Articles
- Page Path
- HOME > Endocrinol Metab > Volume 34(3); 2019 > Article
-
Review ArticleA Review of the Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors on Lean Body Mass in Humans
-
Jack Alistair Sargeant1,2*
 , Joseph Henson1,2*
, Joseph Henson1,2* , James Adam King2,3, Thomas Yates1,2, Kamlesh Khunti1,4, Melanie Jane Davies1,2
, James Adam King2,3, Thomas Yates1,2, Kamlesh Khunti1,4, Melanie Jane Davies1,2
-
Endocrinology and Metabolism 2019;34(3):247-262.
DOI: https://doi.org/10.3803/EnM.2019.34.3.247
Published online: September 26, 2019
1Diabetes Research Centre, University of Leicester, Leicester, UK.
2NIHR Leicester Biomedical Research Centre, University Hospital of Leicester NHS Trust and the University of Leicester, Leicester, UK.
3National Centre for Sport and Exercise Medicine, Loughborough University, Loughborough, UK.
4NIHR Collaborations for Leadership in Applied Health Research and Care (CLAHRC), Leicester, UK.
- Corresponding author: Melanie Jane Davies. Diabetes Research Centre, Leicester General Hospital, Gwendolen Rd, Leicester, LE5 4PW, UK. Tel: +44-116-258-6481, melanie.davies@uhl-tr.nhs.uk
- *These authors contributed equally to this work.
Copyright © 2019 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Weight loss is an important goal in the management of several chronic conditions, including type 2 diabetes mellitus, and pharmacological therapies that aid weight loss are appealing. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) are novel glucose-lowering therapies that have been shown to induce clinically significant reductions in body weight. However, this weight loss may not be attributed solely to fat mass (FM). Given the importance of skeletal muscle and lean body mass (LBM) on cardio-metabolic health and physical function, we reviewed the available literature reporting the effects of GLP-1RAs and SGLT2is on body composition. Results demonstrate that, in most circumstances, the weight loss associated with both therapies predominantly comprises a reduction in FM, although significant heterogeneity exists between studies. In over half of the studies identified, the proportion of LBM reduction ranged between 20% and 50% of total weight lost, which is consistent with diet-induced weight loss and bariatric surgery. No clear differences existed between GLP-1RAs and SGLT2is. Consequently, the loss of LBM and skeletal muscle associated with weight loss induced by GLP-1RAs and SGLT2is warrants attention. Strategies to preserve skeletal muscle and improve physical function, for example through structured exercise, are of great importance.
- Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) are novel glucose-lowering therapies that are prominent in the most recent guidelines for the management of hyperglycaemia in type 2 diabetes mellitus (T2DM) and recommended as preferred second-line pharmacological therapies after metformin, alongside ongoing lifestyle management (predominantly diet and physical activity) [1]. They are particularly recommended when, in addition to glucose lowering, there is a compelling need to reduce body weight (BW) [1].
- In large cardiovascular outcome trials, several GLP-1RAs (liraglutide, semaglutide, exenatide once-weekly, albiglutide and dulaglutide) have shown compelling cardiovascular protection in the form of reduced risk of major adverse cardiovascular events, along with some renal benefits [23]. A number of SGLT2is (canagliflozin, dapagliflozin, and empagliflozin) have also shown cardiovascular benefits and greater renal protection, in addition to reduced hospitalization for heart failure [456]. Both classes of therapy also elicit clinically relevant weight loss [1], with liraglutide gaining an independent license (at a higher dose of 3 mg) for use in obesity management. Importantly, the glucose-dependent mechanisms of action of both therapies mean that their beneficial effects come with low risk of hypoglycaemia, particularly when not used with sulphonylureas or insulin [1].
- The dual effects of weight loss and improved glycaemic control associated with GLP-1RAs and SGLT2is are appealing. Obesity and T2DM are intrinsically linked, and each are associated with increased risk of multiple comorbidities [7]. Notably, however, previous studies demonstrate that when BW is reduced through dietary energy restriction, not all of the resulting weight loss can be attributed to fat mass (FM), with approximately 25% to 33% estimated to comprise of reductions in lean body mass (LBM) [89]. This is important because LBM (predominantly comprised of skeletal muscle) has several important functions. It acts as a primary site of glucose disposal (with lower skeletal muscle mass contributing to poorer glycaemic control [10]), and is a strong determinant of resting metabolic rate; and thus loss of skeletal muscle with weight loss may predispose individuals to a greater chance of weight regain [11].
- Lower muscle mass and function, associated with impaired muscular strength and endurance, also result in a higher risk of falls, hospitalisation and physical frailty [1213]. The important association between T2DM and frailty is becoming increasingly recognised [14], with frailty up to five times more likely in individuals with T2DM compared to those without [14151617]. T2DM represents a state of accelerated metabolic ageing, and some of this frailty risk may be underpinned by an increased loss of LBM and function [181920].
- Consequently, whilst weight loss is an important goal in the management of several obesity-associated comorbidities, including T2DM, and pharmacological therapies that support such weight loss are appealing, it is important to understand the impact of these therapies on body composition. The aim of this narrative review is to describe the effects of GLP-1RAs and SGLT2is upon body composition, with a particular focus on LBM and skeletal muscle.
INTRODUCTION
- To interpret changes in body composition, it is important to understand the basic theory and appreciate the advantages and disadvantages of different measurement methods.
- Broadly, body composition measurement divides the body into “compartments” on the basis of differing physical properties, which commonly include FM, fat free mass (FFM), LBM, skeletal muscle mass, bone mineral content and total body water (TBW). Definitions of these terms are provided in Table 1.
- Direct methods of body composition include computed tomography (CT) and magnetic resonance imaging (MRI) [21]. Whilst these techniques have the highest accuracy, they are also expensive, and not widely used in clinical practice. More common measures, which estimate body composition indirectly, include bioelectrical impedance analysis (BIA), air displacement plethysmography (ADP) and dual-energy X-ray absorptiometry (DXA).
- BIA uses a small alternating current to measure body impedance. Estimates of body composition are provided through in-built equations based on assumed impedance of different biological tissues [22]. BIA is quick, easy and relatively inexpensive, but less precise than other available methods and influenced by factors such as hydration status.
- ADP provides an estimate of body composition by combining body volume, measured using the displacement of air within a sealed measurement chamber, with BW to calculate body density. Body density is then used to estimate body composition using pre-defined formulae [23]. ADP is relatively quick to perform and non-invasive, but requires operation by trained personnel to avoid undue error.
- DXA remains a prominent and preferred technique in clinical trials, balancing a high level of accuracy with comparatively lower costs than MRI and CT. DXA provides whole-body and regional estimates of FM, FFM, LBM, and bone mineral content [21]; using a small safe dose of radiation. It is relatively quick and provides much greater accuracy than BIA.
DEFINITIONS AND MEASUREMENT OF BODY COMPOSITION
- We searched for published studies reporting data on the effects of GLP-1RAs and SGLT2is on body composition using the online database, PubMed, from inception to August 2019, using search terms related to GLP-1RAs, SGLT2is, weight loss and body composition. We included studies using DXA, BIA, ADP, MRI, or CT to measure body composition in any human study population before and after 2 or more weeks of GLP-1RA and/or SGLT2i therapy. Where appropriate, the following assumptions were used during data extraction:
- (1) Where data were reported for mean total BW and mean body fat percentage, we estimated mean FM as; BW divided by 100, multiplied by body fat percentage.
- (2) Where mean BW and mean FM were available (including when FM was estimated as above) we estimated FFM as; BW minus FM.
- We used FFM to cautiously infer changes in LBM, with recognition of their subtle differences as outlined in Table 1. Due to variation in techniques used and methods of reporting, we did not sum different body compartments to estimate total LBM, FFM or BW change.
