Alan A. Aragon, Brad J. Schoenfeld
Nutrients.2022; 14(23): 5022. CrossRef - Sex- and region-specific associations of skeletal muscle mass with metabolic dysfunction-associated fatty liver disease
Pei Xiao, Pu Liang, Panjun Gao, Jinyi Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Donor Skeletal Muscle Quality Affects Graft Mortality After Living Donor Liver Transplantation- A Single Center, Retrospective Study
Takahiro Tomiyama, Noboru Harada, Takeo Toshima, Yuki Nakayama, Katsuya Toshida, Akinari Morinaga, Yukiko Kosai-Fujimoto, Takahiro Tomino, Takeshi Kurihara, Kazuki Takeishi, Yoshihiro Nagao, Kazutoyo Morita, Shinji Itoh, Tomoharu Yoshizumi
Transplant International.2022;[Epub] CrossRef - Handgrip Strength Is Associated with Metabolic Syndrome and Insulin Resistance in Children and Adolescents: Analysis of Korea National Health and Nutrition Examination Survey 2014–2018
Hae Woon Jung, Jieun Lee, Jaehyun Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(4): 334. CrossRef - Diet quality and a traditional dietary pattern predict lean mass in Australian women: Longitudinal data from the Geelong Osteoporosis Study
Jessica A. Davis, Mohammadreza Mohebbi, Fiona Collier, Amy Loughman, Nitin Shivappa, James R. Hébert, Julie A. Pasco, Felice N. Jacka
Preventive Medicine Reports.2021; 21: 101316. CrossRef - Association between serum FGF21 level and sarcopenia in older adults
Hee-Won Jung, Jin Hoon Park, Da Ae Kim, Il-Young Jang, So Jeong Park, Jin Young Lee, Seungjoo Lee, Jeoung Hee Kim, Hyon-Seung Yi, Eunju Lee, Beom-Jun Kim
Bone.2021; 145: 115877. CrossRef - Benchside to the bedside of frailty and cardiovascular aging: Main shared cellular and molecular mechanisms
Sandra Maria Barbalho, Ricardo José Tofano, Eduardo Federigui Baisi Chagas, Cláudia Rucco Penteado Detregiachi, Ricardo de Alvares Goulart, Uri Arian Princ Flato
Experimental Gerontology.2021; 148: 111302. CrossRef - Effect of CCL11 on In Vitro Myogenesis and Its Clinical Relevance for Sarcopenia in Older Adults
Da Ae Kim, So Jeong Park, Jin Young Lee, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Il-Young Jang, Hee-Won Jung, Jin Hoon Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(2): 455. CrossRef - Sarcopenic obesity as a determinant of cardiovascular disease risk in older people: a systematic review
Katherine Evans, Dima Abdelhafiz, Ahmed H Abdelhafiz
Postgraduate Medicine.2021; 133(8): 831. CrossRef - Decreased continuous sitting time increases heart rate variability in patients with cardiovascular risk factors
Natsuki Nakayama, Masahiko Miyachi, Koji Tamakoshi, Toshio Hayashi, Koji Negi, Koji Watanabe, Makoto Hirai, Sharon Mary Brownie
PLOS ONE.2021; 16(6): e0253399. CrossRef - Muskeltraining – ein universelles Medikament
Wolfgang Laube
Manuelle Medizin.2021; 59(3): 179. CrossRef - The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans
Liya Kerem, Elizabeth A. Lawson
International Journal of Molecular Sciences.2021; 22(14): 7737. CrossRef - Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss
David McCarthy, Aloys Berg
Nutrients.2021; 13(7): 2473. CrossRef - Association of FGF‐19 and FGF‐21 levels with primary sarcopenia
Rabia Bag Soytas, Veysel Suzan, Pinar Arman, Tugce Emiroglu Gedik, Damla Unal, Mahir Cengiz, Ibrahim Murat Bolayirli, Deniz Suna Erdincler, Alper Doventas, Hakan Yavuzer
Geriatrics & Gerontology International.2021; 21(10): 959. CrossRef - Der Muskulatur mehr Aufmerksamkeit schenken!
