Search
- Page Path
- HOME > Search
- Miscellaneous
- Fancd2os Reduces Testosterone Production by Inhibiting Steroidogenic Enzymes and Promoting Cellular Apoptosis in Murine Testicular Leydig Cells
- Xiang Zhai, Xin-yang Li, Yu-jing Wang, Ke-ru Qin, Jin-rui Hu, Mei-ning Li, Hai-long Wang, Rui Guo
- Endocrinol Metab. 2022;37(3):533-546. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1431
- 2,385 View
- 86 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
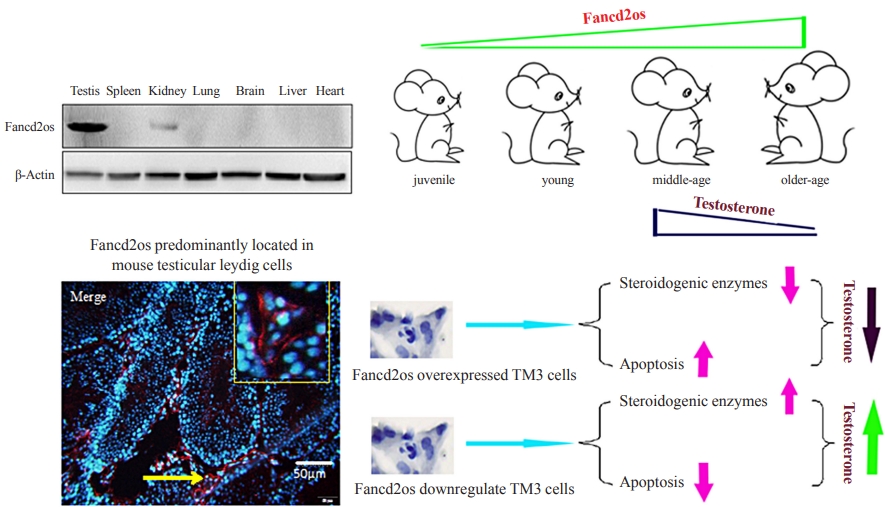
It is well-established that serum testosterone in men decreases with age, yet the underlying mechanism of this change remains elusive.
Methods
The expression patterns of Fancd2 opposite-strand (Fancd2os) in BALB/c male mice and testicular tissue derived cell lines (GC-1, GC-2, TM3, and TM4) were assessed using real-time polymerase chain reaction (RT-PCR), Western blot and immunofluorescence. The Fancd2os-overexpressing or knockdown TM3 cells were constructed by infecting them with lentivirus particles and were used to evaluated the function of Fancd2os. The testosterone production was measured using enzyme linked immunosorbent assay (ELISA) and the steroidogenic enzymes such as steroidogenic acute regulatory protein (StAR), P450 cholesterol side-chain cleavage (P450scc), and 3β-hydroxysteroid dehydrogenase (3β-HSD) were analysed using RT-PCR. The apoptosis of TM3 cells induced by ultraviolet light or testicular tissues was detected using flow cytometry, Western blot or dUTP-biotin nick end labeling (TUNEL) assays. Pearson correlation analysis was used to assess the correlation between the Fancd2os expression and TUNEL-positive staining in mouse testicular Leydig cells.
Results
The Fancd2os protein was predominantly expressed in mouse testicular Leydig cells and its expression increased with age. Fancd2os overexpression inhibited testosterone levels in TM3 Leydig cells, whereas knockdown of Fancd2os elevated testosterone production. Fancd2os overexpression downregulated the levels of StAR, P450scc and 3β-HSD, while Fancd2os knockdown reversed this effect. Fancd2os overexpression promoted ultraviolet light-induced apoptosis of TM3 cells. In contrast, Fancd2os knockdown restrained apoptosis in TM3 cells. In vivo assays revealed that higher Fancd2os levels and mouse age were associated with increased apoptosis in Leydig cells and decreased serum testosterone levels. Pearson correlation analysis exhibited a strong positive correlation between the expression of Fancd2os and TUNEL-positive staining in mouse testicular Leydig cells.