METHODS
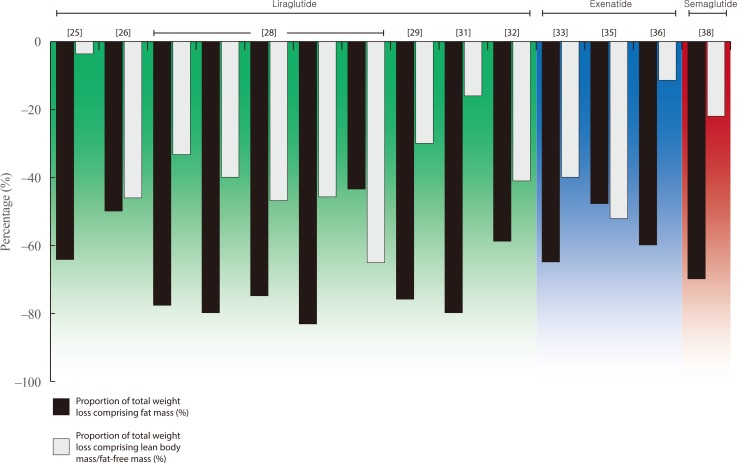
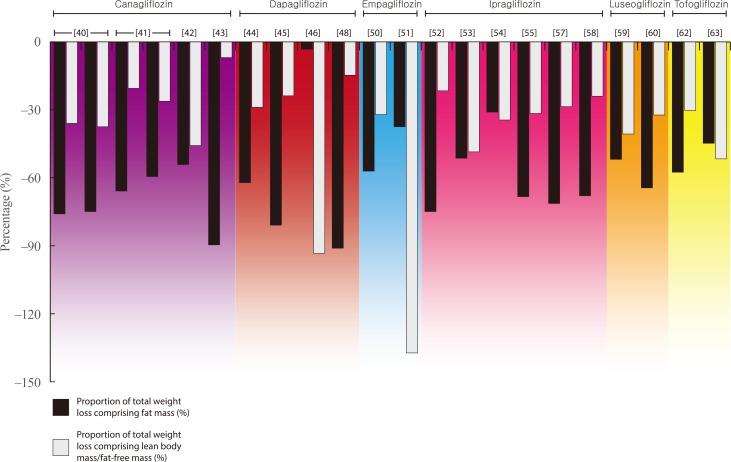
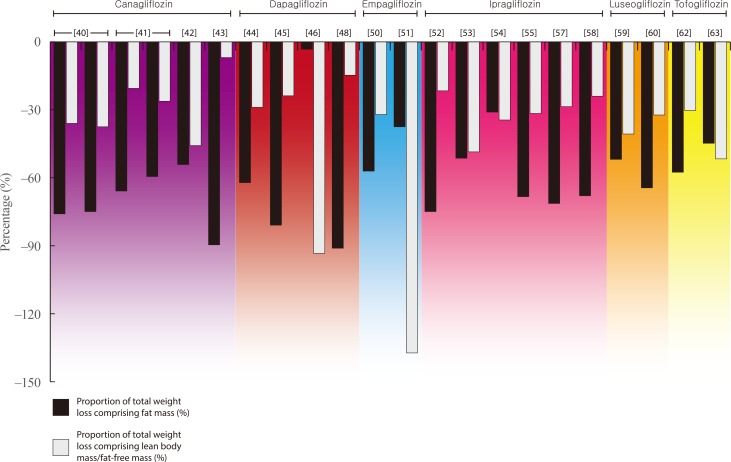
- Details of all eligible studies identified in our search can be found in Tables 2,3,4 [242526272829303132333435363738394041424344454647484950515253545556575859606162636465666768]. The following paragraphs provide an overview of the findings for each individual therapy within both drug classes. For ease of reading, medication doses are not included in text unless providing specific context or comparison. Figs. 1, 2 present the relative proportion of weight loss that could be attributed to FM and LBM/FFM with GLP-1RA and SGLT2i therapy, respectively. Our literature search yielded no data for lixisenatide, albiglutide, or ertugliflozin.
- Glucagon-like peptide-1 receptor agonists
- We identified 17 studies (two within a single manuscript) reporting changes in body composition with liraglutide (n=10), exenatide (n=5), semaglutide (n=1), or dulaglutide (n=1). Thirteen of these studies used DXA to measure body composition, two used BIA alone, one used a combination of BIA and CT, and one used ADP.
- Four manuscripts, containing data from five studies, reported relative contributions of LBM/FFM to total weight loss with liraglutide therapy, ranging from 30% to 47% [26,28,29,32]. This included data from separate sub-studies contained within two phase 3 trials in the Liraglutide Effect and Action in Diabetes (LEAD) programme. In LEAD 2, there was a step-wise increase in the magnitude of both total weight loss (0.9, 2.0, and 3.2 kg) and the relative contribution of LBM (33%, 40%, and 47%) when liraglutide was prescribed at 0.6, 1.2, and 1.8 mg once-daily for 26 weeks [28]. In LEAD 3, 1.2 mg liraglutide elicited 2.4 kg weight loss after 52 weeks, with 46% comprising LBM. Liraglutide 1.8 mg resulted in a similar magnitude of weight loss at 52 weeks (2.3 kg) but the relative contribution of LBM was considerably higher (65.2%). Two further trials using DXA report similar results in individuals with obesity and T2DM (30% of 5.0 kg weight loss over 12 weeks) [29] and polycystic ovarian syndrome (PCOS; 46% of 5.2 kg weight loss over 26 weeks) [26]. Furthermore, a single study, using BIA, reported 5.6 kg weight loss after 24 weeks of liraglutide therapy in individuals with type 1 diabetes mellitus, of which 41% constituted LBM [32].
- Conversely, two studies report a comparatively smaller contribution of LBM/FFM to total weight loss with 24 weeks liraglutide treatment in overweight/obese individuals with T2DM (16% of 2.5 kg weight loss with 3 mg once-daily), and those with T2DM and non-alcoholic fatty liver disease (NAFLD) (4% of 5.6 kg weight loss) [2531]. Furthermore, two studies report no change or marginal increases in LBM after 24 and 8 weeks of treatment, respectively [2730]. The final manuscript identified reductions in LBM in individuals undergoing liraglutide therapy at 1.2, 1.8, 2.4, and 3.0 mg for 20 weeks, but did not report total BW change for the subset of individuals undergoing body composition assessment [24].
- We identified five studies examining the impact of exenatide on body composition, reporting similar, although more heterogeneous, findings than those for liraglutide. Two studies used DXA to assess changes in body composition with 14 weeks of exenatide treatment in individuals with obesity with or without schizophrenia. These studies reported a mean weight loss of 2.0 and 2.3 kg, respectively, of which the relative contribution of FFM to total weight loss was 40% and 52% [3335].
- A separate study reported 3.5 kg weight loss after 16 weeks of treatment in individuals with overweight/obesity and T2DM and reported that 11.4% of weight loss comprised of LBM [36]. One further study reported a small increase (0.3 kg) in LBM after 52 weeks of treatment in individuals with T2DM [34]. The final study identified (utilising BIA to assess changes in body composition over 12 weeks in individuals with obesity and T2DM), did not report changes in LBM, but reported a loss of FM that was greater than the total weight loss induced by exenatide, and a 1.3 kg increase in skeletal muscle [37].
- The only study administering semaglutide reported 5.0 kg weight loss after 12 weeks of treatment in individuals with obesity [38]. Of this weight loss, 20% was estimated, using ADP, to consist of LBM.
- A single study of dulaglutide reported data from a case series of five individuals assessed before and after 12 weeks of treatment, using BIA [39]. This manuscript did not report total weight loss, but outlined 0.1 and 0.2 kg loss of LBM and skeletal muscle, respectively, in comparison to 1.9 kg loss of FM.
- Sodium-glucose cotransporter 2 inhibitors
- We identified 27 studies examining changes in body composition with SGLT2i therapy (canagliflozin=4, dapagliflozin=6, empagliflozin=2, ipragliflozin=7, luseogliflozin=2, tofogliflozin=3, and various=3). Compared to the studies identified for GLP-1RAs, there was much greater use of BIA (17 studies), with the remaining 10 using DXA.
- The most robust evidence of body composition changes with SGLT2i comes from a pair of randomised controlled trials (RCTs) reporting data on the effects of canagliflozin on DXA-derived body composition in individuals with T2DM [4041]. After 26 weeks, canagliflozin resulted in 2.5 and 3.2 kg weight loss at 100 and 300 mg, respectively, with the relative contribution of weight loss attributed to LBM being 36.0% and 37.5% [40]. At 52 weeks, the magnitude of weight loss was numerically greater than at 26 weeks and similar with both doses (−4.4 and −4.2 kg), but the relative contribution attributed to LBM was lower (20.5% and 26.2%, respectively) [41]. A third study using DXA, resulted in 2.4 kg weight loss in individuals with T2DM, of which almost 46% was LBM [42].
- A single study, using BIA to assess body composition changes over a longer period of time (1 year), reported the contribution of LBM to a total 2.9 kg weight loss in individuals with T2DM and NAFLD to be lower than the studies above, at just 6.9% [43].
- Two studies report changes in body composition with dapagliflozin therapy using DXA, providing similar findings to those for canagliflozin. In a sub-study contained within a large multi-site RCT, total weight loss after 104 weeks of dapagliflozin therapy in 69 participants with overweight/obesity and T2DM was 4.5 kg, of which 28.9% consisted of LBM [45]. A smaller, shorter study reported a lower magnitude of weight loss over 12 weeks (2.1 kg) but with a similar relative contribution of LBM (23.8%) [45].
- Three further studies, using BIA, show heterogeneous findings. In a non-randomised trial in which 50 individuals with T2DM were prescribed dapagliflozin or non-SGLT2i therapies for 6 months, the dapagliflozin group lost a mean 3.4 kg of BW, of which 15% constituted LBM [48]. In contrast, a 12-week RCT reported that almost all (94%) of the 3.1 kg weight loss elicited with dapagliflozin was LBM [46], whilst a small cohort study in 11 individuals with T2DM and nonalcoholic steatohepatitis, reported a 1.2 kg increase in LBM after 24 weeks of treatment, despite 3.8 kg weight loss [49].
- Only two studies report the impact of empagliflozin on body composition. One of these provides DXA-derived data from a sub-study nested within the global EMPA-REG H2H-SU trial; a randomised head-to-head trial conducted as part of the empagliflozin phase 3 programme, which compared empagliflozin with the sulphonylurea glimepiride as second line therapy alongside metformin in individuals with T2DM [50]. Using the weight loss observed in the entire population after 104 weeks (2.8 kg), it was estimated that approximately one-third (32.1%) of weight loss elicited in this trial comprised of FFM.
- A separate study using BIA to assess changes with empagliflozin in women with PCOS reported a loss of FFM (1.1 kg) which was greater than the total weight loss elicited (0.8 kg) [51].
- Of the seven studies identified for ipragliflozin, six reported that the contribution of LBM/FFM to total weight loss elicited ranged from 22% to 49% [525354555759]. All of these studies were in individuals with T2DM, and included two studies using DXA, reporting 22% and 49% of 2.8 and 3.5 kg of weight loss over 24 weeks, respectively. The remaining four used BIA and in the largest and longest of these studies (n=217; 104 weeks of treatment), the proportion of weight loss attributed to FFM was approximately one-third (34.5%) [54].