Wolfgang Laube
Manuelle Medizin.2021; 59(4): 302. CrossRef - Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review
Antti Tolonen, Tomppa Pakarinen, Antti Sassi, Jere Kyttä, William Cancino, Irina Rinta-Kiikka, Said Pertuz, Otso Arponen
European Journal of Radiology.2021; 145: 109943. CrossRef - Waist and hip circumference are independently associated with the risk of liver disease in population‐based studies
Oscar Danielsson, Markku J. Nissinen, Antti Jula, Veikko Salomaa, Satu Männistö, Annamari Lundqvist, Markus Perola, Fredrik Åberg
Liver International.2021; 41(12): 2903. CrossRef - Understanding of sarcopenia: from definition to therapeutic strategies
Jee Won Kim, Ryuni Kim, Hyerim Choi, Sang-Jin Lee, Gyu-Un Bae
Archives of Pharmacal Research.2021; 44(9-10): 876. CrossRef - Muscle strength and its association with cardiometabolic variables in adolescents: does the expression of muscle strength values matter?
Tiago Rodrigues de Lima, Xuemei Sui, Luiz Rodrigo Augustemak de Lima, Diego Augusto Santos Silva
World Journal of Pediatrics.2021; 17(6): 597. CrossRef - Musclin Is Related to Insulin Resistance and Body Composition, but Not to Body Mass Index or Cardiorespiratory Capacity in Adults
Yeliana L. Sánchez, Manuela Yepes-Calderón, Luis Valbuena, Andrés F. Milán, María C. Trillos-Almanza, Sergio Granados, Miguel Peña, Mauricio Estrada-Castrillón, Juan C. Aristizábal, Raúl Narvez-Sanchez, Jaime Gallo-Villegas, Juan C. Calderón
Endocrinology and Metabolism.2021; 36(5): 1055. CrossRef - Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications
Sandra Maria Barbalho, Uri Adrian Prync Flato, Ricardo José Tofano, Ricardo de Alvares Goulart, Elen Landgraf Guiguer, Cláudia Rucco P. Detregiachi, Daniela Vieira Buchaim, Adriano Cressoni Araújo, Rogério Leone Buchaim, Fábio Tadeu Rodrigues Reina, Piero
International Journal of Molecular Sciences.2020; 21(10): 3607. CrossRef Prevalence of Metabolic Syndrome and Association with Grip Strength in Older Adults: Findings from the HOPE Study
Reshma Aziz Merchant, Yiong Huak Chan, Jia Yi Lim, John E Morley
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 2677. CrossRef- Lower Serum n-3 Fatty Acid Level in Older Adults with Sarcopenia
Il-Young Jang, Hee-Won Jung, Jin Hoon Park, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Jin Young Lee, So Jeong Park, Da Ae Kim, Su Jung Kim, Hyun Ju Yoo, Beom-Jun Kim
Nutrients.2020; 12(10): 2959. CrossRef - Advances in understanding of health‐promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics
Minmin Yang, Tao Yan, Meng Yu, Jie Kang, Ruoxi Gao, Peng Wang, Yuhuan Zhang, Huafeng Zhang, Lin Shi
Food Frontiers.2020; 1(4): 398. CrossRef - The association of circulating kynurenine, a tryptophan metabolite, with frailty in older adults
Il-Young Jang, Jin Hoon Park, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Jin Young Lee, So Jeong Park, Da Ae Kim, Mark W. Hamrick, Beom-Jun Kim
Aging.2020; 12(21): 22253. CrossRef - Sarcopenia and Muscle Aging: A Brief Overview
Tam Dao, Alexander E. Green, Yun A Kim, Sung-Jin Bae, Ki-Tae Ha, Karim Gariani, Mi-ra Lee, Keir J. Menzies, Dongryeol Ryu
Endocrinology and Metabolism.2020; 35(4): 716. CrossRef
- Sex differences in the association between dual‐energy x‐ray absorptiometry‐measured body composition and periodontitis

- Obesity and Metabolism
- Connecting Myokines and Metabolism
- Rexford S. Ahima, Hyeong-Kyu Park
- Endocrinol Metab. 2015;30(3):235-245. Published online August 4, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.235
- 7,615 View
- 136 Download
- 71 Web of Science
- 71 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Skeletal muscle is the largest organ of the body in non-obese individuals and is now considered to be an endocrine organ. Hormones (myokines) secreted by skeletal muscle mediate communications between muscle and liver, adipose tissue, brain, and other organs. Myokines affect muscle mass and myofiber switching, and have profound effects on glucose and lipid metabolism and inflammation, thus contributing to energy homeostasis and the pathogenesis of obesity, diabetes, and other diseases. In this review, we summarize recent findings on the biology of myokines and provide an assessment of their potential as therapeutic targets.