Conclusion
Our findings suggest that Fancd2os regulates testosterone synthesis via both steroidogenic enzymes and the apoptotic pathway. -
Citations
Citations to this article as recorded by- An EWAS of dementia biomarkers and their associations with age, African ancestry, and PTSD
Mark W. Miller, Erika J. Wolf, Xiang Zhao, Mark W. Logue, Sage E. Hawn
Clinical Epigenetics.2024;[Epub] CrossRef - Gas/Liquid Chromatography–Mass Spectrometry Analysis of Key Functional Substances Regulating Poll Gland Secretion in Male Camels during Seasonal Estrus
Lijun Dai, Bao Yuan, Bohao Zhang, Wenli Chen, Xixue Yuan, Xinhong Liu, Yuan Gao, Yong Zhang, Quanwei Zhang, Xingxu Zhao
Animals.2023; 13(12): 2024. CrossRef - Benzo[b]fluoranthene induces male reproductive toxicity and apoptosis via Akt-Mdm2-p53 signaling axis in mouse Leydig cells: Integrating computational toxicology and experimental approaches
Chao-feng Shi, Fei Han, Xiao Jiang, Zhonghao Zhang, Yingqing Li, Jiankang Wang, Shengqi Sun, Jin-yi Liu, Jia Cao
Food and Chemical Toxicology.2023; 179: 113941. CrossRef - Induction of apoptosis by cannabidiol and its main metabolites in human Leydig cells
Yuxi Li, Xilin Li, Patrick Cournoyer, Supratim Choudhuri, Lei Guo, Si Chen
Archives of Toxicology.2023; 97(12): 3227. CrossRef
- An EWAS of dementia biomarkers and their associations with age, African ancestry, and PTSD

- Hypothalamus and Pituitary gland
- Current National and International Guidelines for the Management of Male Hypogonadism: Helping Clinicians to Navigate Variation in Diagnostic Criteria and Treatment Recommendations
- Ahmed Al-Sharefi, Richard Quinton
- Endocrinol Metab. 2020;35(3):526-540. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.760
- 9,448 View
- 504 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Male hypogonadism—rebadged by some as testosterone deficiency syndrome—is a clinical and biochemical diagnosis of increasing worldwide interest. Organic male hypogonadism—usually permanent—is well-established, but aging men may also exhibit lower serum testosterone levels; principally due to burden of extra-gonadal comorbidities such as obesity, diabetes and metabolic syndrome, but with an underlying intact hypothalamo-pituitary-testicular (HPT) axis capable of springing back into operation once comorbidities are addressed. Despite encouraging observational data and plausible theoretical underpinning, evidence for efficacy and safety of testosterone in this “aging” group of men is lacking; addressing comorbid illnesses remains the key priority instead. Nevertheless, in recent years, accumulation of misleading information online has triggered a global tsunami of testosterone prescriptions. Despite this, many men with organic hypogonadism remain undiagnosed or untreated; many more face a diagnostic odyssey before achieving care by the appropriate specialist. As testosterone therapy is not without risk several clinical practice guidelines have been published specialist societies to guide physicians on best practice. However, these are heterogeneous in key areas, reflecting divergent approaches to the same evidence basis. Herein, we navigate the major clinical practice guidelines on male hypogonadism and test their respective recommendations against current best evidence.
-
Citations
Citations to this article as recorded by- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India
Sanjay Kalra, Jubbin Jacob, A. G. Unnikrishnan, Ganapathi Bantwal, Abhay Sahoo, Rakesh Sahay, Sushil Jindal, Madhu Sudan Agrawal, Nitin Kapoor, Banshi Saboo, Mangesh Tiwaskar, Kapil Kochhar, Henrik Falhammar
International Journal of Endocrinology.2023; 2023: 1. CrossRef - Management Outcomes in Males With Hypogonadotropic Hypogonadism Treated With Gonadotropins
Bahaa O Sahib, Ibrahim H Hussein, Nassar T Alibrahim, Abbas A Mansour
Cureus.2023;[Epub] CrossRef - The Association between Inflammation, Testosterone and SHBG in men: A cross‐sectional Multi‐Ethnic Study of Atherosclerosis
Amar Osmancevic, Bledar Daka, Erin D. Michos, Penelope Trimpou, Matthew Allison
Clinical Endocrinology.2023; 99(2): 190. CrossRef - The Illusory Case for Treatment of an Invented Disease
David J. Handelsman
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Chronic Heart Failure Complicated with Type 2 Diabetes Mellitus on Cognitive Function in the Elderly
Yang Liu, Rui Meng, Jianzeng Dong, Xiaonan Xi
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Different Factors Are Associated With Sex Hormones and Leydig Cell Function in Israelis and Palestinians in Jerusalem
Guy Vishnevsky, Ronit Sinnreich, Hisham Nassar, Dafna Merom, Maya Ish-Shalom, Jeremy D. Kark, Hagai Levine
American Journal of Men's Health.2022; 16(4): 155798832211060. CrossRef - Association of rs9939609 polymorphism in the FTO gene with features of androgen status in men
S. V. Yankovskaya, K. I. Mosalev, I. D. Ivanov, B. B. Pinkhasov, V. G. Selyatitskaya
Сибирский научный медицинский журнал.2022; 42(2): 18. CrossRef - Clinical and pharmacological basis of the use of testosterone drugs for hormonal replacement therapy for hypogonadism in men
N. I. Volkova, A. V. Safronenko, E. V. Gantsgorn, Yu. S. Degtyareva
Obesity and metabolism.2022; 19(2): 233. CrossRef - Monitoring and Management of Bardet-Biedl Syndrome: What the Multi-Disciplinary Team Can Do
Lavinia Caba, Laura Florea, Elena Emanuela Braha, Valeriu Vasile Lupu, Eusebiu Vlad Gorduza
Journal of Multidisciplinary Healthcare.2022; Volume 15: 2153. CrossRef - Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones
Sara Arefhosseini, Mehrangiz Ebrahimi-Mameghani, Farzad Najafipour, Helda Tutunchi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men
Bruno Lunenfeld, George Mskhalaya, Michael Zitzmann, Giovanni Corona, Stefan Arver, Svetlana Kalinchenko, Yuliya Tishova, Abraham Morgentaler
The Aging Male.2021; 24(1): 119. CrossRef
- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India

- Clinical Study
- Associations of Metabolic Syndrome with Total Testosterone and Homocysteine Levels in Male Korean Workers
- Sook Hee Sung, Nam Hee Kim, Sun Pyo Hong, Jong-Keun Lee, Seung Jin Choi
- Endocrinol Metab. 2019;34(2):158-168. Published online June 24, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.2.158
- 4,276 View
- 60 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Low testosterone is associated with metabolic syndrome (MetS), and homocysteine (Hcy) is elevated in individuals with MetS. We investigated the relationships of total testosterone (TT) and serum Hcy levels with MetS in male Korean workers.