- In the final study identified, 24 weeks of treatment with ipragliflozin resulted in a loss of FM that was greater than the total weight loss in individuals with T2DM and NAFLD, inferring a small gain (0.2 kg) in FFM [56].
- Two studies examining the effects of luseogliflozin on body composition using DXA report similar findings to those outlined above. Both studies were in individuals with T2DM, reporting that 12 and 52 weeks of luseogliflozin therapy elicited 2.7 and 3.1 kg weight loss, respectively. Of this weight loss, 41% and 32% could be attributed to a loss of FFM/LBM [5960].
- Three similar studies, each utilising BIA, report data regarding the impact of tofogliflozin in individuals with T2DM. Two assessed changes over 12 weeks, reporting 3.3 and 2.9 kg weight loss, of which 30% and 52%, respectively, could be attributed to LBM [6263]. The third study reported 2.3 kg weight loss in 17 individuals with T2DM despite an estimated 0.2 kg increase in FFM [61].
- Three cohort studies (two retrospective, one prospective) report data from participants prescribed SGLT2i therapy, with variation in the exact agent prescribed [646566]. Each utilised BIA to assess body composition, but only one study reported changes in both total BW and FFM. In this study, almost half (48%) of the 5.2 kg weight loss elicited by dapagliflozin (n=10) or canagliflozin (n=7), in individuals with overweight/obesity, T2DM and NAFLD, could be attributed to FFM [64].
- Dual therapy of GLP-1RAs and SGLT2is in combination
- We identified two studies reporting the effect of GLP-1RAs and SGLT2is in combination. In the first, individuals with obesity and pre-diabetes mellitus were randomised to dapagliflozin-plus-exenatide or placebo for 24 weeks, with an open-label extension (during which all participants underwent combined therapy) for a further 28 weeks [67]. Weight loss after 52 weeks was 5.7 kg, with MRI demonstrating reductions in both adipose tissue and lean tissue volumes (5.3 and 1.4 L, respectively). The second study, using BIA, reported 2.9 kg weight loss after 52 weeks of treatment with luseogliflozin-plus-liraglutide, in individuals with T2DM, of which 14% comprised of LBM [68].
RESULTS
Liraglutide
Exenatide
Semaglutide
Dulaglutide
Canagliflozin
Dapagliflozin
Empagliflozin
Ipragliflozin
Luseogliflozin
Tofogliflozin
Various
- Our review demonstrates that, in most circumstances, the weight loss associated with GLP-1RA and/or SGLT2i therapy comprises predominantly of a reduction in FM. Within 53 groups of individuals prescribed GLP-1RA, SGLT2i or combined therapy, only five groups saw a loss of LBM, FFM, or skeletal muscle mass (depending on reporting) that was greater than FM lost. Thus, in most cases, body composition (i.e., the ratio of FM to FFM/LBM) was more favorable after treatment than before.
- However, out of 43 groups in which it was possible to estimate the proportion of weight loss that could be attributed to LBM/FFM, in 27 groups this proportion of LBM lost ranged from 20% to 50%. Six groups saw a loss of LBM, FFM, or skeletal muscle but with a smaller relative contribution to total weight loss (0% to 19%), whilst another six reported an increase in LBM, FFM, or skeletal muscle mass. These results are in accordance with studies of diet-induced weight loss and bariatric surgery, which have reported similar proportions of LBM/FFM (approximately 25% to 40%) within total weight loss elicited [89697071].
- There were no clear differences between GLP-1RAs and SGLT2is in the magnitude of weight loss that could be attributed to LBM/FFM. However, it should be noted that the number of studies identified for each class of therapy was relatively small, and these were divided further between multiple therapies within each class. There was also heterogeneity of findings within each drug class and within individual therapies. Reasons for this heterogeneity may include (1) the specific therapy used, (2) the dose or duration of treatment, (3) concomitant therapies alongside GLP-1RA or SGLT2i treatment (e.g., if examined as monotherapy or dual-therapy with other glucose-lowering therapies such as metformin), (4) the clinical diagnoses, background medication usage (i.e., use of sulphonylureas or insulin) and other baseline characteristics of the study population, (5) the technique used to assess body composition (including standardisation procedures prior to measurement), and (6) natural inter-individual variation in responses to weight loss. There are no clear patterns within the data collected in this review, including treatment type, dose or duration and the technique used.
- Although the benefits of weight loss in obesity-associated chronic metabolic disease are well established [7273], these favorable outcomes may be limited by losses in LBM, particularly if this constitutes skeletal muscle. Of the studies identified in this review, 12 reported changes in either LBM or FFM, along with changes in skeletal muscle. In seven of these studies, the loss of skeletal muscle comprised between 55% and 100% of the LBM/FFM lost. In one it was twice as great [40], whilst another reported a loss of skeletal muscle despite an increase in FFM [56]. The mechanism of action of SGLT2i, inducing polyuria alongside glycosuria, means that SGLT2i therapy is also associated with fluid loss [1]. Reductions in TBW may impact on body composition measurement, and it could be speculated that reductions in FFM/LBM with weight loss may reflect reductions in water content. Of the 27 studies identified reporting changes in body composition with SGLT2i therapy, 13 reported changes in TBW, 12 of which reported losses; ranging from 0.2 to 2.4 kg (7% to 100% of total weight loss). It is imperative to stress; however, that water is contained within both adipose- and non-adipose tissues [74], and all of the studies reporting TBW in this review utilised BIA. TBW, as provided by BIA, does not allow distinction between water contained in, and thus lost from, different body compartments (i.e., FM or FFM/LBM). No studies reported change in TBW with GLP-1RA or combination therapy.
- Consequently, although yielding a more favorable body composition, the potential LBM and skeletal muscle loss associated with weight loss induced by GLP-1RAs and SGLT2is warrants attention. A more rapid decline in skeletal muscle and consequential increased risk of sarcopenia is concerning, particularly as individuals prescribed these therapies are usually already vulnerable to an increased risk of physical frailty (i.e., those with T2DM and/or obesity) [75]. In turn, strategies to preserve or increase skeletal muscle and physical function in these individuals (e.g., through structured exercise training), are of importance. It is also important to state that the absolute mass of skeletal muscle is not the only factor to consider, and improving muscular function (strength, endurance, flexibility etc.) remains critical to improve physical function and performance in tasks of daily living; to impact positively on an individual's quality of life.
- Previous studies have shown that markers of physical function (including balance, grip strength, and gait speed) improve following diet-induced weight loss [97677], likely by reducing the biomechanical burden of moving around [78]. However, when diet and exercise are combined, improvements in physical function are greater than that elicited by diet alone, which may be underpinned by preserved or improved skeletal muscle mass and function [979]. Whether the same results are observed when exercise is combined with weight loss elicited by GLP-1RAs and SGLT2i therapy is currently unknown, and requires testing in robust experimental research.
- Collectively, the available evidence suggests that the initiation of GLP-1RA or SGLT2i therapy results in weight loss that is primarily derived of FM. However, this is accompanied by reductions in LBM which are not insignificant and should be considered in parallel to changes in FM and overall BW. Differences between therapies are currently unclear. A substantial proportion of LBM loss may be comprised of skeletal muscle, which may be clinically relevant, particularly in the populations to which these therapies are prescribed. Given the heterogeneity, a more consistent approach to measurement and reporting of body composition in future research would be beneficial. As the prevalence of obesity, physical inactivity and associated co-morbidities, including T2DM, continues to rise, it is imperative to explore strategies to preserve LBM and improve physical function, and particularly their interaction with glucose-lowering strategies that positively impact on weight loss.
CONCLUSIONS
-
Acknowledgements
- This research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre (BRC) and the NIHR Collaboration for Leadership in Applied Health Research and Care–East Midlands (CLAHRC–EM). The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: Melanie Jane Davies has acted as a consultant, advisory board member and speaker for Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, an advisory board member for Servier and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. She has received grants in support of investigator and investigator-initiated trials from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, and Janssen.
Kamlesh Khunti has received honoraria from Abbot, AstraZeneca, Berlin-Chemie AG/Menarini Group, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, and Sanofi, and research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, and Sanofi.