-
Citations
Citations to this article as recorded by- Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases
Helena Trevisan Schroeder, Carlos Henrique De Lemos Muller, Thiago Gomes Heck, Mauricio Krause, Paulo Ivo Homem de Bittencourt
Cell Stress and Chaperones.2024; 29(1): 116. CrossRef - Exercise affects high-fat diet-stimulated breast cancer metastasis through irisin secretion by altering cancer stem cell properties
YuJin Lee, SoDam Park, SeungHwa Park, Hye Ji Kwon, Sang-Ho Lee, Yuri Kim, Jung-Hyun Kim
Biochemistry and Biophysics Reports.2024; 38: 101684. CrossRef - Myokines: A central point in managing redox homeostasis and quality of life
Richa Rathor, Geetha Suryakumar
BioFactors.2024;[Epub] CrossRef - Correlations between serum testosterone and irisin levels in a sample of Egyptian men with metabolic syndrome; (case-control study)
Inass Hassan Ahmad, Eman Roshdy Mohamed Mostafa, Shymaa Abdelhafeez Mohammed, Walaa Shipl, Amany Ahmed Soliman, Marwa Said
Archives of Physiology and Biochemistry.2023; 129(1): 180. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef - Myokines: Crosstalk and Consequences on Liver Physiopathology
Aurore Dumond Bourie, Jean-Baptiste Potier, Michel Pinget, Karim Bouzakri
Nutrients.2023; 15(7): 1729. CrossRef - Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia
Archana Arjunan, Juhyun Song
Biomedicine & Pharmacotherapy.2023; 163: 114847. CrossRef - Muscle quality: the assessment, prognosis, and intervention
翔 畑中, 洋祐 大須賀
Nippon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics.2023; 60(2): 103. CrossRef - Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors
Hamed Alizadeh Pahlavani
Frontiers in Endocrinology.2022;[Epub] CrossRef - Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs
Alessandra Renzini, Marco D’Onghia, Dario Coletti, Viviana Moresi
Frontiers in Physiology.2022;[Epub] CrossRef - Irisin response to acute moderate intensity exercise and high intensity interval training in youth of different obesity statuses: A randomized crossover trial
Benjamin H. Colpitts, Brittany V. Rioux, Ashley L. Eadie, Keith R. Brunt, Martin Sénéchal
Physiological Reports.2022;[Epub] CrossRef - The effects of whole‐body vibration amplitude on glucose metabolism, inflammation, and skeletal muscle oxygenation
Adeola A. Sanni, Anson M. Blanks, Cassandra C. Derella, Chase Horsager, Reva H. Crandall, Jacob Looney, Savanna Sanchez, Kimberly Norland, Bingwei Ye, Jeffrey Thomas, Xiaoling Wang, Ryan A. Harris
Physiological Reports.2022;[Epub] CrossRef - Preoperative Thoracic Muscle Mass Predicts Bone Density Change After Parathyroidectomy in Primary Hyperparathyroidism
Seung Won Burm, Namki Hong, Seunghyun Lee, Gi Jeong Kim, Sang Hyun Hwang, Jongju Jeong, Yumie Rhee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(6): e2474. CrossRef - Single skeletal muscle fiber mechanical properties: a muscle quality biomarker of human aging
Jae-Young Lim, Walter R. Frontera
European Journal of Applied Physiology.2022; 122(6): 1383. CrossRef - Hybrid HIIT/isometrics strength training programs: a paradigm shift for physical exercise
Luis Wyche, Guillermo Rojo-Gil, María Marín-Peiró, José Antonio Pérez-Turpin, Jaime Enrique Gómez-Paternina, Carlos Elvira, Duncan Ayers
Scientific Journal of Sport and Performance.2022; 1(1): 37. CrossRef - The Effect of Irisin on Proliferation, Apoptosis, and Expression of Metastasis Markers in Prostate Cancer Cell Lines
Atiye Saeedi Sadr, Hassan Ehteram, Elahe Seyed Hosseini, Marziyeh Alizadeh Zarei, Hassan Hassani Bafrani, Hamed Haddad Kashani
Oncology and Therapy.2022; 10(2): 377. CrossRef - Molecular mechanisms of adaptive and therapeutic effects of physical activity in patients with cardiovascular diseases