Methods We conducted a cross-sectional study including 8,606 male workers, aged 20 to 58 years, who underwent a physical examination in 2015. MetS was diagnosed based on the criteria of the 2009 harmonized definition, while the Korean standard for waist circumference (WC) was used. Participants' biochemical parameters, including TT and serum Hcy, were measured, and participants were divided into quartiles. Multiple logistic regression models were used to estimate the association of MetS and its individual components depending on TT and serum Hcy quartiles.
Results The prevalence of MetS in the study population was 16%. TT was lower in participants with MetS than in those without MetS (
P <0.001). By contrast, Hcy level was similar between groups (P =0.694). In multiple logistic regression analysis, the odds ratio for the lowest TT quartile was 1.29 (95% confidence interval, 1.06 to 1.57) after adjusting for potential confounders. Participants with lower TT were more likely to have high WC, hypertriglyceridemia, and low high density lipoprotein levels. Serum Hcy levels were not significantly associated with MetS. Of the five components of MetS, only WC was significantly associated with serum Hcy.Conclusion In male Korean workers, TT may be an independent predictor of MetS, and serum Hcy levels could be a marker of abdominal obesity. However, future prospective studies are needed.
-
Citations
Citations to this article as recorded by- A negative association between triglyceride glucose-body mass index and testosterone in adult males: a cross-sectional study
Shenghao Wu, Yanhong Wu, Lizi Fang, Junzhao Zhao, Yaoyao Cai, Weiting Xia
Frontiers in Endocrinology.2023;[Epub] CrossRef - Vitamin B12, folate, and homocysteine in metabolic syndrome: a systematic review and meta-analysis
Juan R. Ulloque-Badaracco, Enrique A. Hernandez-Bustamante, Esteban A. Alarcon-Braga, Ali Al-kassab-Córdova, Juan C. Cabrera-Guzmán, Percy Herrera-Añazco, Vicente A. Benites-Zapata
Frontiers in Endocrinology.2023;[Epub] CrossRef
- A negative association between triglyceride glucose-body mass index and testosterone in adult males: a cross-sectional study

- Obesity and Metabolism
- Low Serum Testosterone Concentrations in Hospitalized Men with Poorly Controlled Type 2 Diabetes
- Kyung-Soo Kim, San-Ha Kang, Moon-Jong Kim, Soo-Kyung Kim, Yoo-Lee Kim, Won-Keun Park, Seok Won Park, Yong-Wook Cho
- Endocrinol Metab. 2014;29(4):574-578. Published online December 29, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.4.574
- 3,333 View
- 31 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Our aim was to examine whether serum testosterone concentrations are in fact low in hospitalized men with poorly controlled type 2 diabetes compared with healthy men. In this study, 79 men aged 40 years or older (41 healthy men and 38 men with type 2 diabetes) were included. Total testosterone and sex hormone-binding globulin levels were measured. The average duration of diagnosed diabetes was 10.8 years and the mean glycated hemoglobin value was 10.8%. Total testosterone concentrations were lower in men with type 2 diabetes than in healthy men, after adjusting for age and body mass index (3.83±0.32 ng/mL vs. 5.63±0.31 ng/mL,
P <0.001). In conclusion, this study shows that serum testosterone concentrations are lower in hospitalized men with poorly controlled type 2 diabetes than in healthy men. Therefore, men with poorly controlled type 2 diabetes should undergo further assessment for hypogonadism.-
Citations
Citations to this article as recorded by- Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α
Duaa Bakhshwin, Khadija Abdul Jalil Faddladdeen, Soad Shaker Ali, Samar Mohammed Alsaggaf, Nasra Naeim Ayuob
Molecules.2022; 27(3): 1027. CrossRef - Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis
Jianzhong Zhang, Xiao Li, Zhonglin Cai, Hongjun Li, Bin Yang
The Aging Male.2020; 23(5): 607. CrossRef - Momordica charantia Extract Protects against Diabetes-Related Spermatogenic Dysfunction in Male Rats: Molecular and Biochemical Study
Gamal A. Soliman, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Hassan N. Althurwi, Reham M. Abd-Elsalam, Faisal F. Albaqami, Maged S. Abdel-Kader
Molecules.2020; 25(22): 5255. CrossRef - Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study
Gamal A. Soliman, Abdulaziz S. Saeedan, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Reham M. Abd-Elsalam, Maged S. Abdel-Kader
Saudi Pharmaceutical Journal.2019; 27(3): 326. CrossRef - Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review
Jianzhong Zhang, Bin Yang, Wenhui Xiao, Xiao Li, Hongjun Li
World Journal of Urology.2018; 36(8): 1315. CrossRef - RAS and sex differences in diabetic nephropathy
Sergi Clotet, Marta Riera, Julio Pascual, María José Soler
American Journal of Physiology-Renal Physiology.2016; 310(10): F945. CrossRef
- Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α

- Obesity and Metabolism
- Testosterone Deficiency Associated with Poor Glycemic Control in Korean Male Diabetics
- Joo-Sung Kim, Bong Sun Kim, Ja Young Jeon, Yong Jun Choi, Yoon-Sok Chung
- Endocrinol Metab. 2014;29(3):300-306. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.300
- 3,483 View
- 31 Download
- 8 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Recent studies have shown that men with diabetes have lower testosterone levels than healthy men. However, studies on the correlation between testosterone and diabetes are rare in Korea. We examined the relationship between testosterone deficiency and markers related to diabetes in adult Korean men.