Article information
- 1. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–2498. ArticlePubMedPDF
- 2. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031. ArticlePubMed
- 3. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet 2019;394:121–130. ArticlePubMed
- 4. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. ArticlePubMed
- 5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. ArticlePubMed
- 6. Pollock C, Stefansson B, Reyner D, Rossing P, Sjostrom CD, Wheeler DC, et al. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2019;7:429–441. ArticlePubMed
- 7. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015;38:1161–1172. ArticlePubMed
- 8. Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591–601. ArticlePubMedPMC
- 9. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–1229. ArticlePubMedPMC
- 10. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009;32 Suppl 2:S157–S163. ArticlePubMed
- 11. Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab 2012;97:2489–2496. ArticlePubMedPMCPDF
- 12. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015;14:58–74. ArticlePubMedPDF
- 13. McLeod M, Breen L, Hamilton DL, Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology 2016;17:497–510. ArticlePubMedPMCPDF
- 14. Sinclair AJ, Abdelhafiz A, Dunning T, Izquierdo M, Rodriguez Manas L, Bourdel-Marchasson I, et al. An international position statement on the management of frailty in diabetes mellitus: summary of recommendations 2017. J Frailty Aging 2018;7:10–20. ArticlePubMed
- 15. Morley JE. Frailty: diagnosis and management. J Nutr Health Aging 2011;15:667–670. ArticlePubMedPDF
- 16. Saum KU, Dieffenbach AK, Muller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol 2014;29:171–179. ArticlePubMedPDF
- 17. Morley JE, Malmstrom TK, Rodriguez-Manas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc 2014;15:853–859. ArticlePubMed
- 18. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care 2005;28:2541–2542. ArticlePubMed
- 19. Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–1818. ArticlePubMed
- 20. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–1585. ArticlePubMedPMCPDF
- 21. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. ArticlePubMedPMC
- 22. Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann N Y Acad Sci 1963;110:113–140. ArticlePubMed
- 23. Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr 2002;75:453–467. ArticlePubMedPDF
- 24. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. ArticlePubMedPDF
- 25. Feng WH, Bi Y, Li P, Yin TT, Gao CX, Shen SM, et al. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig 2019;10:399–407.ArticlePubMed
- 26. Frossing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metab 2018;20:215–218. ArticlePubMed
- 27. Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care 2004;27:1915–1921. ArticlePubMed
- 28. Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, During M, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 2009;11:1163–1172. ArticlePubMed
- 29. Li CJ, Yu Q, Yu P, Yu TL, Zhang QM, Lu S, et al. Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 2014;13:36. ArticlePubMedPMC
- 30. Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res 2016;28:1251–1257. ArticlePubMedPDF
- 31. Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence 2016;10:407–413. PubMedPMC
- 32. Dube MC, D'Amours M, Weisnagel SJ. Beyond glycaemic control: a cross-over, double-blinded, 24-week intervention with liraglutide in type 1 diabetes. Diabetes Obes Metab 2018;20:178–184. ArticlePubMed
- 33. Bradley DP, Kulstad R, Racine N, Shenker Y, Meredith M, Schoeller DA. Alterations in energy balance following exenatide administration. Appl Physiol Nutr Metab 2012;37:893–899. ArticlePubMedPMC
- 34. Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–1737. ArticlePubMedPMC
- 35. Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab 2017;19:162–171. ArticlePubMed
- 36. Yin TT, Bi Y, Li P, Shen SM, Wang WM, Jiang C, et al. Effects of exenatide versus insulin glargine on body composition in overweight and obese T2DM patients: a randomized controlled trial. Nutr Metab (Lond) 2018;15:67. ArticlePubMedPMCPDF
- 37. Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short-term exenatide treatment on regional fat distribution, glycated hemoglobin levels, and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab (Seoul) 2016;31:80–85. ArticlePubMedPMC
- 38. Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab 2017;19:1242–1251. ArticlePubMedPMC
- 39. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 2017;47:1206–1211. ArticlePubMed
- 40. Blonde L, Stenlof K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med 2016;128:371–380. ArticlePubMed
- 41. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–950. ArticlePubMed
- 42. Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, et al. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2019;149:140–146. ArticlePubMed
- 43. Inoue M, Hayashi A, Taguchi T, Arai R, Sasaki S, Takano K, et al. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Investig 2019;10:1004–1011.ArticlePubMedPMC
- 44. Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16:159–169. ArticlePubMed
- 45. Kosugi R, Nakatani E, Okamoto K, Aoshima S, Arai H, Inoue T. Effects of sodium-glucose cotransporter 2 inhibitor (dapagliflozin) on food intake and plasma fibroblast growth factor 21 levels in type 2 diabetes patients. Endocr J 2019;66:677–682. ArticlePubMed
- 46. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol 2017;16:42. ArticlePubMedPMCPDF
- 47. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 2019;21:285–292. ArticlePubMed
- 48. Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb 2018;25:467–476. ArticlePubMedPMC
- 49. Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp 2017;87:13–19. ArticlePubMedPMC
- 50. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:691–700. ArticlePubMed
- 51. Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf) 2019;90:805–813. ArticlePubMed
- 52. Inoue H, Morino K, Ugi S, Tanaka-Mizuno S, Fuse K, Miyazawa I, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019;10:1012–1021.ArticlePubMedPMC
- 53. Ohta A, Kato H, Ishii S, Sasaki Y, Nakamura Y, Nakagawa T, et al. Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother 2017;18:1433–1438. ArticlePubMed
- 54. Iemitsu K, Kawata T, Iizuka T, Takihata M, Takai M, Nakajima S, et al. Efficacy and safety of ipragliflozin in patients with type 2 diabetes: ASSIGN-K study. J Endocrinol Metab 2019;9:51–62.Article
- 55. Kato M, Sakai K, Saito K, Tsutsui K, Yamashita S, Kato N. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes receiving conventional therapy: clinical implication of the importance of exercise habits during treatment with ipragliflozin. Diabetol Int 2017;8:275–285. ArticlePubMedPMCPDF
- 56. Miyake T, Yoshida S, Furukawa S, Sakai T, Tada F, Senba H, et al. Ipragliflozin ameliorates liver damage in non-alcoholic fatty liver disease. . Open Med (Wars) 2018;13:402–409. ArticlePubMedPMC
- 57. Osonoi T, Nakamoto S, Saito M, Tamasawa A, Ishida H, Osonoi Y. Efficacy of ipragliflozin as monotherapy or as add-on therapy with other oral antidiabetic medications for treating type 2 diabetes in Japanese patients with inadequate glycemic control: a subgroup analysis based on patient characteristics. J Diabetes Investig 2018;9:341–353.ArticlePubMed
- 58. Yamamoto C, Miyoshi H, Ono K, Sugawara H, Kameda R, Ichiyama M, et al. Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J 2016;63:589–596. ArticlePubMed
- 59. Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol 2017;16:32ArticlePubMedPMCPDF
- 60. Sasaki T, Sugawara M, Fukuda M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig 2019;10:108–117.ArticlePubMed
- 61. Iwahashi Y, Hirose S, Nakajima S, Seo A, Takahashi T, Tamori Y. Evaluation of metabolic parameters and body composition in Japanese patients with type 2 diabetes mellitus who were administered tofogliflozin for 48 weeks. Diabetol Int 2016;8:205–211. ArticlePubMedPMCPDF
- 62. Kamei S, Iwamoto M, Kameyama M, Shimoda M, Kinoshita T, Obata A, et al. Effect of tofogliflozin on body composition and glycemic control in Japanese subjects with type 2 diabetes mellitus. J Diabetes Res 2018;2018:6470137. ArticlePubMedPMCPDF
- 63. Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab 2018;20:1311–1315. ArticlePubMedPMC
- 64. Arase Y, Shiraishi K, Anzai K, Sato H, Teramura E, Tsuruya K, et al. Effect of sodium glucose co-transporter 2 inhibitors on liver fat mass and body composition in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus. Clin Drug Investig 2019;39:631–641.ArticlePubMedPMCPDF
- 65. Kinoshita T, Shimoda M, Sanada J, Fushimi Y, Hirata Y, Irie S, et al. There is a close association between the recovery of liver injury and glycemic control after SGLT2 inhibitor treatment in Japanese subjects with type 2 diabetes: a retrospective clinical study. Diabetes Ther 2018;9:1569–1580. ArticlePubMedPMCPDF
- 66. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 2017;47:1072–1078. ArticlePubMed
- 67. Lundkvist P, Pereira MJ, Katsogiannos P, Sjostrom CD, Johnsson E, Eriksson JW. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab 2017;19:1276–1288. ArticlePubMedPMC
- 68. Seino Y, Yabe D, Sasaki T, Fukatsu A, Imazeki H, Ochiai H, et al. Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52-week, open-label, single-arm study. J Diabetes Investig 2018;9:332–340.ArticlePubMed
- 69. Schneider J, Peterli R, Gass M, Slawik M, Peters T, Wolnerhanssen BK. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis 2016;12:563–570. ArticlePubMed
- 70. Davidson LE, Yu W, Goodpaster BH, DeLany JP, Widen E, Lemos T, et al. Fat-free mass and skeletal muscle mass five years after bariatric surgery. Obesity (Silver Spring) 2018;26:1130–1136. ArticlePubMedPMC
- 71. Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750. ArticlePubMedPDF
- 72. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–551. ArticlePubMed
- 73. Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304. ArticlePubMed
- 74. Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 2014;15:310–321. ArticlePubMedPMC
- 75. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. ArticlePubMed
- 76. Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes 2011;2011:516576. ArticlePubMedPDF
- 77. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr 2015;101:991–999. ArticlePubMedPMCPDF
- 78. Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Kritchevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:80–86. ArticlePubMedPDF
- 79. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985) 2007;102:634–640. ArticlePubMed
References
Relative proportions of fat mass and lean body/fat-free mass within total weight loss elicited by glucagon-like peptide-1 receptor agonist therapy [25262829313233353638].

Relative proportions of fat mass and lean body/fat-free mass within total weight loss elicited by sodium-glucose cotransporter 2 inhibitor therapy [4041424344454648505152535455575859606263].