V.E. Vladimirsky, E.V. Vladimirsky, A.N. Lunina, A.D. Fesyun, A.P. Rachin, O.D. Lebedeva, M.Yu. Yakovlev, M.A. Tubekova
Voprosy kurortologii, fizioterapii i lechebnoi fizicheskoi kul'tury.2022; 99(2): 69. CrossRef - Studies in Rats of Combined Muscle and Liver Perfusion and of Muscle Extract Indicate That Contractions Release a Muscle Hormone Directly Enhancing Hepatic Glycogenolysis
Xiao X. Han, Jens J. Holst, Henrik Galbo
Journal of Personalized Medicine.2022; 12(5): 837. CrossRef - Local and systemic transcriptomic responses from acute exercise induced muscle damage of the human knee extensors
Eric A. Kirk, Christina A. Castellani, Timothy J. Doherty, Charles L. Rice, Shiva M. Singh
Physiological Genomics.2022; 54(8): 305. CrossRef - Upregulation of IL‐8, osteonectin, and myonectin mRNAs by intermittent hypoxia via OCT1‐ and NRF2‐mediated mechanisms in skeletal muscle cells
Shin Takasawa, Ryogo Shobatake, Asako Itaya‐Hironaka, Mai Makino, Tomoko Uchiyama, Sumiyo Sakuramoto‐Tsuchida, Yoshinori Takeda, Hiroyo Ota, Akiyo Yamauchi
Journal of Cellular and Molecular Medicine.2022; 26(24): 6019. CrossRef - Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview
Amritpal Dhaliwal, Jonathan I. Quinlan, Kellie Overthrow, Carolyn Greig, Janet M. Lord, Matthew J. Armstrong, Sheldon C. Cooper
Nutrients.2021; 13(2): 656. CrossRef - Role of Interleukin-6 in Vascular Health and Disease
Paulina Villar-Fincheira, Fernanda Sanhueza-Olivares, Ignacio Norambuena-Soto, Nicole Cancino-Arenas, Felipe Hernandez-Vargas, Rodrigo Troncoso, Luigi Gabrielli, Mario Chiong
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Computed Tomography-Derived Myosteatosis and Metabolic Disorders
Iva Miljkovic, Chantal A. Vella, Matthew Allison
Diabetes & Metabolism Journal.2021; 45(4): 482. CrossRef - Skeletal muscle energy metabolism in obesity
Abel M. Mengeste, Arild C. Rustan, Jenny Lund
Obesity.2021; 29(10): 1582. CrossRef - Myokines and adipomyokines: inflammatory mediators or unique molecules of targeted therapy for obesity?
O. V. Vasyukova, Yu. V. Kasyanova, P. L. Okorokov, O. B. Bezlepkina
Problems of Endocrinology.2021; 67(4): 36. CrossRef - Associations Between Lipoprotein Subfractions and Area and Density of Abdominal Muscle and Intermuscular Adipose Tissue: The Multi-Ethnic Study of Atherosclerosis
Megan M. Marron, Matthew Allison, Alka M. Kanaya, Britta Larsen, Alexis C. Wood, David Herrington, Philip Greenland, Iva Miljkovic
Frontiers in Physiology.2021;[Epub] CrossRef - Musclin Is Related to Insulin Resistance and Body Composition, but Not to Body Mass Index or Cardiorespiratory Capacity in Adults
Yeliana L. Sánchez, Manuela Yepes-Calderón, Luis Valbuena, Andrés F. Milán, María C. Trillos-Almanza, Sergio Granados, Miguel Peña, Mauricio Estrada-Castrillón, Juan C. Aristizábal, Raúl Narvez-Sanchez, Jaime Gallo-Villegas, Juan C. Calderón
Endocrinology and Metabolism.2021; 36(5): 1055. CrossRef - Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial
Hélio José Coelho-Júnior, Ivan de Oliveira Gonçalves, Ricardo Aurélio Carvalho Sampaio, Priscila Yukari Sewo Sampaio, Eduardo Lusa Cadore, Riccardo Calvani, Anna Picca, Mikel Izquierdo, Emanuele Marzetti, Marco Carlos Uchida
International Journal of Environmental Research and Public Health.2020; 17(10): 3435. CrossRef - Prostate tumor–derived GDF11 accelerates androgen deprivation therapy–induced sarcopenia
Chunliu Pan, Neha Jaiswal Agrawal, Yanni Zulia, Shalini Singh, Kai Sha, James L. Mohler, Kevin H. Eng, Joe V. Chakkalakal, John J. Krolewski, Kent L. Nastiuk
JCI Insight.2020;[Epub] CrossRef - Muscle–Organ Crosstalk: The Emerging Roles of Myokines
Mai Charlotte Krogh Severinsen, Bente Klarlund Pedersen
Endocrine Reviews.2020; 41(4): 594. CrossRef - Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets?
Ai Guo, Kai Li, Qian Xiao
Experimental Gerontology.2020; 139: 111022. CrossRef - Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging
Rosaly Correa-de-Araujo, Odessa Addison, Iva Miljkovic, Bret H. Goodpaster, Bryan C. Bergman, Richard V. Clark, Joanne W. Elena, Karyn A. Esser, Luigi Ferrucci, Michael O. Harris-Love, Steve B. Kritchevsky, Amanda Lorbergs, John A. Shepherd, Gerald I. Shu
Frontiers in Physiology.2020;[Epub] CrossRef - Muscle-Organ Crosstalk: Focus on Immunometabolism
Marie Lund Bay, Bente Klarlund Pedersen
Frontiers in Physiology.2020;[Epub] CrossRef - Impact of diets rich in olive oil, palm oil or lard on myokine expression in rats
Chantal Gauze-Gnagne, Fabrice Raynaud, Youzan Ferdinand Djohan, Céline Lauret, Christine Feillet-Coudray, Charles Coudray, Absalome Monde, Gervais Koffi, Marion Morena, Massara Camara-Cisse, Jean Paul Cristol, Eric Badia
Food & Function.2020;[Epub] CrossRef - Long-term treatment with insulin and retinoic acid increased glucose utilization in L6 muscle cells via glycogenesis
Matthew Goff, Guoxun Chen
Biochemistry and Cell Biology.2020; 98(6): 683. CrossRef - Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review
Vahid Maleki, Hamed Jafari-Vayghan, Sevda Saleh-Ghadimi, Mahsa Adibian, Sorayya Kheirouri, Mohammad Alizadeh
Complementary Therapies in Medicine.2019; 43: 20. CrossRef - Effect of a Very-Low-Calorie Ketogenic Diet on Circulating Myokine Levels Compared with the Effect of Bariatric Surgery or a Low-Calorie Diet in Patients with Obesity
Sajoux, Lorenzo, Gomez-Arbelaez, Zulet, Abete, Castro, Baltar, Portillo, Tinahones, Martinez, Crujeiras, Casanueva
Nutrients.2019; 11(10): 2368. CrossRef - Extracellular Vesicles: Delivery Vehicles of Myokines
Eleonora Trovato, Valentina Di Felice, Rosario Barone
Frontiers in Physiology.2019;[Epub] CrossRef - Effects of Exercise to Improve Cardiovascular Health
Kelsey Pinckard, Kedryn K. Baskin, Kristin I. Stanford
Frontiers in Cardiovascular Medicine.2019;[Epub] CrossRef - Does exercise-induced apelin affect sarcopenia? A systematic review and meta-analysis
Jun Hyun Bae, Seong Eun Kwak, Ji Hyun Lee, Zhang Yangjie, Wook Song
Hormones.2019; 18(4): 383. CrossRef - Skeletal muscle denervation investigations: selecting an experimental control wisely
Haiming Liu, LaDora V. Thompson
American Journal of Physiology-Cell Physiology.2019; 316(3): C456. CrossRef - Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease
Yung-Shuen Lin, Fang-Yu Lin, Ya-Hsin Hsiao
Molecular Neurobiology.2019; 56(3): 1984. CrossRef - Skeletal muscle as a protagonist in the pregnancy metabolic syndrome
Raul Narvaez-Sanchez, Juan C. Calderón, Gloria Vega, Maria Camila Trillos, Sara Ospina
Medical Hypotheses.2019; 126: 26. CrossRef - Oncostatin M, a muscle-secreted myokine, recovers high-glucose-induced impairment of Akt phosphorylation by Fos induction in hippocampal neuron cells
William Won Seok Hyung, Sung Gon Lee, Keun Tae Kim, Hyeon Soo Kim
NeuroReport.2019; 30(11): 765. CrossRef - If my muscle could talk: Myokines as a biomarker of frailty
Hélio J. Coelho-Junior, Anna Picca, Riccardo Calvani, Marco C. Uchida, Emanuele Marzetti
Experimental Gerontology.2019; 127: 110715. CrossRef - Impact of Sarcopenia as a Prognostic Biomarker of Bladder Cancer
Hiroshi Fukushima, Kosuke Takemura, Hiroaki Suzuki, Fumitaka Koga
International Journal of Molecular Sciences.2018; 19(10): 2999. CrossRef - Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes
Hye Soo Chung, Soon Young Hwang, Ju Hee Choi, Hyun Jung Lee, Nam Hoon Kim, Hye Jin Yoo, Ji-A Seo, Sin Gon Kim, Nan Hee Kim, Sei Hyun Baik, Kyung Mook Choi
Diabetes Research and Clinical Practice.2018; 136: 100. CrossRef - Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non‐massaged hindlimb
Benjamin F. Miller, Karyn L. Hamilton, Zana R. Majeed, Sarah M. Abshire, Amy L. Confides, Amanda M. Hayek, Emily R. Hunt, Patrick Shipman, Frederick F. Peelor, Timothy A. Butterfield, Esther E. Dupont‐Versteegden
The Journal of Physiology.2018; 596(1): 83. CrossRef - La funzione endocrina del muscolo scheletrico
Francesco Marampon, Clara Crescioli
L'Endocrinologo.2018; 19(1): 10. CrossRef - RESPUESTA INFLAMATORIA Y ANTIINFLAMATORIA TRAS EL ESFUERZO AGUDO EN NATACIÓN // INFLAMMATORY AND ANTINFLAMMATOY RESPONSE AFTER SWIMMING ACUTE EFFORT
G.A. Pussieldi, C.E. Veneroso, J.A. de Paz
Revista Internacional de Medicina y Ciencias de la Actividad Física y del Deporte.2018; 18(71): 413. CrossRef - Alpha-linolenic acid and linoleic acid differentially regulate the skeletal muscle secretome of obese Zucker rats
Alex Rajna, Heather Gibling, Ousseynou Sarr, Sarthak Matravadia, Graham P. Holloway, David M. Mutch
Physiological Genomics.2018; 50(8): 580. CrossRef - Serum irisin associates with breast cancer to spinal metastasis
Zheng-ping Zhang, Xue-fang Zhang, Hui Li, Tuan-jiang Liu, Qin-peng Zhao, Lin-hong Huang, Zi-jun Cao, Li-min He, Ding-jun Hao
Medicine.2018; 97(17): e0524. CrossRef - Causes and solutions to “globesity”: The new fa(s)t alarming global epidemic
Liliya V. Vasileva, Andrey S. Marchev, Milen I. Georgiev
Food and Chemical Toxicology.2018; 121: 173. CrossRef - Chronic inflammation and sarcopenia: A regenerative cell therapy perspective
Jagadish K. Chhetri, Philipe de Souto Barreto, Bertrand Fougère, Yves Rolland, Bruno Vellas, Matteo Cesari
Experimental Gerontology.2018; 103: 115. CrossRef - Level of Interleukins IL-6 and IL-15 in Blood Plasma of Mice after Forced Swimming Test
L. V. Kapilevich, T. A. Kironenko, A. N. Zakharova, A. V. Kabachkova, S. N. Orlov
Bulletin of Experimental Biology and Medicine.2017; 163(1): 10. CrossRef - Exercise Inhibits the Effects of Smoke-Induced COPD Involving Modulation of STAT3
Maysa Alves Rodrigues Brandao-Rangel, Andre Luis Lacerda Bachi, Manoel Carneiro Oliveira-Junior, Asghar Abbasi, Adriano Silva-Renno, Auriléia Aparecida de Brito, Ana Paula Ligeiro de Oliveira, Alessandra Choqueta Toledo-Arruda, Maria Gabriela Belvisi, Rod
Oxidative Medicine and Cellular Longevity.2017; 2017: 1. CrossRef - The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report
Rosaly Correa-de-Araujo, Michael O. Harris-Love, Iva Miljkovic, Maren S. Fragala, Brian W. Anthony, Todd M. Manini
Frontiers in Physiology.2017;[Epub] CrossRef - Myostatin and carbohydrate disturbances
Yavor S. Assyov, Tsvetelina V. Velikova, Zdravko A. Kamenov
Endocrine Research.2017; 42(2): 102. CrossRef -
OPA
1 deficiency promotes secretion of
FGF
21 from muscle that prevents obesity and insulin resistance
Renata Oliveira Pereira, Satya M Tadinada, Frederick M Zasadny, Karen Jesus Oliveira, Karla Maria Pereira Pires, Angela Olvera, Jennifer Jeffers, Rhonda Souvenir, Rose Mcglauflin, Alec Seei, Trevor Funari, Hiromi Sesaki, Matthew J Potthoff, Christopher M
The EMBO Journal.2017; 36(14): 2126. CrossRef - Low muscle mass is associated with cardiometabolic risk regardless of nutritional status in adolescents: A cross-sectional study in a Chilean birth cohort
R Burrows, P Correa-Burrows, M Reyes, E Blanco, C Albala, S Gahagan
Pediatric Diabetes.2017; 18(8): 895. CrossRef - Impact of sarcopenia in the management of urological cancer patients
Hiroshi Fukushima, Fumitaka Koga
Expert Review of Anticancer Therapy.2017; 17(5): 455. CrossRef - Prognostic value of preoperative total psoas muscle area on long-term outcome in surgically treated oesophageal cancer patients
Seong Yong Park, Joon-Kee Yoon, Su Jin Lee, Seokjin Haam, Joonho Jung
Interactive CardioVascular and Thoracic Surgery.2017; 24(1): 13. CrossRef - Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide
F. Scaldaferri, M. Pizzoferrato, L. R. Lopetuso, T. Musca, F. Ingravalle, L. L. Sicignano, M. Mentella, G. Miggiano, M. C. Mele, E. Gaetani, C. Graziani, V. Petito, G. Cammarota, E. Marzetti, A. Martone, F. Landi, A. Gasbarrini
Gastroenterology Research and Practice.2017; 2017: 1. CrossRef - Circulating Irisin Is Reduced in Male Patients with Type 1 and Type 2 Myotonic Dystrophies
Elena Dozio, Elena Passeri, Rosanna Cardani, Stefano Benedini, Carmen Aresta, Rea Valaperta, Massimiliano Corsi Romanelli, Giovanni Meola, Valeria Sansone, Sabrina Corbetta
Frontiers in Endocrinology.2017;[Epub] CrossRef - Implication of hepatokines in metabolic disorders and cardiovascular diseases
Tae Woo Jung, Hye Jin Yoo, Kyung Mook Choi
BBA Clinical.2016; 5: 108. CrossRef - Development of a high‐throughput method for real‐time assessment of cellular metabolism in intact long skeletal muscle fibre bundles
Rui Li, Frederik J. Steyn, Michael B. Stout, Kevin Lee, Tanya R. Cully, Juan C. Calderón, Shyuan T. Ngo
The Journal of Physiology.2016; 594(24): 7197. CrossRef - Integrated data mining of transcriptomic and proteomic datasets to predict the secretome of adipose tissue and muscle in ruminants
M. Bonnet, J. Tournayre, I. Cassar-Malek
Molecular BioSystems.2016; 12(9): 2722. CrossRef - CHI3L1 - a novel myokine
H. Kainulainen
Acta Physiologica.2016; 216(3): 260. CrossRef - Elevated circulating irisin is associated with lower risk of insulin resistance: association and path analyses of obese Chinese adults
Xiulin Shi, Mingzhu Lin, Changqin Liu, Fangsen Xiao, Yongwen Liu, Peiying Huang, Xin Zeng, Bing Yan, Suhuan Liu, Xiaoying Li, Shuyu Yang, Xuejun Li, Zhibin Li
BMC Endocrine Disorders.2016;[Epub] CrossRef - Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia
Kyoung Min Kim, Hak Chul Jang, Soo Lim
The Korean Journal of Internal Medicine.2016; 31(4): 643. CrossRef - Relation of serum irisin level with metabolic and antropometric parameters in obese children
Gönül Çatlı, Tuncay Küme, Hale Ünver Tuhan, Ahmet Anık, Özlem Gürsoy Çalan, Ece Böber, Ayhan Abacı
Journal of Diabetes and its Complications.2016; 30(8): 1560. CrossRef
- Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases

- Thyroid
- Expression of Thyroid Stimulating Hormone Receptor mRNA in Mouse C2C12 Skeletal Muscle Cells
- Jung Hun Ohn, Sun Kyoung Han, Do Joon Park, Kyong Soo Park, Young Joo Park
- Endocrinol Metab. 2013;28(2):119-124. Published online June 18, 2013
- DOI: https://doi.org/10.3803/EnM.2013.28.2.119
- 3,478 View
- 39 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We analyzed whether thyroid stimulating hormone receptor (TSH-R) is expressed in a skeletal muscle cell line and if TSH has influence on the differentiation of muscle cells or on the determination of muscle fiber types.
Methods TSH-R gene expression was detected with nested real-time polymerase chain reaction (RT-PCR) in C2C12, a mouse skeletal muscle cell line. The effect of TSH on myotube differentiation was assessed by microscopic examination of myotube formation and through the measurement of expression of muscle differentiation markers, i.e., myogenin and myoD, and muscle type-specific genes, i.e., MyHC1, MyHC2a, and MyHC2b, with quantitative RT-PCR before and after incubation of C2C12 myotube with TSH.
Results TSH-R was expressed in the mouse skeletal muscle cell line. However, treatment with TSH had little effect on the differentiation of muscle cells, although the expression of the muscle differention marker myogenin was significantly increased after TSH treatment. Treatment of TSH did not affect the expression of muscle type-specific genes.
Conclusion TSH-R is expressed in a mouse skeletal muscle cell line, but the role of TSH receptor signaling in skeletal muscle needs further investigation.
-
Citations
Citations to this article as recorded by- Associations between thyroid hormones and appendicular skeletal muscle index, and hand grip strength in people with diabetes: The KAMOGAWA-A study
Shinnosuke Hata, Hiroshi Okada, Megumi Minamida, Junya Hironaka, Yuka Hasegawa, Yuriko Kondo, Hanako Nakajima, Nobuko Kitagawa, Takuro Okamura, Yoshitaka Hashimoto, Takafumi Osaka, Noriyuki Kitagawa, Saori Majima, Takafumi Senmaru, Emi Ushigome, Naoko Nak
Diabetes Research and Clinical Practice.2024; 209: 111573. CrossRef - Clinical parameters correlated with the psoas muscle index in Japanese individuals with type 2 diabetes mellitus
Emi Asano-Hayami, Yoshiaki Morishita, Tomohide Hayami, Yuka Shibata, Toshiki Kiyose, Sachiko Sasajima, Yusuke Hayashi, Mikio Motegi, Makoto Kato, Saeko Asano, Hiromi Nakai-Shimoda, Yuichiro Yamada, Emiri Miura-Yura, Tatsuhito Himeno, Masaki Kondo, Shin Ts
Diabetology International.2023; 14(1): 76. CrossRef - Effect of Thyroid-Stimulating Hormone Suppression on Muscle Function After Total Thyroidectomy in Patients With Thyroid Cancer
Jun Choul Lee, Byong-Sop Song, Young Mi Kang, Yu-Ri Kim, Yea Eun Kang, Ju Hee Lee, Minho Shong, Hyon-Seung Yi
Frontiers in Endocrinology.2021;[Epub] CrossRef - Different Relationships Between Thyrotropin and Muscle Strength According to Sex and Age in Euthyroid Koreans (The 6th Korea National Health and Nutritional Examination Survey 2014–2015)
Seong Hee Ahn, Da Hea Seo, Yongin Cho, Mihye Jung, So Hun Kim, Seongbin Hong
Thyroid.2020; 30(12): 1710. CrossRef - A Significant Association of Upper Limb Muscle Strength with Thyroid Function in Overweight and Obese Population: A Study of the Sixth Korea National Health and Nutrition Examination Survey (KNHANES 2014-2015)
Jeongmin Lee, Kwanhoon Jo, Jeonghoon Ha, Dong-Jun Lim, Jung Min Lee, Sang-Ah Chang, Moo Il Kang, Min-Hee Kim, Flavia Magri
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Muscle-derived IL-6 improved insulin resistance of C2C12 cells through activating AMPK and inhibiting p38MAPK signal pathway in vitro
Hui Tang, Shuai Deng, Jian-guang Cai, Xue-nan Ma, Man Liu, Liang Zhou
International Journal of Diabetes in Developing Countries.2019; 39(3): 486. CrossRef - Thyroid Function as a Predictor of Handgrip Strength Among Middle-Aged and Older Euthyroid Adults: The TCLSIH Cohort Study
Yeqing Gu, Ge Meng, Hongmei Wu, Qing Zhang, Li Liu, Xue Bao, Yawen Wang, Shunming Zhang, Shaomei Sun, Xing Wang, Ming Zhou, Qiyu Jia, Kun Song, Kaijun Niu
Journal of the American Medical Directors Association.2019; 20(10): 1236. CrossRef - Association of Serum TSH With Handgrip Strength in Community-Dwelling Euthyroid Elderly
Beom-Jun Kim, Seung Hun Lee, Carlos M Isales, Mark W Hamrick, Mi Kyung Kwak, Jung-Min Koh
The Journal of Clinical Endocrinology & Metabolism.2018; 103(11): 3986. CrossRef - Changes in Thyroid Hormone Levels Within the Normal and/or Subclinical Hyper- or Hypothyroid Range Do Not Affect Circulating Irisin Levels in Humans
Grigorios Panagiotou, Kalliopi Pazaitou-Panayiotou, Stavroula A. Paschou, Despina Komninou, Nikolaos Kalogeris, Andromachi Vryonidou, Christos S. Mantzoros
Thyroid.2016; 26(8): 1039. CrossRef - Thyroid-stimulating hormone improves insulin sensitivity in skeletal muscle cells via cAMP/PKA/CREB pathway-dependent upregulation of insulin receptor substrate-1 expression
Min Kyong Moon, Geun Hyung Kang, Hwan Hee Kim, Sun Kyoung Han, Young Do Koo, Sun Wook Cho, Ye An Kim, Byung-Chul Oh, Do Joon Park, Sung Soo Chung, Kyong Soo Park, Young Joo Park
Molecular and Cellular Endocrinology.2016; 436: 50. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef
- Associations between thyroid hormones and appendicular skeletal muscle index, and hand grip strength in people with diabetes: The KAMOGAWA-A study


 KES
KES

 First
First Prev
Prev