Methods A total 464 men with diabetes who visited an outpatient clinic at Ajou University Hospital and had serum total testosterone and serum insulin levels measured between January 2000 and September 2013 were selected. Blood samples were collected after the subjects had fasted overnight. We divided the participants into testosterone deficient and normal groups. Testosterone deficiency was defined as having a serum total testosterone level <3.5 ng/mL.
Results Of 464 subjects, 34.9% had a testosterone deficiency. The mean levels of fasting plasma glucose (
P =0.007) and glycated hemoglobin (HbA1c;P =0.038) were significantly higher in the testosterone deficiency group than in the normal group. To clarify the relationship between serum total testosterone level and fasting plasma glucose or HbA1c values, Pearson's correlation test was performed. Fasting plasma glucose levels (r =-0.142,P =0.002) and HbA1c values (r =-0.097,P =0.040) showed a significant negative correlation with serum testosterone levels in men with diabetes.Conclusion Major markers of diabetes that are associated with testosterone deficiency are fasting plasma glucose and HbA1c values. Poor glycemic control appears to be associated with testosterone deficiency in Korean men with diabetes.
-
Citations
Citations to this article as recorded by- Association between T2DM and the lowering of testosterone levels among Kashmiri males
Rabia Farooq, Mohammad Hayat Bhat, Sabhiya Majid, Mohammad Muzaffar Mir
Archives of Endocrinology and Metabolism.2020;[Epub] CrossRef - Hypogonadism and associated risk factors in male patients with type 2 diabetes mellitus attending the diabetic clinic of Tikur Anbessa Specialized Teaching Hospital, Addis Ababa, Ethiopia
Sisay Teka, Samuel Kinde, Gobena Dedefo, Kissi Mudi, Getahun Tarekegn
Journal of Endocrinology, Metabolism and Diabetes of South Africa.2019; 24(1): 16. CrossRef - Hypogonadism in Nigerian men with type 2 diabetes mellitus
S. I. Onung, E. E. Young, T. E. Ugwu, O. A. Fasanmade
International Journal of Diabetes in Developing Countries.2017; 37(3): 254. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Association between T2DM and the lowering of testosterone levels among Kashmiri males

- Bone Metabolism
- Testosterone Replacement and Bone Mineral Density in Male Pituitary Tumor Patients
- Min Jeong Lee, Hyoung Kyu Ryu, So-Yeon An, Ja Young Jeon, Ji In Lee, Yoon-Sok Chung
- Endocrinol Metab. 2014;29(1):48-53. Published online March 14, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.1.48
- 3,493 View
- 32 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Hypopituitarism is associated with osteoporosis and osteopenia especially when hypogonadotropic hypogonadism is present. Despite hypopituitarism being an important cause of secondary osteoporosis, osteoporosis in patients receiving surgery for pituitary tumors in Korea has not been studied. In this study, we evaluated the effects of testosterone replacement therapy (TRT) on bone mineral density (BMD) in postoperative hypogonadal patients with pituitary tumors.
Methods To examine the effect of TRT on BMD, we performed a retrospective observational study in 21 postoperative male patients who underwent pituitary tumor surgery between 2003 and 2012 at the Ajou University Hospital. Testosterone was replaced in postoperative hypogonadal patients by regular intramuscular injection, daily oral medication, or application of transdermal gel. BMD (g/cm2) measurements of central skeletal sites (lumbar spine, femoral neck, and total femur) were obtained using dual-energy X-ray absorptiometry (GE Lunar). For lumbar spine BMD, L1 to L4 values were chosen for analysis. Femur neck and total femur were also analyzed.