Definitions of Body Composition Compartments
Studies Reporting Changes in Body Composition with GLP-1RA Therapy
| Study (location) | Study design | No. prescribed GLP-1RA therapy | Population | Dose (all subcutaneous injection) | Duration, wk | Tech. used | Total BW change, kg (% change from baseline) | FM change, kg | LBM change, kg | Skeletal muscle mass change, kg | Total body water change, kg | Proportion of weight loss from FM, % | Proportion of weight loss from LBM, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liraglutide | |||||||||||||
| Astrup et al. (2012) [24] (Europe; multi-site) | Six-arm RCT (sub-study) | 15 | Obesity | 1.2 mg once-daily | 20 | DXA | NR | −6.1 | −0.5 | NR | NR | - | - |
| 13 | 1.8 mg once-daily | NR | −5.9 | −1.5 | NR | NR | - | - | |||||
| 15 | 2.4 mg once-daily | NR | −7.0 | −1.3 | NR | NR | - | - | |||||
| 15 | 3.0 mg once-daily | NR | −6.8 | −1.1 | NR | NR | - | - | |||||
| Feng et al. (2019) [25] (China) | Three-arm RCT | 29 | T2DM and NAFLD | 1.8 mg once-daily (titrated from 0.6 mg as tolerated) | 24 | DXA | −5.6 (−7.4%) | −3.6 | −0.2 | NR | NR | 64.3 | 3.6 |
| Frossing et al. (2018) [26] (Denmark) | Two-arm RCT | 44 | PCOS | 1.8 mg once-daily | 26 | DXA | −5.2 (−5.5%) | −2.6 | −2.4 | NR | NR | 50 | 46.1 |
| Harder et al. (2004) [27] (Denmark) | Two-arm RCT | 21 | T2DM | 0.6 mg once-daily | 8 | DXA | −2.1 (−2.0%) | −1.6 | +0.6 | NR | NR | 76.2 | LBM increased |
| Jendle et al., (2009) [28] (Global; multi-site) | Five-arm RCT Study 1; LEAD 2 (sub-study) | 35 | T2DM | 0.6 mg once-daily (with 1.5–2.0 g metformin) | 26 | DXA | −0.9 (−1.0%) | −0.7 | −0.3 | NR | NR | 77.8 | 33.3 |
| 31 | 1.2 mg once-daily (with 1.5–2.0 g metformin) | −2.0 (−2.3%) | −1.6 | −0.8 | NR | NR | 80 | 40 | |||||
| 37 | 1.8 mg once-daily (with 1.5–2.0 g metformin) | −3.2 (−3.6%) | −2.4 | −1.5 | NR | NR | 75.0 | 46.9 | |||||
| Three-arm RCT Study 2; LEAD 3 (sub-study) | 23 | T2DM | 1.2 mg once-daily | 52 | DXA | −2.4 (−2.6%) | −2.0 | −1.1 | NR | NR | 83.3 | 45.8 | |
| 20 | 1.8 mg once-daily | −2.3 (−2.5%) | −1.0 | −1.5 | NR | NR | 43.5 | 65.2 | |||||
| Li et al. (2014) [29] (China) | Case series (prospective) | 31 | Obesity and T2DM | 1.2 mg once-daily (titrated from 0.6 mg as tolerated) | 12 | DXA | −5.0 (−5.5%) | −3.8 | −1.5 | NR | NR | 76.0 | 30.0 |
| Perna et al. (2016) [30] (Italy) | Case series (retrospective) | 9 | Obesity and T2DM (elderly) | 3 mg once-daily (titrated from 1.2 mg as tolerated) | 24 | DXA | −2.0 (−2.3%) | −1.5 | +0.1a | NR | NR | 75.0 | FFM increased |
| Rondanelli et al. (2016) [31] (Italy) | Case series (prospective) | 28 | Overweight/ obese and T2DM | 3 mg once-daily (titrated from 1.2 mg as tolerated) | 24 | DXA | −2.5 (−2.6%) | −2.0 | −0.4a | NR | NR | 80.0 | 16.0 |
| Dube et al. (2018) [32] (Canada) | Two-arm RCT | 15 | T1DM | 1.8 mg once-daily (titrated from 0.6 mg as tolerated) | 24 | BIA/ CT | −5.6 (−6.3%) | −3.3 | −2.3a | NR | NR | 58.9 | 41.1 |
| Exenatide | |||||||||||||
| Bradley et al. (2012) [33] (USA) | Case series (prospective) | 18 | Obesity | 10 μg twice-daily (titrated from 5 μg as tolerated) | 14 | DXA | −2.0 (−2.0%) | −1.3 | −0.8a | NR | NR | 65 | 40 |
| Bunck et al. (2010) [34] (Sweden) | Two-arm RCT | 29 | T2DM | 20 μg twice-daily (titrated from 5 μg as tolerated) | 52 | DXA | −3.9 (−4.3%) | −2.4 | +0.3 | NR | NR | 61.5 | LBM increased |
| Ishoy et al. (2017) [35] (Denmark) | Two-arm RCT | 20 | Obesity and schizophrenia (treated with anti-psychotic medication) | 2 mg weekly | 14 | DXA | −2.3 (−1.9%) | −1.1 | −1.2a | −0.7 | NR | 47.8 | 52.2 |
| Yin et al. (2018) [36] (China) | Two-arm RCT | 19 | Overweight/ obesity and T2DM | 10 μg twice-daily (titrated from 5 μg as tolerated) | 16 | DXA | −3.5 (−4.3%) | −2.1 | −0.4 | NR | NR | 60 | 11.4 |
| Hong et al. (2016) [37] (Korea) | Case series (prospective) | 32 | Obesity and T2DM | 10 μg twice-daily (titrated from 5 μg as tolerated) | 12 | BIA | −1.0 (−1.4%) | −2.1 | NR | +1.3 | NR | 210 | - |
| Semaglutide | |||||||||||||
| Blundell et al. (2017) [38] (UK) | Randomised, placebo-controlled corss-over trial | 30 | Obesity | 1.0 mg (titrated up from 0.25 mg) | 12 | ADP | −5.0 (−4.9%) | −3.5 | −1.1 | NR | NR | 70.0 | 22.0 |
| Dulaglutide | |||||||||||||
| Seko et al. (2017) [39] (Japan) | Case series (retrospective) | 5 | T2DM and NAFLD | 0.75 mg once-daily | 12 | BIA | NR | −1.9 | −0.1 | −0.2 | −0.1 | - | - |
GLP-1RA, glucagon-like peptide-1 receptor agonist; BW, body weight; FM, fat mass; LBM, lean body mass; RCT, randomised controlled trial; DXA, dual-energy X-ray absorptiometry; NR, not reported; T2DM, type 2 diabetes mellitus; NAFLD, nonalcoholic fatty liver disease; PCOS, polycystic ovarian syndrome; LEAD, Liraglutide Effect and Action in Diabetes phase 3 programme for liraglutide; FFM, fat-free mass; T1DM, type 1 diabetes mellitus; BIA, bioelectrical impedance analysis; CT, computed tomography; ADP, air displacement plethysmography.
aFFM.
Studies Reporting Changes in Body Composition with SGLT2i Therapy
| Study (location) | Study design | No. (in group prescribed SGLT2i therapy) | Population | Dose (all once-daily, oral tablet unless otherwise specified) | Duration | Tech. used | Total BW change, kg (% change from baseline) | FM change, kg | LBM change, kg | Skeletal muscle mass change, kg | Total body water change, kg | Proportion of weight loss from FM, % | Proportion of weight loss from LBM, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Various | |||||||||||||
| Arase et al. (2019) [64] (Japan) | Case series (retrospective) | 17 (DAPA=10; CANA=7) | Overweight, T2DM and NAFLD | DAPA: 5 mg | 24 wk | BIA | −5.2 (−7.1%) | −2.7 | −2.5a | −0.7 | −1.2 | 51.9 | 48.1 |
| CANA: 100 mg | |||||||||||||
| Kinoshita et al. (2018) [65] (Japan) | Case series (prospective) | 156 (n=108 for body composition) (LUSEO=52%; TOFO=29%; IPRA=15%; DAPA=4%) | T2DM | NR | 12 wk | BIA | −2.8 (−3.6%) | NR | NR | −0.1 | NR | - | - |
| Seko et al. (2017) [66] (Japan) | Case series (retrospective) | 24 (n=17 for body composition) (CANA=18; IPRA=6) | T2DM and biopsy-proven NAFLD/NASH | CANA: 100 mg | 24 wk | BIA | NR | −2.4 | −1.1 | −0.6 | −0.8 | - | - |
| IPRA: 50 mg | |||||||||||||
| Canagliflozin | |||||||||||||
| Blonde et al. (2016) [40] Study 2 (Global; multi-site) | Three-arm RCT (sub-study) | CANA 100=63 | T2DM | 100 mg | 26 wk | DXA | −2.5 (−2.8%) | −1.9 | −0.9 | NR | NR | 76 | 36.0 |
| CANA 300=71 | 300 mg | −3.2 (−3.4%) | −2.4 | −1.2 | NR | NR | 75 | 37.5 | |||||
| Cefalu et al. (2013) [41]b (Global; multi-site) | Three-arm RCT (sub-study) | CANA 100=111 | T2DM | 100 mg | 52 wk | DXA | −4.4 (−5.2%) | −2.9 | −0.9 | NR | NR | 65.9 | 20.5 |
| CANA 300=102 | 300 mg | −4.2 (−4.9%) | −2.5 | −1.1 | NR | NR | 59.5 | 26.2 | |||||
| Koike et al. (2019) [42] (Japan) | Case series (prospective) | 38 | T2DM | 100 mg | 24 wk | DXA | −2.4 (−3.3%) | −1.3 | −1.1 | NR | NR | 54.2 | 45.8 |
| Inoue et al. (2019) [43] (Japan) | Case series (prospective) | 20 | T2DM and NAFLD | 100 mg | 12 mo | BIA | −2.9 (−3.5%) | −2.6 | −0.2 | −0.2 | −0.2 | 89.7 | 6.9 |
| Dapagliflozin | |||||||||||||
| Bolinder et al. (2014) [44] (Europe; multi-site) | Two-arm RCT | 69 | Overweight/obese with T2DM | 10 mg (added to open-label metformin) | 104 wk | DXA | −4.5 (−4.9%) | −2.8 | −1.3 | NR | NR | 62.2 | 28.9 |
| Kosugi et al. (2019) [45] (Japan) | Case series (prospective) | 26 | T2DM | 5 mg | 12 wk | DXA | −2.1 (−2.9%) | −1.7 | −0.5 | NR | NR | 81 | 23.8 |
| Fadini et al. (2017) [46] (Italy) | Two-arm RCT | 16 | T2DM | 10 mg | 12 wk | BIA | −3.1 (baseline NR) | −0.1 | −2.9a | NR | −2.4 | 3.2 | 93.5 |
| Shimizu et al. (2019) [47] (Japan) | Two-arm RCT | 33 | T2DM and NAFLD | 5 mg | 24 wk | BIA | −2.9 (−3.9%) | NR | NR | −0.9 | −1.1 | - | - |
| Sugiyama et al. (2018) [48] (Japan) | Two-arm non-randomised cohort study | 28 | T2DM | 5 mg | 6 mo | BIA | −3.4 (−4.4%) | −3.1 | −0.5 | −0.2 | NR | 91.2 | 14.7 |
| Tobita et al. (2017) [49] (Japan) | Case series (prospective) | 11 | T2DM and NASH | 5 mg | 24 wk | BIA | −3.8 (−4.8%) | −6.1 | 1.2 | 0.1 | 1.2 | 160.5 | LBM increased Fluctuation over 24 wk |
| Empagliflozin | |||||||||||||
| Ridderstrale et al. (2014) [50] (Global; multi-site) | Two-arm RCT (sub-study) | 46 | T2DM | 25 mg | 104 wk | DXA | NR (−2.8 kg [–3.4%] in entire EMPA group; n=765) | −1.6 | −0.9a | NR | NR | 57.1 | 32.1c |
| Javed et al. (2019) [51] (UK) | Two-arm RCT | 19 | PCOS | 25 mg | 12 wk | BIA | −0.8 (−0.8%) | −0.3 | −1.1a | NR | −0.8 | 37.5 | 137.5 |
| Ipragliflozin | |||||||||||||
| Inoue et al. (2019) [52] (Japan) | Two-arm RCT | 24 | T2DM (on insulin therapy) | 50 mg (insulin dose reduced by 20%) | 24 wk | DXA | −2.8 (−4.4%) | −2.1 | −0.6 | NR | NR | 75 | 21.6 |
| Ohta et al. (2017) [53] (Japan) | Case series (prospective) | 20 | T2DM | 50 mg | 24 wk | DXA | −3.5 (−4.3%) | −1.8 | −1.7 | NR | NR | 51.4 | 48.6 |
| Iemitsu et al. (2019) [54] (Japan) | Case series (prospective) | 217 | T2DM | 50 mg | 104 wk | BIA | −2.9 (−3.7%) | −1.9 | −1.0a | −0.9 | −0.7 | 31 | 34.0 |
| Kato et al. (2017) [55] (Japan) | Case series (prospective) | 20 | T2DM | 50 mg | 12 wk | BIA | −1.9 (−2.3%) | −1.3 | −0.6a | −0.6 | −0.7 | 68.4 | 31.6 |
| Miyake et al. (2018) [56] (Japan) | Case series (prospective) | 12 | T2DM+NAFLD | 50 mg | 24 wk | BIA | −1.4 (−2.1%) | −1.6 | +0.2a | −0.5 | NR | 114.3 | FFM increased |
| Osonoi et al. (2018) [57] (Japan) | Case series (prospective) | 98 | T2DM | 50 mg | 24 wk | BIA | −2.1 (−3.2%) | −1.5 | −0.6a | NR | NR | 71.4 | 28.6 |
| Yamamoto et al. (2016) [58] (Japan) | Case series (prospective) | 24 | T2DM | 50 mg | 16 wk | BIA | −2.5 (−3.3%) | −1.7 | −0.6 | NR | −0.6 | 68 | 24.0 |
| Luseogliflozin | |||||||||||||
| Bouchi et al. (2017) [59] (Japan) | Case series (prospective) | 19 | T2DM | 2.5 mg, titrated up to 5 mg where tolerated and safe | 12 wk | DXA | −2.7 (−3.3%) | −1.4 | −1.1a | NR | NR | 51.9 | 40.7 |
| Sasaki et al. (2019) [60] (Japan) | Case series (prospective) | 37 | T2DM | 2.5 mg, titrated up to 5 mg where tolerated and safe | 52 wk | DXA | −3.1 (−3.9%) | −2.0 | −1.0 | NR | NR | 64.5 | 32.3 |
| Tofogliflozin | |||||||||||||
| Iwahashi et al. (2016) [61] (Japan) | Case series (prospective) | 17 | T2DM | 20 mg | 48 wk | BIA | −2.3 (−3.0%) | −2.6 | +0.2a | NR | −0.3 | 113 | FFM increased |
| Kamei et al. (2018) [62] (Japan) | Case series (retrospective) | 37 | T2DM | 20 mg | 12 wk | BIA | −3.3 (−3.7%) | −1.9 | −1.0 | −0.8 | −0.9 | 57.6 | 30.3 |
| Matsuba et al. (2018) [63] (Japan) | Case series (prospective) | 14 | T2DM | 20 mg | 12 wk | BIA | −2.9 (baseline NR) | −1.3 | −1.5 | −1.4 | −1.1 | 44.8 | 51.7 |
SGLT2i, sodium-glucose cotransporter 2 inhibitor; BW, body weight; FM, fat mass; LBM, lean body mass; DAPA, dapagliflozin; CANA, canagliflozin; T2DM, type 2 diabetes mellitus; NAFLD, nonalcoholic fatty liver disease; BIA, bioelectrical impedance analysis; LUSEO, luseogliflozin; TOFO, tofogliflozin; IPRA, ipragliflozin; NR, not reported; NASH, nonalcoholic steatohepatitis; RCT, randomised controlled trial; DXA, dual-energy X-ray absorptiometry; EMPA, empagliflozin; PCOS, polycystic ovarian syndrome; FFM, fat-free mass.
aFFM; bSame as “Study 1” in Blonde et al. (2016) [41]; cCalculated using mean weight loss in entire cohort.
Studies Reporting Changes in Body Composition with GLP-1RA and SGLT2i Combination Therapy
| Drug/author (location) | Study design | No. (in group prescribed SGLT2i+ GLP-1RA therapy) | Population | Dose (all once-daily, unless otherwise specified; SGLT2i taken as oral tablet, GLP-1RA as subcutaneous injection) | Duration, wk | Tech. used | Total BW change, kg (% change from baseline) | FM change | LBM change | Skeletal muscle mass change, kg | Total body water change, kg | Proportion of weight loss from FM, % | Proportion of weight loss from LBM, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dapagliflozin+Exenatide | |||||||||||||
| Lundkvist et al. (2017) [67] (Sweden) | Two-arm RCT | 16 | Obesity and pre-diabetes mellitus | DAPA: 10 mg | 52 | MRI | −5.7 (−5.4%) | −5.3 La | −1.4 L | NR | NR | - | - |
| EXEN: 2 mg weekly | |||||||||||||
| Luseogliflozin+Liraglutide | |||||||||||||
| Seino et al. (2018) [68] (Japan) | Case series (prospective) (sub-study) | 22 | T2DM | LUSEO: 2.5 mg, titrated up to 5 mg where tolerated and safe (n=17 reached 5 mg dose) | 52 | BIA | −2.9 (−4.3%) | −2.5 kg | −0.4 kg | NR | NR | 86.2 | 13.8 |
| LIRA: 0.6 mg (n=1) or 0.9 mg (n=21) |
GLP-1RA, glucagon-like peptide-1 receptor agonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor; BW, body weight; FM, fat mass; LBM, lean body mass; RCT, randomised controlled trial; DAPA, dapagliflozin; EXEN, exenatide; MRI, magnetic resonance imaging; NR, not reported; T2DM, type 2 diabetes mellitus; LUSEO, luseogliflozin; LIRA, liraglutide; BIA, bioelectrical impedance analysis.
aTotal adipose tissue.
Figure & Data
References
Citations

- Drug‐related sarcopenia as a secondary sarcopenia
Masafumi Kuzuya
Geriatrics & Gerontology International.2024; 24(2): 195. CrossRef - Exercise induces tissue-specific adaptations to enhance cardiometabolic health
Stephen P. Ashcroft, Ben Stocks, Brendan Egan, Juleen R. Zierath
Cell Metabolism.2024; 36(2): 278. CrossRef - Once-weekly semaglutide administered after laparoscopic sleeve gastrectomy: Effects on body weight, glycemic control, and measured nutritional metrics in Japanese patients having both obesity and type 2 diabetes
Rieko Kanai, Sachiho Kinoshita, Izumi Kanbe, Mariko Sameda, Shuhei Yamaoka, Osamu Horikawa, Yasuhiro Watanabe, Ichiro Tatsuno, Kohji Shirai, Takashi Oshiro, Atsuhito Saiki
Obesity Pillars.2024; 9: 100098. CrossRef - Twenty‐four‐hour physical behaviour profiles across type 2 diabetes mellitus subtypes
Joseph Henson, Aikaterina Tziannou, Alex V. Rowlands, Charlotte L. Edwardson, Andrew P. Hall, Melanie J. Davies, Thomas Yates
Diabetes, Obesity and Metabolism.2024; 26(4): 1355. CrossRef - The Current Landscape of Pharmacotherapies for Sarcopenia
Gulistan Bahat, Serdar Ozkok
Drugs & Aging.2024; 41(2): 83. CrossRef - Malnutrition and Sarcopenia as Reasons for Caution with GLP-1 Receptor Agonist Use in HFpEF
ELISSA DRIGGIN, PARAG GOYAL
Journal of Cardiac Failure.2024; 30(4): 610. CrossRef - Is the GLP-1 receptor agonist, semaglutide, a good option for weight loss in persons with HIV?