Results During the follow-up period (mean, 56 months; range, 12 to 99 months) serum testosterone levels increased with the administration of TRT (
P =0.007). There was significant improvement (4.56%±9.81%) in the lumbar spine BMD compared to baseline BMD. There were no significant changes in the femur neck BMD or total femur BMD. We did not find any statistically significant relationships between changes in testosterone levels and BMD using Spearman correlation analysis.Conclusion Our results indicated that TRT used in the postoperative period for hypogonadal pituitary tumor surgery patients may have beneficial effects on the BMD of the spine.
-
Citations
Citations to this article as recorded by- Testosterone supplementation and bone parameters: a systematic review and meta-analysis study
G. Corona, W. Vena, A. Pizzocaro, V. A. Giagulli, D. Francomano, G. Rastrelli, G. Mazziotti, A. Aversa, A. M. Isidori, R. Pivonello, L. Vignozzi, E. Mannucci, M. Maggi, A. Ferlin
Journal of Endocrinological Investigation.2022; 45(5): 911. CrossRef - Physiological testosterone replacement effects on male aged rats with orchiectomy-induced osteoporosis in advanced stage: a tomographic and biomechanical pilot study
Vinícius de Paiva Gonçalves, Adriana Alicia Cabrera-Ortega, Jhonatan de Souza Carvalho, Dania Ramadan, Luís Carlos Spolidorio
The Aging Male.2021; 24(1): 139. CrossRef - Androgens and Androgen Receptor Actions on Bone Health and Disease: From Androgen Deficiency to Androgen Therapy
Jia-Feng Chen, Pei-Wen Lin, Yi-Ru Tsai, Yi-Chien Yang, Hong-Yo Kang
Cells.2019; 8(11): 1318. CrossRef - Testosterone and male rejuvenation
Sevann Helo, Peyton Thomas, Nicholas N. Tadros
Panminerva Medica.2019;[Epub] CrossRef - Systemic Non-Reproductive Effects of Sex Steroids in Adult Males and Females
Syed Imran Ali Shah
Human Physiology.2018; 44(1): 83. CrossRef - Benefits and Health Implications of Testosterone Therapy in Men With Testosterone Deficiency
Abdulmaged M. Traish
Sexual Medicine Reviews.2018; 6(1): 86. CrossRef - Multiple Fractures in Patient with Graves' Disease Accompanied by Isolated Hypogonadotropic Hypogonadism
Hyon-Seung Yi, Ji Min Kim, Sang Hyeon Ju, Younghak Lee, Hyun Jin Kim, Koon Soon Kim
Journal of Bone Metabolism.2016; 23(1): 40. CrossRef - Severity and pattern of bone mineral loss in endocrine causes of osteoporosis as compared to age-related bone mineral loss
D Dutta, P Dharmshaktu, A Aggarwal, K Gaurav, R Bansal, N Devru, UC Garga, B Kulshreshtha
Journal of Postgraduate Medicine.2016; 62(3): 162. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Bone health in hypogonadal men
Michael S. Irwig
Current Opinion in Urology.2014; 24(6): 608. CrossRef - Testosterone Replacement Therapy and Bone Mineral Density in Men with Hypogonadism
Se Hwa Kim
Endocrinology and Metabolism.2014; 29(1): 30. CrossRef
- Testosterone supplementation and bone parameters: a systematic review and meta-analysis study

- Androgen Receptor Gene CAG Repeat Polymorphism and Effect of Testosterone Therapy in Hypogonadal Men in Korea.
- Min Joo Kim, Jin Taek Kim, Sun Wook Cho, Sang Wan Kim, Chan Soo Shin, Kyong Soo Park, Seong Yeon Kim
- Endocrinol Metab. 2011;26(3):225-231. Published online September 1, 2011
- DOI: https://doi.org/10.3803/EnM.2011.26.3.225
- 2,018 View
- 25 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
As the number of CAG repeats in the androgen receptor (AR) gene increases, transcriptional activities decrease and the effects of testosterone decline. In this study, we evaluated the importance of the CAG repeat polymorphism in regard to the effect/safety of testosterone therapy in hypogonadal Korean men. METHODS: The number of CAG repeats was determined in 42 hypogonadal men who underwent testosterone therapy for more than 24 months between December 1999 and August 2007. Body mass index, lean body mass, body fat, bone mineral density, type I collagen N-telopeptide (NTx), osteocalcin, lipid profile, hematocrit and PSA levels prior to and after 24 months of testosterone therapy were identified in our medical record review. RESULTS: Twenty-four months of testosterone therapy increased lean body mass, hematocrit, and PSA levels and reduced body fat, NTx, and HDL cholesterol levels. The mean number of CAG repeats in the AR gene was 23 +/- 3 (range, 15-29) in hypogonadal Korean men. The number of CAG repeats was not found to be associated with changes in lean body mass, body fat, NTx, HDL cholesterol, hematocrit, or PSA levels during testosterone therapy. CONCLUSIONS: No association between the number of CAG repeats in the AR gene and the effect/safety of testosterone therapy was detected in hypogonadal Korean men. -
Citations
Citations to this article as recorded by- Androgen Receptor CAG Repeat Length as a Risk Factor of Late-Onset Hypogonadism in a Korean Male Population
Jong Wook Kim, Young Dae Bae, Sun Tae Ahn, Jin Wook Kim, Je Jong Kim, Du Geon Moon
Sexual Medicine.2018; 6(3): 203. CrossRef - Positive Correlation between Androgen Receptor CAG Repeat Length and Metabolic Syndrome in a Korean Male Population
Jong Wook Kim, Young Dae Bae, Sun Tae Ahn, Jin Wook Kim, Je Jong Kim, Du Geon Moon
The World Journal of Men's Health.2018; 36(1): 73. CrossRef - Genome-Based Approaches in Endocrinology and Metabolism: From Clinical and Research Aspects
Sihoon Lee
Endocrinology and Metabolism.2011; 26(3): 208. CrossRef
- Androgen Receptor CAG Repeat Length as a Risk Factor of Late-Onset Hypogonadism in a Korean Male Population

- Transcriptional Regulation of the Estrogen Receptor alpha Gene by Testosterone in Cultures of Primary Rat Sertoli Cells.
- Sang Kuk Yang, Kyung Ah Yoon, Eun Jin Yun, Kyoung Sub Song, Jong Seok Kim, Young Rae Kim, Jong Il Park, Seung Kiel Park, Byung Doo Hwang, Kyu Lim
- J Korean Endocr Soc. 2006;21(2):106-115. Published online April 1, 2006
- DOI: https://doi.org/10.3803/jkes.2006.21.2.106
- 1,640 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
We wanted to identify the presence of the estrogen receptor (ER) alpha in Sertoli cells and gain insight on the regulation of the ER alpha gene expression by testosterone in Sertoli cells. The transcriptional regulation of the ER alpha gene was investigated in primary Sertoli cell cultures by in situ hybridization and reverse transcription-polymerase chain reaction (RT-PCR). METHODS: Primary Sertoli cell culture was performed. The expression levels of ER alpha and ER beta mRNA in Sertoli cells were detected by Northern blot, RT-PCR, immunocytochemistry and in situ hybridization. RESULTS: The ovary, testis and epididymis showed a moderate to high expression of ER alpha while the prostate, ovary and LNCap cells showed the ER beta expression. ER alpha mRNA and protein were detected in the germ cells and Sertoli cells by in situ hybridization and immunocytochemistry. The level of ER alpha mRNA was gradually decreased in a time-dependent manner after testosterone treatment, and the changes of ER alpha mRNA were dependent on the concentration of testosterone. Androgen binding protein and testosterone-repressive prostate message-2 (TRPM-2) mRNA were reduced at 24 hour by estradiol, while the transferrin mRNA was not affected. ER alpha mRNA was strongly detectable in the testes of 7 days-old-rats, but it was gradually decreased from 14 to 21 days of age. The primary Sertoli cells also showed the same pattern. The ER alpha gene expression was also regulated by testosterone in the Sertoli cells prepared from the 14- and 21-day old rats. CONCLUSIONS: These results suggest that ER alpha is transcriptionally regulated by testosterone and it may play some role in the Sertoli cells.

- The Effects on Visceral Fat and Cardiovascular Risk Factors of Testosterone Replacement in Secondary Hypogonadal Men.
- Eui Sil Hong, Sung Yeon Kim, Young Ju Choi, Sang Wan Kim, Chan Soo Shin, Kyong Soo Park, Hak Chul Jang, Seong Yeon Kim, Bo Youn Cho, Hong Kyu Lee
- J Korean Endocr Soc. 2005;20(3):252-260. Published online June 1, 2005
- DOI: https://doi.org/10.3803/jkes.2005.20.3.252
- 1,840 View
- 19 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Increased body fat, abdominal obesity and insulin resistance are important clinical features in hypogonadal men. Several studies have demonstrated that a low testosterone concentration in men is associated with coronary heart disease, visceral obesity and insulin resistance. In this study, the effects of testosterone replacement therapy on the abdominal visceral fat and cardiovascular risk factors in hypogonadal men were investigated. METHODS: We selected 26 men with secondary hypogonadism (mean serum testosterone+/-SD 0.39+/- 0.57ng/mL), who were then treated with testosterone for 12 months. We measured the body composition, including the abdominal visceral fat area by abdominal CT at the L4 level, both before and 12 months after treatment, and the lipid profile, fasting plasma insulin, HOMA-IR and the serum homocysteine, CRP and IL-6 before and 6, 12 months after treatment. RESULTS: With respect to the body composition, the lean body mass had significantly increased 12 months after treatment(P= 0.002), but there were no significant changes in the body fat mass and abdominal visceral fat area. There was a trend toward a decreased fasting plasma insulin and HOMA-IR, but this did not reach statistical significance. The total cholesterol had decreased significantly at 12 months(P=0.04) and the HDL cholesterol decreased significantly over the course of study(P=0.02). There were no significant changes in the serum homocysteine, CRP and IL-6 after treatment. CONCLUSIONS: After 12 months testosterone replacement therapy in the 26 men with hypogonadism, the lean body mass had increased significantly, but there was no significant change on the abdominal visceral fat during the treatment period. Testosterone replacement had deleterious effect on HDL cholesterol, but not significant effects on insulin resistance and the serum homocysteine, CRP and IL-6. These results suggest that testosterone replacement therapy may have a few adverse effects on cardiovascular diseases in hypogonadal men. However, it will be necessary to examine the long-term effects of testosterone replacement on the incidence of cardiovascular events as well as the cardiovascular risk factors in men with hypogonadism -
Citations
Citations to this article as recorded by- The Association of Level of Testosterone and Parameters of Obesity
Chong Hwa Kim
The Korean Journal of Obesity.2015; 24(1): 28. CrossRef - The Relationship between Various Obesity Indices and Level of Male Hormone according to Different Age Groups
Yoo-Jung Lee, Hyeon-Ju Kim, Mi-Hee Kong
The Korean Journal of Obesity.2014; 23(4): 245. CrossRef - Androgen Receptor Gene CAG Repeat Polymorphism and Effect of Testosterone Therapy in Hypogonadal Men in Korea
Min Joo Kim, Jin Taek Kim, Sun Wook Cho, Sang Wan Kim, Chan Soo Shin, Kyong Soo Park, Seong Yeon Kim
Endocrinology and Metabolism.2011; 26(3): 225. CrossRef - Effects of Androgen on the Cardiovascular System in the Aging Male
Jin Wook Kim, Je Jong Kim, Du Geon Moon
Korean Journal of Andrology.2011; 29(1): 10. CrossRef
- The Association of Level of Testosterone and Parameters of Obesity

- Relationship between Adiponectin, Leptin and Body Fat in Men with Hypogonadism Before and After Testosterone Treatment.
- Sang Wan Kim, Joon Ku Kang, Do Joon Park, Chan Soo Shin, Kyung Soo Park, Seong Yeon Kim, Bo Youn Cho, Hong Kyu Lee
- J Korean Endocr Soc. 2004;19(5):473-484. Published online October 1, 2004
- 1,102 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Testosterone replacement therapy in men with hypogonadism improves sexual function, decreases body fat, and increases the mass and function of lean muscle. These beneficial effects of testosterone replacement therapy are accompanied by slight lowering of the high density lipoprotein (HDL) cholesterol levels, increase in the hematocrit/hemoglobin ratio and size of the prostate gland. It is presently unknown whether the effect of testosterone on body fat could also reduce the risk of atherosclerotic disease associated with obesity. We investigated the relationship between body fat and blood leptin and adiponectin levels to elucidate the effect of testosterone on body fat metabolism, as well as the effect of testosterone on lipid and bone metabolism. METHODS: We selected 28 men, who were hypogonadal (mean serum testosterone+/-SD, 22.3+/-35.3 ng/dL) due to an organic disease, and them with oral testosterone (testosterone undecanoate) for 12 months. We measured the body composition, serum leptin, plasma adiponectin, biochemical bone markers, bone mineral density, prostate-specific antigen, and serum lipids before and 3, 6 and 12 months after treatment. We analyzed the relationship between body fat and blood leptin and adiponectin levels. RESULTS: The mean serum testosterone concentration reached the subnormal range after 6 months of treatment, which remained for the duration of treatment. The fat mass decreased and muscle mass increased, not within the first 6 months, but principally within 12 months (p<0.05). Although the decrease in the serum leptin level was not statistically significant, there were positive correlations between the leptin level and fat mass before and after 6 months of treatment (p<0.05). The plasma adiponectin did not increase or correlate with body fat parameters. The bone mineral densities of the lumbar spine (L2-L4) and femoral neck did not increased, but the serum osteocalcin and urine N-telopeptide were significantly decreased (p<0.05 and <0.01, respectively). The HDL-cholesterol decreased, principally within the first 6 months (p<0.01), but the total and LDL cholesterols, and the triglycerides remained unchanged during the course of treatment. There was also no change in prostate-specific antigen. CONCLUSION: Twelve months of oral testosterone replacement in men with hypogonadism improved body composition and bone metabolism, but demonstrated subnormal serum testosterone levels, had no effect on the leptin and adiponectin levels and decrease in HDL-cholesterol levels. It will be necessary to examine the long-term effects of testosterone replacement on the incidence of cardiovascular events as well as cardiovascular risk factors in men with hypogonadism

- Clonical Experience on Non-Scrotal Testosterone Transdermal Patch in the Middle Aged Male.
- Young Chan Kim, Jong Ho Park, Suk Ki Lee, Young Jin Lee, Chul Young Bae, Yong Wook Cho, Myung Seo Kang, Jung Hoon Kim
- J Korean Endocr Soc. 1999;14(1):102-121. Published online January 1, 2001
- 1,013 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
To evaluate metabolic effects of testosterone on whole bodily systems, non-scrotal testosterone transdermal patch was given to middle aged men. METHODS: Sixteen impotent patients with serum testosterone levels between 300 and 500 ng/dL, were recruited for 6 month of treatment with non-scrotal testosterone transdermal delivery system, and six patients dropped during the study. All patients have a non-organic impotence (mean age:48 +/- 7). After 1 month placebo patch running period, patients were given 1 or 2 patches. The parameters were evaluated at each stage; before treatment, after placebo patch, and after testosterone patch for 3 months and 6 months. The evaluation parameters included body weight, blood pressure, heart rate, body mass index (BMI), body fat, haemoglobin, haematocrit, RBC, lipid profiles, Prostatic Specific Antigen (PSA), Transrectal Ultrasonography (TRUS), International Prostatic Symptom Score (IPSS), bone markers such as osteocalcin and Deoxypyridinoline (dPyr), Bone Mineral Density (BMD), psychological evaluation with Questionnaire and hormones such as cortisol, Dehydroepiandrosterone sulfate (DHEA-S), Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), prolactin, testosterone and Sex Hormone Binding Globulin (SHBG). Sexual functions were evaluated by means of sexual Questionnaire which has grade systems (high grade means good response) on each domain. RESULTS: Hormonal, hematopoietic, lipid and prostatic parametem were not changed with statistical insignificance. There were no significant changes in BMD. But mean osteocalcin values increased about 31.5% (p<0.05). Bone resorption marker, D-Pyr values were also decreased significantly about 18.6% after 4 montbs treatment, but such changes were not shown after 6 months. Tendencies of improvement in all domains of Sexual Questionnaire were noticed, even though they were not statistically significant except in frequency of coitus and satisfaction with ejaculation (p<0.05), CONCLUSION: Decreased bone resorption was noticed while persistent increased bone formation occurred after 4 months treatment of testosterone. Testosterone supplementation has a beneficial effects on mood and sexual function in the impotent patients with lower borderline testosterone level. And it can be concluded that 6 months testosterone treatment dose not produce any adverse reactions on bodily system.

- A Study About Correlation Between Urinary Androgen Metabolites and Bone Mineral Density in Psstmenopausal Women.
- Kyoung Rae Kim, Ji Hyun Lee, Sung Kil Lim, Young Jun Won, Seok Ho Kwon, Bong Soo Cha, Young Duk Song, Hyun Chul Lee, Kap Bum Huh, Su Youn Nam, Bong Chul Jung
- J Korean Endocr Soc. 1997;12(3):450-461. Published online January 1, 2001
- 1,142 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Positive correlations between bone mass and androgen levels have been observed in premenopausal and postmenopausal women as well as in men. Androgen production was decreased in women with osteoporosis compared to that in age-matched controls. We hypothesized that androgen metabolism might be also deranged in osteoporosis. To clarify our hypothesis, we investigated the relationship between urinary metabolites of androgen and bone mineral density (BMD) in Korean postmenopausal osteoporotics. METHODS: We examined the anthropometry and bone turnover marker in 67 postmenopausal women. BMD was measured by dual energy X-ray absorptiometry (DEXA). Serurn levels of estrone, estradiol, free testosterone were measured by radioirnmunoassay and serum level of sex hormone binding globulin (SHBG) was measured by two site immunoradiometric assay. The urinary metabolites of androgen were determined by gas chromatography-mass spectrometry (GC-MS) at Korean Institute of Science and Technology Doping Control Center. RESULTS: 1. Spinal BMD had a positive correlation with height (r 0.3049, p<0.05), weight (r=0.4114, p<0.001) and body mass index (BMI, r=0.2638, p<0,05). 2. Spinal and femoral neck BMD had no correlation with serum levels of estrone, estradiol and ten major urinary metabolites of androgen, but serum free testosterone had positive correlation with spinal BMD (r=0.3622, p<0.01) and SHBG had negative correlation with femoral neck BMD (r=-0.2625, p< (0.05). 3. Serum free testosterone in osteoporotics was lower than non-osteoporotics with spinal BMD (p<0.05) and SHBG in patients with osteopenia was higher than non-osteopenic subjects with femoral neck BMD (p <0.05). 4. In multiple stepwise regression analysis, weight and serum free testosterone were statistically significant for spinal BMD (R =0.3072). As for femoral neck BMD, weight was the independent determinant (R 0.1307). 5. Serum level of osteo#ealcin and urinary deoxypyridinoline/creatinine had a positive correlation with urinary 11-ketoandrosterone (p<0.05). SHBG was positive correlation with osteocalcin (r=0.3190, p<0.05). 6. Serum free testosterone (r=-0.2740, p<0.05) decreased with aging. CONCLUSION: Our data suggest that androgen metabolism is not deranged in osteoporotics, but serum free testosterone is important than estrogen on postmenopausal osteoporosis after 5-10 years menopause.


 KES
KES

 First
First Prev
Prev