Daniel Lee, Jacqueline Capeau
AIDS.2024; 38(4): 603. CrossRef - Efficacy and safety of tirzepatide, GLP‐1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta‐analysis
Xin‐Hui Pan, Bryan Tan, Yip Han Chin, Ethan Cheng Zhe Lee, Gwyneth Kong, Bryan Chong, Martin Kueh, Chin Meng Khoo, Anurag Mehta, Priyanka Majety, Gowtham R. Grandhi, Georgios K. Dimitriadis, Roger Foo, Nicholas W. S. Chew, Carel W. Le Roux, Mamas A. Mamas
Obesity.2024;[Epub] CrossRef - Dual and Triple Incretin-Based Co-agonists: Novel Therapeutics for Obesity and Diabetes
Robert M. Gutgesell, Rubén Nogueiras, Matthias H. Tschöp, Timo D. Müller
Diabetes Therapy.2024;[Epub] CrossRef - Malnutrition in real-world patients hospitalized for heart failure with preserved ejection fraction and its potential impact on generalizability of EMPEROR-Preserved trial
Shinsuke Takeuchi, Takashi Kohno, Ayumi Goda, Yasuyuki Shiraishi, Mike Saji, Yuji Nagatomo, Toshikazu D. Tanaka, Makoto Takei, Shintaro Nakano, Kyoko Soejima, Shun Kohsaka, Tsutomu Yoshikawa
International Journal of Cardiology.2023; 370: 263. CrossRef - Marked weight loss on liraglutide 3.0 mg: Real‐life experience of a Swiss cohort with obesity
Sara Santini, Nathalie Vionnet, Jérôme Pasquier, Elena Gonzalez‐Rodriguez, Montserrat Fraga, Nelly Pitteloud, Lucie Favre
Obesity.2023; 31(1): 74. CrossRef - Early type 2 diabetes treatment intensification with glucagon‐like peptide‐1 receptor agonists in primary care: An Australian perspective on guidelines and the global evidence
Roy Rasalam, Sarah Abdo, Gary Deed, Richard O'Brien, Jane Overland
Diabetes, Obesity and Metabolism.2023; 25(4): 901. CrossRef - The effects of weight‐lowering pharmacotherapies on physical activity, function and fitness: A systematic review and meta‐analysis of randomized controlled trials
Rishi Jobanputra, Jack A. Sargeant, Abdullah Almaqhawi, Ehtasham Ahmad, Franciskos Arsenyadis, David R. Webb, Louisa Y. Herring, Kamlesh Khunti, Melanie J. Davies, Thomas Yates
Obesity Reviews.2023;[Epub] CrossRef - Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: a randomized controlled trial
Rasmus M. Sandsdal, Christian R. Juhl, Simon B. K. Jensen, Julie R. Lundgren, Charlotte Janus, Martin B. Blond, Mads Rosenkilde, Adrian F. Bogh, Lasse Gliemann, Jens-Erik B. Jensen, Charalambos Antoniades, Bente M. Stallknecht, Jens J. Holst, Sten Madsbad
Cardiovascular Diabetology.2023;[Epub] CrossRef - Impact of novel glucose‐lowering therapies on physical function in people with type 2 diabetes: A systematic review and meta‐analysis of randomised placebo‐controlled trials
Ehtasham Ahmad, Franciskos Arsenyadis, Abdullah Almaqhawi, Mary Barker, Rishi Jobanputra, Jack A. Sargeant, David R. Webb, Thomas Yates, Melanie J. Davies
Diabetic Medicine.2023;[Epub] CrossRef - Cancer cachexia as a blueprint for treating obesity
Nikolai P. Jaschke, Tilman D. Rachner
Trends in Endocrinology & Metabolism.2023; 34(7): 395. CrossRef - The sun is rising on a new era of pharmacotherapy for obesity: some words of caution
Peter N. Benotti, Bruce R. Bistrian
Surgery for Obesity and Related Diseases.2023; 19(9): 1075. CrossRef - Liraglutide Protects Against Diastolic Dysfunction and Improves Ventricular Protein Translation
Cody Rutledge, Angela Enriquez, Kevin Redding, Mabel Lopez, Steven Mullett, Stacy L. Gelhaus, Michael Jurczak, Eric Goetzman, Brett A. Kaufman
Cardiovascular Drugs and Therapy.2023;[Epub] CrossRef - Effect of sodium-glucose transporter 2 inhibitors on sarcopenia in patients with type 2 diabetes mellitus: a systematic review and meta-analysis
Sha Zhang, Zhan Qi, Yidong Wang, Danfei Song, Deqiu Zhu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Cysteine‐lowering treatment with mesna against obesity: Proof of concept and results from a human phase I, dose‐finding study
Kathrine J. Vinknes, Thomas Olsen, Hasse Khiabani Zaré, Nasser E. Bastani, Emma Stolt, Anja F. Dahl, Roger D. Cox, Helga Refsum, Kjetil Retterstøl, Anders Åsberg, Amany Elshorbagy
Diabetes, Obesity and Metabolism.2023; 25(11): 3161. CrossRef - Repurposing Drugs for Diabetes Mellitus as Potential Pharmacological Treatments for Sarcopenia – A Narrative Review
Miles D. Witham, Antoneta Granic, Ewan Pearson, Sian M. Robinson, Avan A. Sayer
Drugs & Aging.2023; 40(8): 703. CrossRef - Introduction to the dietary management of obesity in adults
Vivian Lee
Clinical Medicine.2023; 23(4): 304. CrossRef - Efficacy and safety of the sodium‐glucose co‐transporter‐2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: A randomized, double‐blind, placebo‐controlled, 52‐week clinical trial (EMPA‐ELDERLY)
Daisuke Yabe, Kosuke Shiki, Gosuke Homma, Thomas Meinicke, Yuji Ogura, Yutaka Seino
Diabetes, Obesity and Metabolism.2023; 25(12): 3538. CrossRef - Independent Link Between Use of Mineralocorticoid Receptor Antagonists and Muscle Wasting in Heart Failure Patients Not Receiving Renin-Angiotensin System Inhibitors
Ryo Numazawa, Satoshi Katano, Toshiyuki Yano, Ryohei Nagaoka, Katsuhiko Ohori, Hidemichi Kouzu, Suguru Honma, Yusuke Fujisawa, Kotaro Yamano, Arata Osanami, Masayuki Koyama, Akiyoshi Hashimoto, Masato Furuhashi
Circulation Journal.2023; 88(1): 10. CrossRef - Sodium-glucose co-transporter 2 inhibitors and Sarcopenia: A controversy that must be solved
Baris Afsar, Rengin Elsurer Afsar
Clinical Nutrition.2023; 42(12): 2338. CrossRef - Oral semaglutide improves body composition and preserves lean mass in patients with type 2 diabetes: a 26-week prospective real-life study
Sara Volpe, Giuseppe Lisco, Margherita Fanelli, Davide Racaniello, Valentina Colaianni, Valentina Lavarra, Domenico Triggiani, Lucilla Crudele, Vincenzo Triggiani, Carlo Sabbà, Giovanni De Pergola, Giuseppina Piazzolla
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Effects of Sodium–Glucose Cotransporter 2 Inhibitors on Body Composition in Type 2 Diabetes Mellitus: A Narrative Review
Soodeh Jahangiri, Mojtaba Malek, Sanjay Kalra, Mohammad E. Khamseh
Diabetes Therapy.2023; 14(12): 2015. CrossRef - Relationship between sodium–glucose cotransporter-2 inhibitors and muscle atrophy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis
Chengdong Xia, Yufeng Han, Chunhui Yin, Ruyue Geng, Zhenfei Liu, Yongle Du, Mingkun Yu
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Role of Lifestyle Modification with Second-Generation Anti-obesity Medications: Comparisons, Questions, and Clinical Opportunities
Thomas A. Wadden, Ariana M. Chao, Molly Moore, Jena S. Tronieri, Adam Gilden, Anastassia Amaro, Sharon Leonard, John M. Jakicic
Current Obesity Reports.2023; 12(4): 453. CrossRef - Sarcopenia and Diabetes: A Detrimental Liaison of Advancing Age
Giuseppe Lisco, Olga Eugenia Disoteo, Anna De Tullio, Vincenzo De Geronimo, Vito Angelo Giagulli, Fabio Monzani, Emilio Jirillo, Renato Cozzi, Edoardo Guastamacchia, Giovanni De Pergola, Vincenzo Triggiani
Nutrients.2023; 16(1): 63. CrossRef - Novel Antidiabetic Strategies and Diabetologists' Views in Nonalcoholic Steatohepatitis
Sabine Kahl, Jennifer Pützer, Michael Roden
Seminars in Liver Disease.2022; 42(01): 048. CrossRef - Effect of Empagliflozin Versus Placebo on Body Fluid Balance in Patients With Acute Myocardial Infarction and Type 2 Diabetes Mellitus: Subgroup Analysis of the EMBODY Trial
Yu Hoshika, Yoshiaki Kubota, Kosuke Mozawa, Shuhei Tara, Yukichi Tokita, Kenji Yodogawa, Yu-Ki Iwasaki, Takeshi Yamamoto, Hitoshi Takano, Yayoi Tsukada, Kuniya Asai, Masaaki Miyamoto, Yasushi Miyauchi, Eitaro Kodani, Mitsunori Maruyama, Jun Tanabe, Wataru
Journal of Cardiac Failure.2022; 28(1): 56. CrossRef - Effect of GLP-1 receptor agonist, liraglutide, on muscle in spontaneously diabetic torii fatty rats
Shohei Yamada, Yuji Ogura, Kazuho Inoue, Jun Tanabe, Takeshi Sugaya, Keiichi Ohata, Yoshio Nagai, Yasunori Natsuki, Seiko Hoshino, Shiika Watanabe, Daisuke Ichikawa, Kenjiro Kimura, Yugo Shibagaki, Atsuko Kamijo-Ikemori
Molecular and Cellular Endocrinology.2022; 539: 111472. CrossRef - Exendin-4 alleviates steatosis in an in vitro cell model by lowering FABP1 and FOXA1 expression via the Wnt/-catenin signaling pathway
Olfa Khalifa, Neyla S. AL-Akl, Khaoula Errafii, Abdelilah Arredouani
Scientific Reports.2022;[Epub] CrossRef - Body composition changes at 12 months following different surgical weight loss interventions in adults with obesity: A systematic review and meta‐analysis of randomized control trials
Amy Sylivris, Jakub Mesinovic, David Scott, Paul Jansons
Obesity Reviews.2022;[Epub] CrossRef - Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance study
Kohei Kaku, Kazuhiro Yamamoto, Yumiko Fukushima, Hristo Lliev, Atsutaka Yasui
Expert Opinion on Drug Safety.2022; 21(10): 1315. CrossRef - Safety and effectiveness of empagliflozin according to body mass index in Japanese patients with type 2 diabetes: a subgroup analysis of a 3-year post-marketing surveillance study
Kohei Kaku, Kazuhiro Yamamoto, Yumiko Fukushima, Seiko Mizuno, Daisuke Nitta
Expert Opinion on Drug Safety.2022; 21(11): 1411. CrossRef - Le risque de dénutrition chez le sujet âgé diabétique : une limite à l’utilisation des « nouvelles » classes thérapeutiques ?
Lyse Bordier, Jean Doucet, Bernard Bauduceau
Médecine des Maladies Métaboliques.2022; 16(5): 422. CrossRef - Emerging evidence of the relationship between fat-free mass and ghrelin, glucagon-like peptide-1, and peptide-YY
Austin J. Graybeal, Jada L. Willis, Elisa Morales-Marroquin, Grant M. Tinsley, Sarah E. Messiah, Meena Shah
Nutrition.2022; 103-104: 111815. CrossRef - The Effectiveness of GLP-1 Receptor Agonist Semaglutide on Body Composition in Elderly Obese Diabetic Patients: A Pilot Study
Yoshinori Ozeki, Takayuki Masaki, Akari Kamata, Shotaro Miyamoto, Yuichi Yoshida, Mitsuhiro Okamoto, Koro Gotoh, Hirotaka Shibata
Medicines.2022; 9(9): 47. CrossRef - Distribution of lean mass and mortality risk in patients with type 2 diabetes
Li Ding, Yuxin Fan, Jingting Qiao, Jing He, Ruodan Wang, Qing He, Jingqiu Cui, Zhongshu Ma, Fangqiu Zheng, Hua Gao, Chenlin Dai, Hongyan Wei, Jun Li, Yuming Cao, Gang Hu, Ming Liu
Primary Care Diabetes.2022; 16(6): 824. CrossRef - Cardio-sarcopenia: A syndrome of concern in aging
De Rong Loh, Ru-San Tan, Wee Shiong Lim, Angela S. Koh
Frontiers in Medicine.2022;[Epub] CrossRef - Type 2 diabetes
Ehtasham Ahmad, Soo Lim, Roberta Lamptey, David R Webb, Melanie J Davies
The Lancet.2022; 400(10365): 1803. CrossRef - Elevated circulating level of β-aminoisobutyric acid (BAIBA) in heart failure patients with type 2 diabetes receiving sodium-glucose cotransporter 2 inhibitors
Satoshi Katano, Toshiyuki Yano, Hidemichi Kouzu, Ryohei Nagaoka, Ryo Numazawa, Kotaro Yamano, Yusuke Fujisawa, Katsuhiko Ohori, Nobutaka Nagano, Takefumi Fujito, Ryo Nishikawa, Wataru Ohwada, Masaki Katayose, Tatsuya Sato, Atsushi Kuno, Masato Furuhashi
Cardiovascular Diabetology.2022;[Epub] CrossRef - An overview of anamorelin as a treatment option for cancer-associated anorexia and cachexia
Guilherme Wesley Peixoto Da Fonseca, Stephan von Haehling
Expert Opinion on Pharmacotherapy.2021; 22(7): 889. CrossRef - Liraglutide Does Not Adversely Impact Fat‐Free Mass Loss
Andrew Grannell, William P. Martin, Babak Dehestani, Werd Al‐Najim, John C. Murphy, Carel W. le Roux
Obesity.2021; 29(3): 529. CrossRef - Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium–glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabet
Daisuke Yabe, Kosuke Shiki, Keiko Suzaki, Thomas Meinicke, Yutaro Kotobuki, Kenichiro Nishida, Douglas Clark, Atsutaka Yasui, Yutaka Seino
BMJ Open.2021; 11(4): e045844. CrossRef - Cancer Risk in Normal Weight Individuals with Metabolic Obesity: A Narrative Review
Bethina Liu, Hugh E. Giffney, Rhonda S. Arthur, Thomas E. Rohan, Andrew J. Dannenberg
Cancer Prevention Research.2021; 14(5): 509. CrossRef - Comprehensive analysis of LncRNAs expression profiles in an in vitro model of steatosis treated with Exendin-4
Khaoula Errafii, Neyla S. Al-Akl, Olfa Khalifa, Abdelilah Arredouani
Journal of Translational Medicine.2021;[Epub] CrossRef - Dapagliflozin increases the lean-to total mass ratio in type 2 diabetes mellitus
Vaneza Lira W. Wolf, Ikaro Breder, Luiz Sérgio F. de Carvalho, Alexandre A. S. Soares, Riobaldo M. Cintra, Joaquim Barreto, Daniel B. Munhoz, Sheila T. Kimura-Medorima, Wilson Nadruz, Gil Guerra-Júnior, Thiago Quinaglia, Elza Muscelli, Andrei C. Sposito
Nutrition & Diabetes.2021;[Epub] CrossRef - Optimising the Heart Failure Treatment Pathway: The Role of SGLT2 Inhibitors
Marc Evans, Angharad R. Morgan, Zaheer Yousef, Gethin Ellis, Umesh Dashora, Dipesh C. Patel, Pam Brown, Wasim Hanif, Johnathan N. Townend, Naresh Kanumilli, Jim Moore, John P. H. Wilding, Stephen C. Bain
Drugs.2021; 81(11): 1243. CrossRef - Glucose-lowering Drugs and Hospitalization for Heart Failure: A Systematic Review and Additive-effects Network Meta-analysis With More Than 500 000 Patient-years
Riobaldo M Cintra, Ana Claudia Nogueira, Isabella Bonilha, Beatriz M Luchiari, Otavio R Coelho-Filho, Otavio R Coelho, Pedro Schwartzmann, Elza Muscellie, Wilson Nadruz, Luiz Sergio F Carvalho, Andrei C Sposito
The Journal of Clinical Endocrinology & Metabolism.2021; 106(10): 3060. CrossRef - Physical activity and exercise in the management of type 2 diabetes: where to start?
Deirdre Harrington, Joe Henson
Practical Diabetes.2021; 38(5): 35. CrossRef - Efpeglenatide and Heart and Kidney Outcomes in Type 2 Diabetes
New England Journal of Medicine.2021; 385(22): 2105. CrossRef - Effects of Antidiabetic Drugs on Muscle Mass in Type 2 Diabetes Mellitus
Satoshi Ida, Ryutaro Kaneko, Kanako Imataka, Kaoru Okubo, Yoshitaka Shirakura, Kentaro Azuma, Ryoko Fujiwara, Kazuya Murata
Current Diabetes Reviews.2021; 17(3): 293. CrossRef - Effects of liraglutide and empagliflozin added to insulin therapy in patients with type 2 diabetes: A randomized controlled study
Hirotatsu Nakaguchi, Yoshinobu Kondo, Mayu Kyohara, Hiromi Konishi, Koji Oiwa, Yasuo Terauchi
Journal of Diabetes Investigation.2020; 11(6): 1542. CrossRef - Sodium Glucose Co-Transporter 2 Inhibition Does Not Favorably Modify the Physiological Responses to Dietary Counselling in Diabetes-Free, Sedentary Overweight and Obese Adult Humans
Shane P.P. Ryan, Alissa A. Newman, Jessie R. Wilburn, Lauren D. Rhoades, S. Raj J. Trikha, Ellen C. Godwin, Hayden M. Schoenberg, Micah L. Battson, Taylor R. Ewell, Gary J. Luckasen, Laurie M. Biela, Christopher L. Melby, Christopher Bell
Nutrients.2020; 12(2): 510. CrossRef - GLP-1 Receptor Agonist Treatment in Morbid Obesity and Type 2 Diabetes Due to Pathogenic Homozygous Melanocortin-4 Receptor Mutation: A Case Report
Eva W. Iepsen, Christian T. Have, Simon Veedfald, Sten Madsbad, Jens J. Holst, Niels Grarup, Oluf Pedersen, Ivan Brandslund, Jens-Christian Holm, Torben Hansen, Signe S. Torekov
Cell Reports Medicine.2020; 1(1): 100006. CrossRef - Glucagon‐like peptide 1 infusions overcome anabolic resistance to feeding in older human muscle
Haitham Abdulla, Bethan E. Phillips, Daniel J. Wilkinson, Marie Limb, Tereza Jandova, Joseph J. Bass, Debbie Rankin, Jessica Cegielski, Mariwan Sayda, Hannah Crossland, John P. Williams, Kenneth Smith, Iskandar Idris, Philip J. Atherton
Aging Cell.2020;[Epub] CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite