Articles
- Page Path
- HOME > Endocrinol Metab > Volume 28(4); 2013 > Article
-
Original ArticleEffects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice
- Joo Yeon Jeong, Dong Hoon Lee, Sang Soo Kang
-
Endocrinology and Metabolism 2013;28(4):288-296.
DOI: https://doi.org/10.3803/EnM.2013.28.4.288
Published online: December 12, 2013
Department of Anatomy and Neurobiology, Institute of Health Sciences, Medical Research Center for Neural Dysfunction, Gyeongsang National University School of Medicine, Jinju, Korea.
- Corresponding author: Sang Soo Kang. Department of Anatomy and Neurobiology, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 660-751, Korea. Tel: +82-55-772-8033, Fax: +82-55-772-8039, kangss@gnu.ac.kr
• Received: April 1, 2013 • Accepted: August 9, 2013
Copyright © 2013 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 6,697 Views
- 144 Download
- 130 Crossref
ABSTRACT
-
Background
- Stress affects body weight and food intake, but the underlying mechanisms are not well understood.
-
Methods
- We evaluated the changes in body weight and food intake of ICR male mice subjected to daily 2 hours restraint stress for 15 days. Hypothalamic gene expression profiling was analyzed by cDNA microarray.
-
Results
- Daily body weight and food intake measurements revealed that both parameters decreased rapidly after initiating daily restraint stress. Body weights of stressed mice then remained significantly lower than the control body weights, even though food intake slowly recovered to 90% of the control intake at the end of the experiment. cDNA microarray analysis revealed that chronic restraint stress affects the expression of hypothalamic genes possibly related to body weight control. Since decreases of daily food intake and body weight were remarkable in days 1 to 4 of restraint, we examined the expression of food intake-related genes in the hypothalamus. During these periods, the expressions of ghrelin and pro-opiomelanocortin mRNA were significantly changed in mice undergoing restraint stress. Moreover, daily serum corticosterone levels gradually increased, while leptin levels significantly decreased.
-
Conclusion
- The present study demonstrates that restraint stress affects body weight and food intake by initially modifying canonical food intake-related genes and then later modifying other genes involved in energy metabolism. These genetic changes appear to be mediated, at least in part, by corticosterone.
- Stress is well known to change body weight and food intake in animal models. Of the various stress models available for the study of the effects of stress, the restraint stress model is most commonly employed, as it effectively mimics potent physical and psychological stress [1]. The restraint stress model has also been used as an animal model of depression and anorexia nervosa. Thus, many studies have shown that restraint stress suppresses body weight gain and food intake in rodents [2,3].
- The central regulation of body weight and food intake occurs in the hypothalamus, which contains multiple neuronal systems that play important roles in the regulation of energy homeostasis [4]. These systems involve the interaction of multiple neuropeptides. Food intake reflects a functional balance between hypothalamic orexigenic peptides (such as neuropeptide Y [NPY] and agouti-related protein [AgRP]) and anorexigenic peptides (such as pro-opiomelanocortin [POMC] and cocaine- and amphetamine-regulated transcript [CART]) [5]. In addition, ghrelin, a peptide that is predominantly produced by the stomach, is also expressed by the hypothalamus and regulates growth hormone secretion, food intake, and energy homeostasis [6,7]. Another factor that regulates food intake and energy homeostasis is leptin, an anorexigenic hormone secreted by adipose tissue [8]. Leptin is well known for its critical role in the regulation of food intake in adult mammals. Furthermore, leptin participates in the control of several neuroendocrine functions, including those of the hypothalamicpituitary-adrenal (HPA) axis. In response to the nutritional status and energy storage levels, leptin signals hypothalamic feeding centers by controlling the expression and release of orexigenic and anorexigenic neuropeptides [9,10].
- Chronic stress increases serum corticosterone levels. However, the effects of chronic stress-induced elevated corticosterone on food intake and body weight are not clear [11]. Furthermore, the precise mechanism by which stress affects energy metabolism as well as food intake and body weight control is not well understood, especially at the hypothalamic gene expression level. In this study, to identify the central genes that regulate body weight and food intake and to characterize the molecular mechanisms involved, we extensively analyzed the hypothalamic gene expression profiles of chronically restraint stressed mice using large-scale cDNA microarray analysis.
INTRODUCTION
- Animals and restraint stress
- Male 7-week-old ICR mice were purchased from Central Laboratory Animal Inc. (Seoul, Korea) and housed individually in clear plastic cages in a temperature- and humidity-controlled environment under a 12 hours light/dark cycle (light on at 0600 hour) with free access to lab chow and water. The experiments were performed after the animals had been habituated to the experimental environment for 1 week. The mice were divided into two weight-matched (31 to 33 g) groups, controls, and stressed mice. The stressed mice were exposed daily for 15 days to 2 hours of restraint (0930 to 1130 hours) in an acrylic cylindrical animal restrainer (Φ25×[H] 85 mm, Daejong Instrument Industry, Seoul, Korea) with holes that permit the restrainer to be adjusted according to the size of the subject. The restrainer allows unlimited breathing but restricts the movement of the limbs. After being restrained, the mice were returned to their home cage and given food and water ad libitum. The food consumption and body weight of the mice were monitored daily (0830 to 0900 hours). All animal procedures adhered to the Animal Care and Use Guidelines of Gyeongsang National University (Approval No., GLA-060502-M0002 and GLA-070802-M0035).
- Preparation of hypothalamic RNAs
- One day after stress ended, days 1 to 5 or day 16 (depending on the experiment), the animals were sacrificed and their hypothalami were rapidly extracted (0930 to 1130 hours). Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The purity and quantity of the RNAs were assess ed by spectrophotometry.
- Microarray analysis
- Gene expression analysis was conducted on the day 16 hypothalamic mRNAs using the Agilent Mouse oligo microarray kit (Digital Genomics, Seoul, Korea). The scanned images were analyzed with GenePix Pro 6.0 software (Axon Instruments, Union City, CA, USA) to obtain gene expression ratios. The transformed data were normalized by LOWESS regression and analyzed using GeneSpring GX 7.3 software program (Agilent Technologies Inc., Santa Clara, CA, USA).
- Elevated plus maze
- The elevated plus maze (EPM) has two open arms and two closed arms (30×7 cm each) and a connecting central platform (7×7 cm) mounted 50 cm above the floor. Tested mice were placed in the center of the maze facing the open arm, and behavior was recorded for 5 minutes. Arm entry was scored if a mouse moved into the arm.
- Measurements of serum corticosterone and leptin levels
- To measure the basal levels of serum corticosterone and leptin, blood was collected the morning after stress via decapitation. Serum corticosterone and leptin concentrations were determined using an EIA kit (Assay Designs Inc., Ann Arbor, MI, USA) and an ELISA kit (Millpore, St Charles, MO, USA), respectively.
- Reverse transcriptase polymerase chain reaction
- For the day 0 to 4 samples of 16 hypothalamic mRNAs, a M-MLV RT kit (Promega, Madison, WI, USA) was used to convert the RNAs (1 µg) to cDNAs. The ghrelin and POMC mRNA levels were then determined using a real-time polymerase chain reaction (PCR) kit (LightCycler FastStart DNA Master SYBR Green I, Roche Applied Science, Mannheim, Germany) with the following primers: ghrelin (292 bp) forward, 5'-CAGTTTGCTGCTACTCAG-3', reverse, 5'-GATATCCTGAAGAAACTTCC-3'; POMC (497 bp) forward, 5'-ATGCCGAGATTCTGCTAC-3', reverse, 5'-AGCTCCCTCTTGAACTCT-3'; glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 172 bp) forward, 5'-TGCCGCCTGGAGAAACCTGC-3', reverse, 5'-TGAGAGCAATGCCAGCCCCA-3'. The hydroxysteroid (17-β) dehydrogenase 1 (Hsd17b1), cytochrome P450, family 11, subfamily a, polypeptide 1 (Cyp11a1), glycoprotein hormones, α subunit (Cga), and growth hormone (Gh) mRNA levels were then determined using a conventional PCR with the following primers: Hsd17b1 (370 bp) forward, 5'-ACTACCTGCGTGGTTATGAG-3', reverse, 5'-TGGTAACATGAATTGTCCTG-3'; Cyp11a1 (375 bp) forward, 5'-CCAAGATGGTACAGTTGGTT-3', reverse, 5'-CATCACGGAGATTTTGAACT-3'; Cga (317 bp) forward, 5'-AGCTAGGAGCCCCCATCTAC-3', reverse, 5'-GCGTCAGAAGTCTGGTAGGG-3'; Gh (255 bp) forward, 5'-TTCTGCTTCTCAGAGACCAT-3', reverse, 5'-TCATAGGTTTGCTTGAGGAT-3'. The expression levels of each mRNA are presented throughout as arbitrary units.
- Data analysis and statistics
- Real-time PCR data were analyzed by LightCycler software version 4.0 (LightCycler 2.0 Instrument, Roche Applied Science). Conventional PCR data were analyzed by Gel Doc (Bio-Rad, Hercules, CA, USA) and Quantity One version 4.6.3. Messenger RNA levels were normalized to the levels of the GAPDH reference gene. EPM test were analyzed using a computerized video-tracking system (EthoVision version 3.0, Noldus Information Technology, Wageningen, the Netherlands). Statistical analysis were performed using Student unpaired t test and one or two-way analysis of variance (GraphPad Prism, La Jolla, CA, USA). All data are shown as mean±SE.
METHODS
- Effects of restraint stress on body weight and food intake
- The effects of daily restraint stress for 15 days on body weight and food intake shown in Fig. 1. While the body weights of the control mice gradually increased over the 15 day experiment, the body weights of the stressed mice dropped sharply during the first 5 days. As a result, the stressed mice had significantly lower body weights than the control mice during the entire experimental period (Fig. 1A).
- The total food intake of the stressed mice also decreased markedly during the first few days of the experiment. The daily food intake of the stressed mice then gradually recovered substantially. However, while food intake of the stressed mice was almost 90% of control intake by day 7, it remained significantly lower at nearly every time point (Fig. 1B).
- Serum corticosterone levels were measured on day 16 (without stress) and were significantly higher in the stressed mice (Fig. 1C). In addition, we measured anxiety levels using the EPM test. The frequency of entries in open arm was significantly lower in the stressed group (Fig. 1D). However, the frequency of entries into the closed arm tended to be increased compared with control group, although the difference was not significant (data not shown).
- Gene expression profiles of stressed and control mice
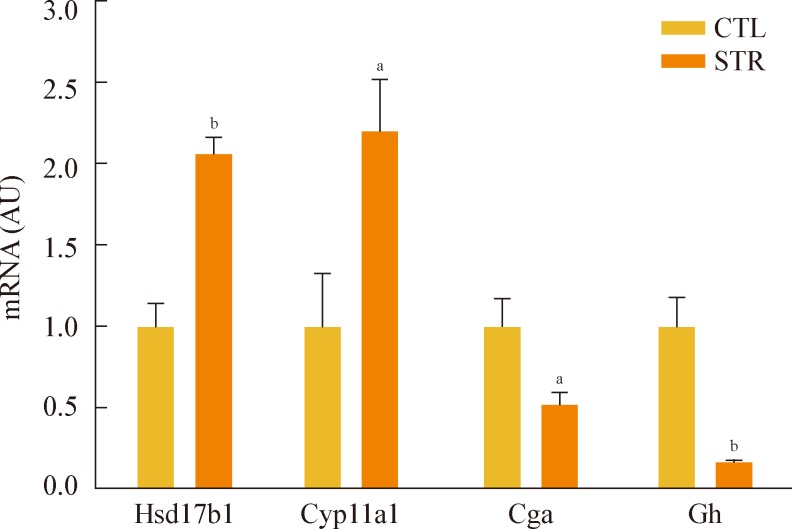
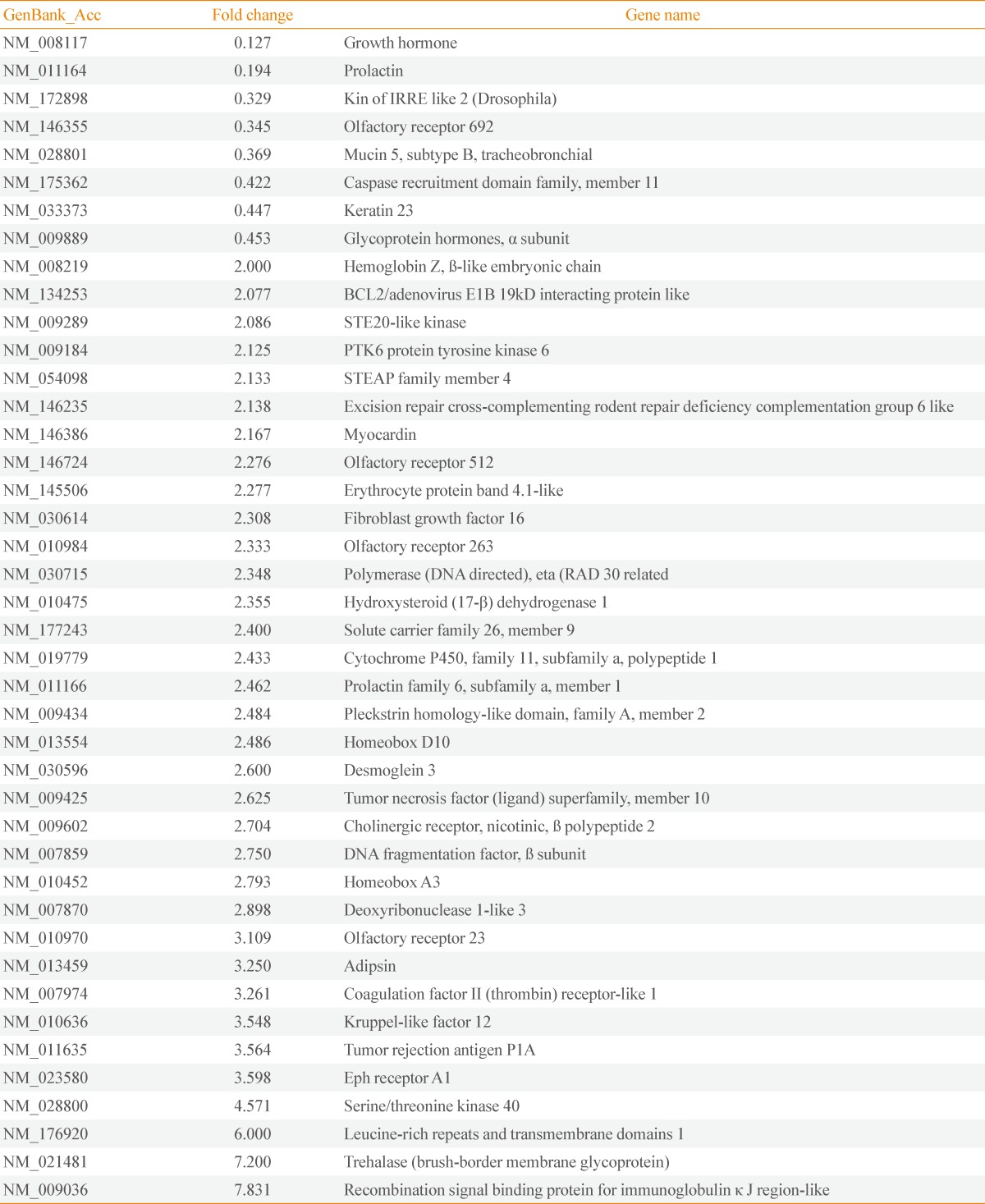
- The restraint stressed or control mice (n=10 in each group) were sacrificed on day 16 without stress, and their hypothalamic mRNAs were subjected to large-scale cDNA microarray analysis. Among the 20,868 detected genes, 42 genes showed a significant greater than 2.0-fold increase or 0.5-fold decrease in expression in the stressed mice (Table 1). To confirm the microarray data using conventional PCR analysis, we randomly selected four genes, two that were up-regulated and two that were down-regulated. The Hsd17b1 and Cyp11a1 mRNA levels were significantly increased, while the Cga and Gh mRNA levels showed a significant decrease (Fig. 2).
- Effects of restraint stress on hypothalamic ghrelin and POMC mRNA expression
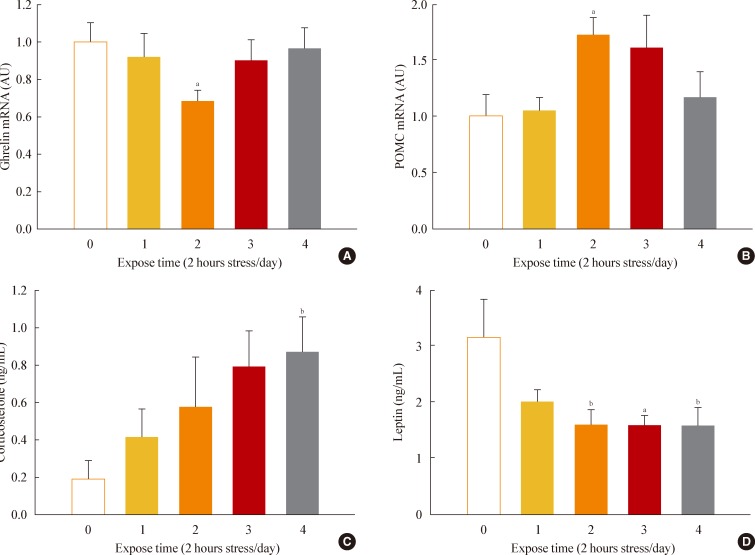
- Chronic restraint stress reduced body weight and food intake. Particularly, in days 1 to 4 of restraint, food intake and body weight were dramatically decreased. Thus, we analyzed the expression of canonical food intake-related genes in this period. To determine the expression of hypothalamic neuropeptides known to be involved in energy homeostasis, such as ghrelin and POMC, the hypothalamic mRNAs from the group of mice sacrificed on each of the 4 days after initiating restraint stress were subjected to real-time PCR analysis. On day 2 of restraint stress, the ghrelin mRNA levels showed a significant decrease, while the POMC mRNA levels were significantly elevated (Fig. 3A, B).
- Effects of restraint stress on serum corticosterone and leptin levels
- To elucidate the changes of food intake-related hormones levels after stress, we collected blood from the animals daily and measured serum corticosterone and leptin levels. Restraint stress showed gradually increasing serum corticosterone levels (Fig. 3C) and significantly decreased leptin levels on days 2 to 4 of restraint (Fig. 3D).
RESULTS
- In the present study, we investigated the effects of restraint stress on body weight, food intake, and hypothalamic gene expression levels in mice. Several studies have demonstrated that chronic exposure to restraint stress reduces the body weight and food intake of rodents [12-14]. However, the mechanisms underlying these restraint-induced changes in body weight and food intake remain to be elucidated.
- Our results here showed that restraint stress rapidly induces a marked decrease in body weight that may be due to a reduction of food intake. However, while food intake recovered to 90% of control intake, this was not matched by an equivalent recovery in body weight for the duration of the exposure to restraint. The stress-induced decrease in body weight may be due initially to an early decrease in food intake but then may be subsequently maintained by increases in energy expenditure and body temperature during restraint [15]. Especially, a previous report has also shown that rats chronically exposed to restraint showed rapid weight loss that did not recover even after removal of the stress [13]. Moreover, this study also showed that, while exposure to restraint stress significantly lowered food intake, once the stress ended, the food intake of the stressed group returned to the level of the control group; there was no attempt to overeat to compensate for the energy deficiency experienced during the restraint period [13]. This may be because stress somehow modifies the pathways that would normally sense and respond to a reduction in weight. It has been reported that stress-related pathways, once activated, act in opposition to the mechanisms that would normally promote the recovery of weight to normal levels [16].
- Increased serum corticosterone levels are consistent with the suggestion that physiological responses to repeated stress are associated with the activation of the HPA axis [17]. Also, we showed increasing anxiety levels in the stressed mice; chronic stress has been shown to increase anxiety and depression-like behavior in animal models [18,19]. Consequently, these results indicate that chronic restraint stress changes physical and psychological responses.
- To determine whether chronic restraint stress affects hypothalamic gene expression in the mice, we subjected the hypothalami of mice that had been exposed to 2 hours of restraint stress daily for 15 consecutive days to cDNA microarray analysis. Many of the genes that showed stress-related changes in expression were related to body weight control. Thus, these genes such as Gh, Prolactin, Cga, STEAP family member 4, Hsd17b1, Cyp11a1, adipsin, and trehalase (see the Genbank_Acc. No.) may participate in the chronic restraint stress-induced reduction of body weight, although this notion remains to be tested (Table 1).
- Supporting the possible involvement of metabolism-related genes, a recent study showed that restraint stress affects lipid metabolism. In that study, rats exposed to acute or chronic restraint stress show remarkable changes in plasma lipid and lipoprotein levels; plasma fatty acid, glycerol, and cholesterol levels are increased, while plasma triacylglyceride levels are decreased [20]. Supporting this notion is the finding that chronic restraint stress increases serum corticosterone levels, which may stimulate the catabolism of skeletal muscle proteins, which in turn may, at least in part, lead to body weight loss [21]. Recently, psychological stress has been shown to attenuate body size and lean body mass by reducing muscle mass [22].
- The mice that were exposed to chronic restraint stress for 15 days showed sustained reductions in body weight and food intake. As the initial dramatic decreases in daily food intake and body weight were observed in days 1 to 4 of restraint, we hypothesized that canonical food intake-related genes may only participate in this period.
- Thus, we analyzed the expression of food intake-related genes, such as NPY, AgRP, POMC, CART, ghrelin, corticotropin-releasing factor (CRF), CRF receptors, leptin receptor, insulin receptor, and melanocortin receptor, using real-time PCR. Only the hypothalamic mRNA expression of the ghrelin and POMC showed a significant decrease and increase on day 2, respectively. It has been shown that ghrelin is an orexigenic factor, as the central administration of ghrelin strongly stimulates food intake and increases body weight [23]. Moreover, when ghrelin is injected in an intracerebroventricular manner, NPY, and AgRP mRNA expressions are increased in the arcuate nucleus (Arc) [24]. In relation to the latter observation, hypothalamic ghrelin neurons are located within the Arc and innervate NPY/AgRP neurons [25]. Thus, it seems that the orexigenic effect of ghrelin is dependent on NPY/AgRP neurons. Moreover, NPY/AgRP neurons innervate POMC/α-melanocyte-stimulating hormone (α-MSH) neurons, indicating that the melanocortin system also seems to be involved in the action of ghrelin [26]. It has been suggested that POMC neurons act anorexigenically by producing and releasing α-MSH, a peptide that activates melanocortin-3, -4 receptors and inhibits food intake [27]. That we observed decreased ghrelin and increased POMC mRNAs early after restraint stress initiation suggests that these proteins may be responsible, at least in part, for the initial weight loss observed after restraint stress induction.
- We also observed that the serum corticosterone and leptin levels increased and decreased, respectively, in the 4 days after restraint stress was initiated. Serum leptin levels were decreased from day 2. In another report, restraint stress decreased serum leptin levels, which were sustained even after restraint stress was eliminated [28]. Sustained reduced leptin levels may recover food intake, as shown in our results. During a period of chronic restraint stress, despite the nearly recovered food intake, discrepancy in body weight between stressed and control mice was not reduced. This continued discrepancy of body weight may be possibly due to the action of increasing corticosterone levels. Glucocorticoids have a broad range of activity that affects the expression and regulation of genes throughout the body; these glucocorticoid-mediated effects lead to changes in the energy and metabolism requirements of the organism [29]. In our study, initial loss of body weight might be caused by reduction of food intake after stress, and this finding is well match with other reports [12,30]. According to several studies, exposure to chronic stress in rats resulted in an increase in basal corticosterone levels [31,32]. These results probably reflect a modified sensitivity to the negative feedback effects of circulating glucocorticoid [33]. In addition, food intake and many metabolic processes are mediated by glucocorticoids. Thus, chronic stress has been related to changes in body weight and physiology of different organs [15,32]. In our study, serum corticosterone levels were increased by repeated restraint stress. Thus, we suggest that the increased serum corticosterone levels after restraint stress could be due to a continuous stress state. This increased serum corticosterone level might affect discrepancies in body weight and food intake recovery in a direct or indirect manner. Daily increased pattern of serum corticosterone levels may affect the serum leptin levels. Although decreased serum leptin levels in early days of stress seemed to have a role in the recovered food intake, the precise role of serum leptin and the correlation of leptin with corticosterone needs further evaluation.
- In summary, restraint stress affects the body weight and food intake in mice. Chronic restraint stress-induced reduction of body weight is caused by reduction of initial daily food intake through modification of canonical food intake-related genes. However, chronic restraint stress-induced sustained discrepancy of body weight without reduction of food intake may be due to expression of other genes related to body weight control and regulation of stress response through corticosterone.
DISCUSSION
-
Acknowledgements
- This work was supported by the Korea Science and Engineering Foundation (KOSEF) for the Basic Research Program (R01-2006-000-10259-0) and partially supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science and Technology (R13-2005-012-02001-0).
ACKNOWLEDGMENTS
- 1. Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev 1994;18:223–249. ArticlePubMed
- 2. Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry 1990;27:1094–1102. ArticlePubMed
- 3. Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 1992;4:517–526. ArticlePubMed
- 4. Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 1998;280:1378–1383. ArticlePubMed
- 5. Korner J, Savontaus E, Chua SC Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 2001;13:959–966. ArticlePubMed
- 6. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–913. ArticlePubMedPDF
- 7. van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004;25:426–457. ArticlePubMedPDF
- 8. Proulx K, Richard D, Walker CD. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology 2002;143:4683–4692. ArticlePubMedPDF
- 9. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 1996;382:250–252. ArticlePubMedPDF
- 10. Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1998;1:445–450. ArticlePubMedPDF
- 11. Smagin GN, Howell LA, Redmann S Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol 1999;276(5 Pt 2):R1461–R1468. ArticlePubMed
- 12. Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 1994;55:747–753. ArticlePubMed
- 13. Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol 1998;275(6 Pt 2):R1928–R1938. ArticlePubMed
- 14. Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int 2003;42:107–114. ArticlePubMed
- 15. Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 2006;18:13–24. ArticlePubMed
- 16. Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. C hronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav 2006;49:615–625. ArticlePubMed
- 17. Ottenweller JE, Servatius RJ, Tapp WN, Drastal SD, Bergen MT, Natelson BH. A chronic stress state in rats: effects of repeated stress on basal corticosterone and behavior. Physiol Behav 1992;51:689–698. ArticlePubMed
- 18. Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience 2002;115:229–242. ArticlePubMed
- 19. Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol 2005;16:171–180. ArticlePubMed
- 20. Ricart-Jane D, Cejudo-Martin P, Peinado-Onsurbe J, Lopez-Tejero MD, Llobera M. Changes in lipoprotein lipase modulate tissue energy supply during stress. J Appl Physiol (1985) 2005;99:1343–1351. ArticlePubMed
- 21. Sato T, Yamamoto H, Sawada N, Nashiki K, Tsuji M, Muto K, Kume H, Sasaki H, Arai H, Nikawa T, Taketani Y, Takeda E. Restraint stress alters the duodenal expression of genes important for lipid metabolism in rat. Toxicology 2006;227:248–261. ArticlePubMed
- 22. Allen DL, McCall GE, Loh AS, Madden MC, Mehan RS. Acute daily psychological stress causes increased atrophic gene expression and myostatin-dependent muscle atrophy. Am J Physiol Regul Integr Comp Physiol 2010;299:R889–R898. ArticlePubMedPMC
- 23. Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002;143:155–162. ArticlePubMedPDF
- 24. Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 2003;52:948–956. ArticlePubMed
- 25. Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003;37:649–661. ArticlePubMed
- 26. Chan CB, Cheng CH. Identification and functional characterization of two alternatively spliced growth hormone secretagogue receptor transcripts from the pituitary of black seabream Acanthopagrus schlegeli. Mol Cell Endocrinol 2004;214:81–95. ArticlePubMed
- 27. Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 2007;92:263–271. ArticlePubMed
- 28. Harris RB, Mitchell TD, Simpson J, Redmann SM Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol 2002;282:R77–R88. ArticlePubMed
- 29. Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 2005;30:939–946. ArticlePubMed
- 30. Marti O, Harbuz MS, Andres R, Lightman SL, Armario A. Activation of the hypothalamic-pituitary axis in adrenalectomised rats: potentiation by chronic stress. Brain Res 1999;821:1–7. ArticlePubMed
- 31. Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 1998;84:1025–1039. ArticlePubMed
- 32. Dal-Zotto S, Marti O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res 2000;114:175–181. ArticlePubMed
- 33. Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 2001;26:443–459. ArticlePubMed
References
Fig. 1Effects of restraint stress on body weight, food intake, serum corticosterone, and anxiety level. (A) Daily body weight and (B) food intake of mice exposed daily to 2 hours of restraint for 15 consecutive days. (C) Serum corticosterone levels were significantly increased in stressed mice (STR) at the end of the restraint stress period. (D) Stressed mice showed a significant reduction in frequency of open arm entry in the elevated plus maze test. Statistical differences were evaluated by (A, B) two-way analysis of variance and (C, D) Student unpaired t test. Data are presented as mean±SE. aP<0.05; bP<0.01 vs. control mice (CTL) (n=10 in each group).
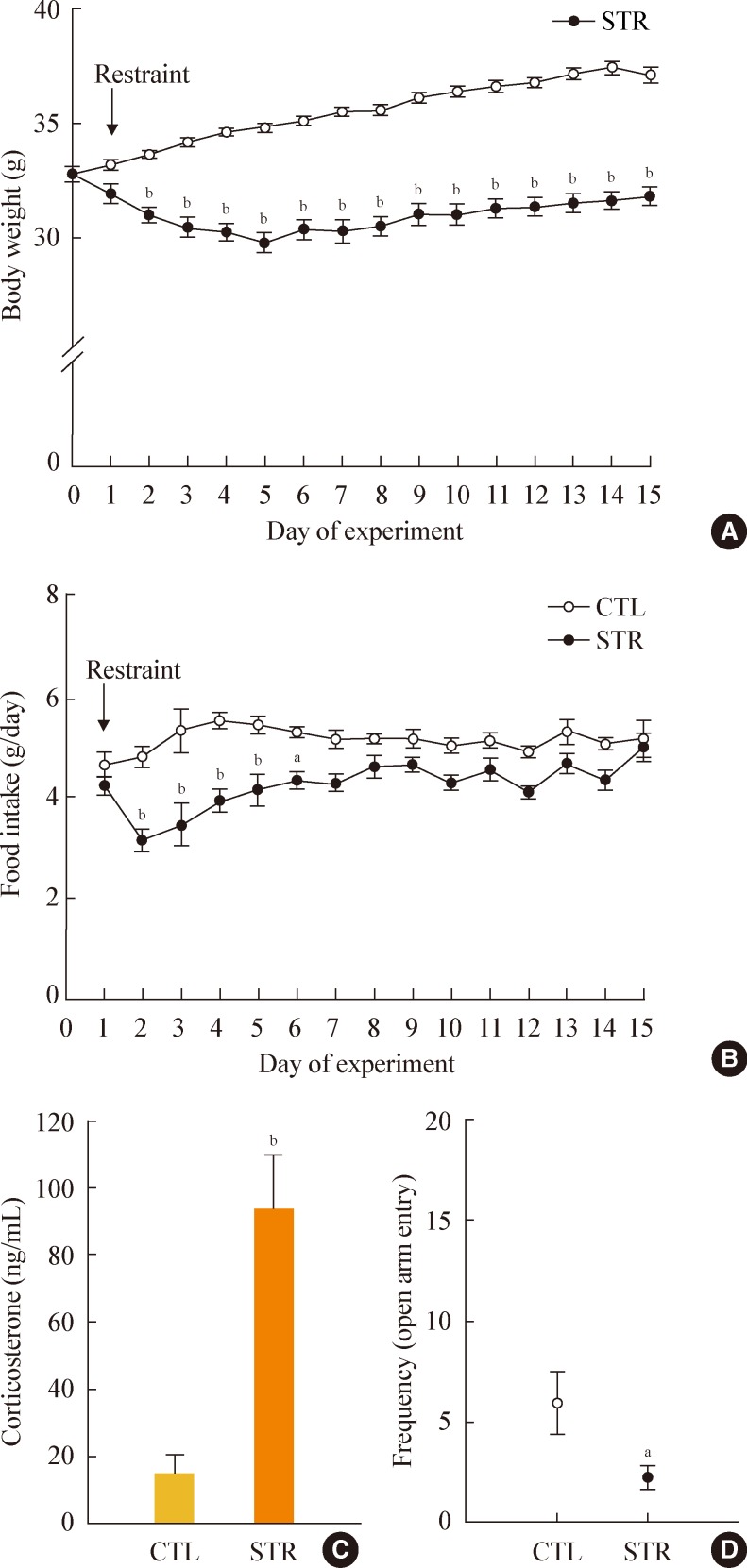

Fig. 2Reverse transcriptase polymerase chain reaction analysis of the altered gene expression identified from microarray analysis. The levels of hydroxysteroid (17-β) dehydrogenase 1 (Hsd17b1) and cytochrome P450, family 11, subfamily a, polypeptide 1 (Cyp11a1) mRNA were increased in stressed mice (STR), while glycoprotein hormones, α subunit (Cga) and growth hormone (Gh) mRNA levels were decreased. Statistical difference was evaluated by Student unpaired t test. Data are presented as mean±SE. aP<0.05; bP<0.01 vs. control mice (CTL) (n=6 in each group).


Fig. 3Effects of restraint stress on hypothalamic gene expression and serum hormone levels. (A) Hypothalamic mRNA expression of ghrelin and (B) pro-opiomelanocortin (POMC) in mice exposed daily to 2 hours of restraint for 4 days. (C) Serum corticosterone and (D) leptin levels for mice exposed daily to 2 hours of restraint for 4 days. Statistical differences were evaluated by one-way analysis of variance and Dunnett t test. Data are presented as mean±SE. aP<0.05; bP<0.01 vs. control mice (n=6 in each group).
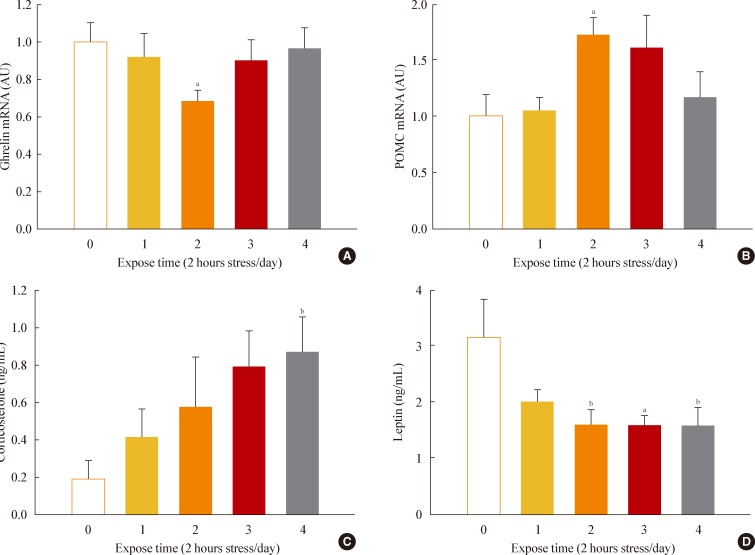

Figure & Data
References
Citations
Citations to this article as recorded by 

- Potential antimicrobial activity of camel milk as a traditional functional food against foodborne pathogens in vivo and in vitro
Amro Abdelazez, Sherif Melak, Heba Abdelmotaal, Garsa Alshehry, Huda Al-jumayi, Eman Algarni, Xiang-Chen Meng
Food Science and Technology International.2024; 30(3): 239. CrossRef - Effects of home‐cage elevation on behavioral tests in mice
Hiroshi Ueno, Yu Takahashi, Shinji Murakami, Kenta Wani, Yosuke Matsumoto, Motoi Okamoto, Takeshi Ishihara
Brain and Behavior.2024;[Epub] CrossRef - Anaesthesia-induced Changes in Genomic Expression Leading to
Neurodegeneration
Khalid Saad Alharbi, Waleed Hassan Almalki, Sami I. Alzarea, Imran Kazmi, Fahad A. Al-Abbasi, Obaid Afzal, Abdulmalik Saleh Alfawaz Altamimi, Mohammed Albratty, Asim Najmi, Gaurav Gupta
CNS & Neurological Disorders - Drug Targets.2024; 23(4): 411. CrossRef - Stress integration by an ascending adrenergic-melanocortin circuit
Connor Laule, Nilufer Sayar-Atasoy, Iltan Aklan, Hyojin Kim, Tayfun Ates, Debbie Davis, Deniz Atasoy
Neuropsychopharmacology.2024;[Epub] CrossRef - Stress-induced changes in cognitive function and intestinal barrier integrity can be ameliorated by venlafaxine and synbiotic supplementations
Sarawut Lapmanee, Nattapon Supkamonseni, Sakkarin Bhubhanil, Nattakan Treesaksrisakul, Chaiyos Sirithanakorn, Mattaka Khongkow, Katawut Namdee, Piyaporn Surinlert, Chittipong Tipbunjong, Prapimpun Wongchitrat
PeerJ.2024; 12: e17033. CrossRef - Effects of prenatal THC vapor exposure on body weight, glucose metabolism, and feeding behaviors in chow and high-fat diet fed rats
Catherine Hume, Samantha L. Baglot, Lucia Javorcikova, Savannah H. M. Lightfoot, Jessica Scheufen, Matthew N. Hill
International Journal of Obesity.2024;[Epub] CrossRef - The inhalation effect of Osmanthus fragrans var. Aurantiacus on physiological parameters in chronically stressed rats
Moon Yeon Youn, Jin-Ju Cho, Seong Jun Hong, Seong Min Jo, Hyangyeon Jeong, Sojeong Yoon, Younglan Ban, Hyeonjin Park, Jae Kyeom Kim, Young Jun Kim, Eui-Cheol Shin
Food Chemistry: X.2024; 22: 101304. CrossRef - Intranasal delivery of paroxetine: A preclinical study on pharmacokinetics, depressive-like behaviour, and neurochemical sex differences
Soraia Silva, Joana Bicker, S. Fialho, Susana Cunha, Amílcar Falcão, Ana Fortuna
Biochemical Pharmacology.2024; 223: 116184. CrossRef - Translational models of stress and resilience: An applied neuroscience methodology review
Zeynep Seda Albayrak, Andreia de Fátima da Silva Vaz, Joeri Bordes, Selen Ünlü, Milou S.C. Sep, Christiaan H. Vinkers, Luisa Pinto, Hale Yapıcı Eser
Neuroscience Applied.2024; : 104064. CrossRef - Stress exacerbates pancreatic cancer both directly and indirectly by creating an immunosuppressive environment
Takahiro Ikeda, Tomohiko Adachi, Takayuki Tanaka, Daisuke Miyamoto, Hajime Imamura, Hajime Matsushima, Kazuo Yamamoto, Masaaki Hidaka, Kengo Kanetaka, Susumu Eguchi
Journal of Hepato-Biliary-Pancreatic Sciences.2023; 30(7): 935. CrossRef - Up-regulation of BDNF/TrkB signaling by δ opioid receptor agonist SNC80 modulates depressive-like behaviors in chronic restraint-stressed mice
Shuo Wu, Kuan Ning, Yujun Wang, Lesha Zhang, Jinggen Liu
European Journal of Pharmacology.2023; 942: 175532. CrossRef - Assessment of Stress Caused by Environmental Changes for Improving the Welfare of Laboratory Beagle Dogs
Gwang-Hoon Lee, Woori Jo, Tae-Ku Kang, Taeho Oh, KilSoo Kim
Animals.2023; 13(6): 1095. CrossRef - Butein ameliorates chronic stress induced atherosclerosis via targeting anti-inflammatory, anti-fibrotic and BDNF pathways
Mujeeba Rehman, Rishabh Chaudhary, Sonu Rajput, Vipul Agarwal, Arjun Singh Kaushik, Siddhi Srivastava, Sukriti Srivastava, Rohit Singh, Irfan Aziz, Sanjay Singh, Vikas Mishra
Physiology & Behavior.2023; 267: 114207. CrossRef - Impact of exercise on brain-bone marrow interactions in chronic stress: potential mechanisms preventing stress-induced hypertension
Thu Van Nguyen, Ko Yamanaka, Keisuke Tomita, Jasenka Zubcevic, Sabine S. S. Gouraud, Hidefumi Waki
Physiological Genomics.2023; 55(5): 222. CrossRef - Effect of mobile phone radiofrequency electromagnetic radiations on oxidative stress and feeding behaviour in Sprague Dawley (SD) rats
Pravallika Pagadala, Vinutha Shankar M S, Sumathi M E
Indian Journal of Physiology and Pharmacology.2023; 67: 131. CrossRef - Mechanical loading and autophagy: A study on the BoNT-A injection-induced condylar cartilage degeneration
Siyu Hou, Sisi Peng, Hongwei Dai, Jinlin Song, Ling Xu, Jianping Zhou, Lingjie Li
Archives of Biochemistry and Biophysics.2023; 749: 109788. CrossRef - Continuous deep brain stimulation of the nucleus accumbens reduces food intake but does not affect body weight in mice fed a high-fat diet
Harold F. Hounchonou, Hui Tang, Raik Paulat, Andrea Kühn, Joachim Spranger, Christoph van Riesen, Lukas Maurer
Scientific Reports.2023;[Epub] CrossRef - Chronic stress predisposes to the aggravation of inflammation in autoimmune diseases with focus on rheumatoid arthritis and psoriasis
Rishabh Chaudhary, Ajay Prasad, Vipul Agarwal, Mujeeba Rehman, Anand Kumar, Arjun Singh Kaushik, Siddhi Srivastava, Sukriti Srivastava, Vikas Mishra
International Immunopharmacology.2023; 125: 111046. CrossRef - Venlafaxine and synbiotic attenuated learned fear-like behavior and recognition memory impairment in immobilized-stressed rats
Sarawut Lapmanee, Sakkarin Bhubhanil, Siriwan Sriwong, Mattaka Khongkow, Katawut Namdee, Prapimpun Wongchitrat, Pawin Pongkorpsakol
Physiology and Pharmacology.2023; 27(2): 171. CrossRef - Gonadotropin levels reduced in seven days immobilization stress-induced depressive-like behavior in female rats
Zafer Sahin, Alpaslan Ozkurkculer, Omer Faruk Kalkan, Funda Gulcu Bulmus, Ozgur Bulmus, Selim Kutlu
Journal of Basic and Clinical Physiology and Pharmacology.2022; 33(2): 199. CrossRef - Impact of the Sound of Magnetic Resonance Imaging Pulse Sequences in Awake Mice
Joana Almeida, Frederico Severo, Daniel Nunes
Journal of Applied Animal Welfare Science.2022; 25(1): 75. CrossRef - Virgin coconut oil abrogates depression-associated cognitive deficits by modulating hippocampal antioxidant balance, GABAergic and glutamatergic receptors in mice
Edem Ekpenyong Edem, Blessing Eghosa Ihaza, Adedamola Adediran Fafure, Azeez Olakunle Ishola, Kate Eberechukwu Nebo, Linus Anderson Enye, Elizabeth Toyin Akinluyi
Drug Metabolism and Personalized Therapy.2022; 37(2): 177. CrossRef - Characterizing the behavioral and neuroendocrine features of susceptibility and resilience to social stress
Dalia Murra, Kathryn L. Hilde, Anne Fitzpatrick, Pamela M. Maras, Stanley J. Watson, Huda Akil
Neurobiology of Stress.2022; 17: 100437. CrossRef - VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors
Soo Ji Baek, Jin Sung Park, Jinhyun Kim, Yukio Yamamoto, Keiko Tanaka-Yamamoto
eLife.2022;[Epub] CrossRef - Repeated Restraint Stress and Binge Alcohol during Adolescence Induce Long-Term Effects on Anxiety-like Behavior and the Expression of the Endocannabinoid System in Male Rats
Laura Sánchez-Marín, María Flores-López, Ana L. Gavito, Juan Suárez, Francisco Javier Pavón-Morón, Fernando Rodríguez de Fonseca, Antonia Serrano
Biomedicines.2022; 10(3): 593. CrossRef - “Sibling” battle or harmony: crosstalk between nesfatin-1 and ghrelin
Xi Chen, Jing Dong, Qian Jiao, Xixun Du, Mingxia Bi, Hong Jiang
Cellular and Molecular Life Sciences.2022;[Epub] CrossRef - Pharmacological Enhancement of Extinction Retention in Non-stressed Adolescent Rats but Not Those Exposed to Chronic Corticosterone
Anthea A. Stylianakis, Kathryn D. Baker, Rick Richardson
Frontiers in Neuroscience.2022;[Epub] CrossRef - The effect of choline alphoscerate on non spatial memory and neuronal differentiation in a rat model of dual stress
Hyo Jeong Yu, Ye Lin Kim, Min Jung Kim, Jung Mee Park, So Young Park, Shi Nae Park, Dong Won Yang
Brain Research.2022; 1786: 147900. CrossRef - Stress and the brain transcriptome: Identifying commonalities and clusters in standardized data from published experiments
Adrian M. Stankiewicz, Aneta Jaszczyk, Joanna Goscik, Grzegorz R. Juszczak
Progress in Neuro-Psychopharmacology and Biological Psychiatry.2022; 119: 110558. CrossRef - Repeated restraint stress modifies fatty acid and amino acid metabolism in the mouse skin
Yume KITAGAWA, Kaho HAYAKAWA, Daichi OIKAWA, Kazuki IKEDA, Maki IKEDA, Daiki HARADA, Mitsuhiro FURUSE
Journal of Veterinary Medical Science.2022; 84(4): 511. CrossRef - Antidiabetic Properties and Toxicological Assessment of Antidesma celebicum Miq: Ethanolic Leaves Extract in Sprague–Dawley Rats
Raysa Y. Pratiwi, Berna Elya, Heri Setiawan, Roshamur C. Forestrania, Rizna T. Dewi, Kim Wei Chan
Advances in Pharmacological and Pharmaceutical Sciences.2022; 2022: 1. CrossRef - Proteomic and microbiota analyses of the oral cavity during psychological stress
Durga Paudel, Yasuhiro Kuramitsu, Osamu Uehara, Tetsuro Morikawa, Koki Yoshida, Sarita Giri, Syed Taufiqul Islam, Takao Kitagawa, Tadashi Kondo, Kazuki Sasaki, Hirofumi Matsuoka, Hiroko Miura, Yoshihiro Abiko, Jon M. Jacobs
PLOS ONE.2022; 17(5): e0268155. CrossRef - Long-lasting effects of postweaning sleep deprivation on cognitive function and social behaviors in adult mice
Boya Huang, Binhuang Sun, Rui Yang, Shihao Liang, Xinrui Li, Yi Guo, Qian Meng, Yaling Fu, Wenshuya Li, Penghui Zhao, Miao Gong, Yun Shi, Li Song, Sheng Wang, Fang Yuan, Haishui Shi
Neuropharmacology.2022; 215: 109164. CrossRef - High Housing Density-Induced Chronic Stress Diminishes Ovarian Reserve via Granulosa Cell Apoptosis by Angiotensin II Overexpression in Mice
Jihyun Kim, Sooseong You
International Journal of Molecular Sciences.2022; 23(15): 8614. CrossRef - The effect of chronic stress and its preconditioning on spatial memory as well as hippocampal LRP1 and RAGE expression in a streptozotocin-induced rat model of Alzheimer’s disease
Zohreh Taghadosi, Asadollah Zarifkar, Vahid Razban, Hadi Aligholi
Metabolic Brain Disease.2022; 37(8): 2699. CrossRef - Validity of the peak velocity to detect physical training improvements in athymic mice
Maurício Beitia Kraemer, Karen Christine Silva, Camila Cunha França Kraemer, Juliana Silva Pereira, Ivan Gustavo Masseli dos Reis, Denise Gonçalves Priolli, Leonardo Henrique Dalcheco Messias
Frontiers in Physiology.2022;[Epub] CrossRef - Nicotine exposure through breastfeeding affects brain‐derived neurotrophic factor and synaptic proteins levels in the brain of stressed adult female mice
Antonio Alves Pereira Júnior, Gabriel Estevam Santos de Amorim, Raphael Caio Tamborelli Garcia, Jéssyca Milene Ribeiro, Alessandra Oliveira Silva, Carolina Aparecida de Faria Almeida, Carla Speroni Ceron, Silvia Graciela Ruginsk, José Antunes‐Rodrigues, L
International Journal of Developmental Neuroscience.2022; 82(8): 758. CrossRef - Fluoxetine treatment supports predictive validity of the three hit model of depression in male PACAP heterozygous mice and underpins the impact of early life adversity on therapeutic efficacy
Tamás Gaszner, József Farkas, Dániel Kun, Balázs Ujvári, Gergely Berta, Valér Csernus, Nóra Füredi, László Ákos Kovács, Hitoshi Hashimoto, Dóra Reglődi, Viktória Kormos, Balázs Gaszner
Frontiers in Endocrinology.2022;[Epub] CrossRef - Abnormal expression of cortical cell cycle regulators underlying anxiety and depressive-like behavior in mice exposed to chronic stress
Ana Paula Mendes-Silva, Thomas Damien Prevot, Mounira Banasr, Etienne Sibille, Breno Satler Diniz
Frontiers in Cellular Neuroscience.2022;[Epub] CrossRef - Chronic Inhibition of Aggressive Behavior Induces Behavioral Change in Mice
Hiroshi Ueno, Yu Takahashi, Shinji Murakami, Kenta Wani, Tetsuji Miyazaki, Yosuke Matsumoto, Motoi Okamoto, Takeshi Ishihara, Enzo Emanuele
Behavioural Neurology.2022; 2022: 1. CrossRef - Korean Red Ginseng reduces chronic social defeat stress-induced mood disorders via N-methyl-D-aspartate receptor modulation in mice
Bo-Ram Lee, Ju-Hyun Lee, Yong-Hyun Ko, Jee-Yeon Seo, Kwang-Hyun Hur, Young-Jung Kim, Seon-Kyung Kim, Seong-Eon Kim, Seok-Yong Lee, Choon-Gon Jang
Journal of Ginseng Research.2021; 45(2): 254. CrossRef - The Rap1 small GTPase is a critical mediator of the effects of stress on prefrontal cortical dysfunction
B. A. Kermath, A. M. Vanderplow, K. J. Bjornson, E. N. Seablom, A. M. Novak, C. R. Bernhardt, M. E. Cahill
Molecular Psychiatry.2021; 26(7): 3223. CrossRef - Anxiolytic activity of Psidium guajava in mice subjected to chronic restraint stress and effect on neurotransmitters in brain
Swati Sahoo, Prashant S. Kharkar, Niteshkumar U. Sahu, Brijesh S.
Phytotherapy Research.2021; 35(3): 1399. CrossRef - Changes in nitric oxide, carbon monoxide, hydrogen sulfide and male reproductive hormones in response to chronic restraint stress in rats
Amira Moustafa
Free Radical Biology and Medicine.2021; 162: 353. CrossRef - Behavioral and neurological improvement by Cydonia oblonga fruit extract in chronic immobilization stress rats
Fatma Z. Sakhri, Naoki Adachi, Sakina Zerizer, Yusuke Ohashi, Hideshi Ikemoto, Mana Tsukada, Zahia Kabouche, Tadashi Hisamitsu, Masataka Sunagawa
Phytotherapy Research.2021; 35(4): 2074. CrossRef - Restraint stress induces uterine microenvironment disorder in mice during early pregnancy through the β2-AR/cAMP/PKA pathway
Jiayin Lu, Guanhui Liu, Zixu Wang, Jing Cao, Yaoxing Chen, Yulan Dong
Stress.2021; 24(5): 514. CrossRef - A model of negative emotional contagion between male-female rat dyads: Effects of voluntary exercise on stress-induced behavior and BDNF-TrkB signaling
Gavin M. Meade, Lily S. Charron, Lantz W. Kilburn, Zhe Pei, Hoau-Yan Wang, Siobhan Robinson
Physiology & Behavior.2021; 234: 113286. CrossRef - Chronic stress promotes acute myeloid leukemia progression through HMGB1/NLRP3/IL-1β signaling pathway
Na Liu, Yifan Wu, Xin Wen, Peng Li, Fei Lu, Hong Shang
Journal of Molecular Medicine.2021; 99(3): 403. CrossRef - Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice
Jinho Kim, Yoo-Hun Suh, Keun-A Chang
Molecular Brain.2021;[Epub] CrossRef - Amelioration on oxidative stress, testosterone, and cortisol levels after administration of Vitamins C and E in albino rats with chronic variable stress
Nanik Hidayatik, Agus Purnomo, Faisal Fikri, Muhammad Thohawi Elziyad Purnama
Veterinary World.2021; 14(1): 137. CrossRef - Decreased expression of AKAP4 and TyrPho proteins in testis, epididymis, and spermatozoa with low sexual performance of mice induced by modified CUMS
Pannawat Choowong‐in, Jintana Sattayasai, Chanasorn Poodendaen, Sitthichai Iamsaard
Andrologia.2021;[Epub] CrossRef - Chronic Mild Unpredictable Stress and High-Fat Diet Given during Adolescence Impact Both Cognitive and Noncognitive Behaviors in Young Adult Mice
Stephen L. P. Lippi
Brain Sciences.2021; 11(2): 260. CrossRef - Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats
Tina Kroll, Nikola Kornadt-Beck, Angela Oskamp, David Elmenhorst, Chadi Touma, Rupert Palme, Andreas Bauer
Animals.2021; 11(3): 710. CrossRef - COVID-19 Stress and Food Intake: Protective and Risk Factors for Stress-Related Palatable Food Intake in U.S. Adults
Jennifer R. Sadler, Gita Thapaliya, Elena Jansen, Anahys H. Aghababian, Kimberly R. Smith, Susan Carnell
Nutrients.2021; 13(3): 901. CrossRef - Effects of Long‐Term Exposure of Intermediate Frequency Magnetic Fields (20 kHz, 360 µT) on the Development, Pathological Findings, and Behavior of Female Mice
Alexander Lerchl, Karen Drees (née Grote), Isabel Gronau, Dirk Fischer, Julia Bauch, Axel Hoppe
Bioelectromagnetics.2021; 42(4): 309. CrossRef - Impact of whole‐body versus nose‐only inhalation exposure systems on systemic, respiratory, and cardiovascular endpoints in a 2‐month cigarette smoke exposure study in the ApoE−/− mouse model
Ulrike Kogel, Ee Tsin Wong, Justyna Szostak, Wei Teck Tan, Francesco Lucci, Patrice Leroy, Bjoern Titz, Yang Xiang, Tiffany Low, Sin Kei Wong, Emmanuel Guedj, Nikolai V. Ivanov, Walter K. Schlage, Manuel C. Peitsch, Arkadiusz Kuczaj, Patrick Vanscheeuwijc
Journal of Applied Toxicology.2021; 41(10): 1598. CrossRef - Neuroprotective Effects of Acrocomia aculeata Pulp Oil Microcapsules on Rats Subjected to Chronic Stress
Ana Cristina Jacobowski, Eduardo Benedetti Parisotto, Leonardo Recena Aydos, Roberta Serafim de Souza, Sandra Viveros, Ana Laura Colín-Gonzalez, Iandara Schettert Silva, Eliana Janet Sanjinez-Argandoña, Danilo Wilhelm Filho, Abel Santamaría-Del Angel, Mar
Journal of Medicinal Food.2021; 24(10): 1068. CrossRef - Chronic Sulfasalazine Treatment in Mice Induces System xc− - Independent Adverse Effects
Lise Verbruggen, Lindsay Sprimont, Eduard Bentea, Pauline Janssen, Azzedine Gharib, Lauren Deneyer, Laura De Pauw, Olaya Lara, Hideyo Sato, Charles Nicaise, Ann Massie
Frontiers in Pharmacology.2021;[Epub] CrossRef - Effects of Achillea tenuifolia Lam. hydro-alcoholic extract on anxiety-like behavior and reproductive parameters in rat model of chronic restraint stress
Y Bagheri, E Fathi, A Maghoul, S Moshtagh, K Mokhtari, A Abdollahpour, S Montazersaheb, A Bagheri
Human & Experimental Toxicology.2021; 40(11): 1852. CrossRef - Pain sensitivity increases with sleep disturbance under predictable chronic mild stress in mice
Junhel Dalanon, Sachiko Chikahisa, Tetsuya Shiuchi, Noriyuki Shimizu, Parimal Chavan, Yoshitaka Suzuki, Kazuo Okura, Hiroyoshi Séi, Yoshizo Matsuka
Scientific Reports.2021;[Epub] CrossRef - Stress Diminishes BDNF-stimulated TrkB Signaling, TrkB-NMDA Receptor Linkage and Neuronal Activity in the Rat Brain
Siobhan Robinson, Allison S. Mogul, Elisa M. Taylor-Yeremeeva, Amber Khan, Anthony D. Tirabassi, Hoau-Yan Wang
Neuroscience.2021; 473: 142. CrossRef - Improved Training and Semen Collection Outcomes Using the Closed Box Chair for Macaques
Lisa A. Houser, Cathy Ramsey, Fernanda M. de Carvalho, Breanna Kolwitz, Chelsey Naito, Kristine Coleman, Carol B. Hanna
Animals.2021; 11(8): 2384. CrossRef - Ethologically relevant repeated acute social stress induces maternal neglect in the lactating female mouse
Zachary J. Rosinger, Heather S. Mayer, Jacqueline I. Geyfen, Mable K. Orser, Danielle S. Stolzenberg
Developmental Psychobiology.2021;[Epub] CrossRef - Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming
Xiaoguo Zheng, Zhenhua Li, Guishuan Wang, Hanshu Wang, Yuchuan Zhou, Xinzhi Zhao, C. Yan Cheng, Yunbo Qiao, Fei Sun
Cell Discovery.2021;[Epub] CrossRef - Involvement of Ghrelin Dynamics in Stress-Induced Eating Disorder: Effects of Sex and Aging
Chihiro Yamada
International Journal of Molecular Sciences.2021; 22(21): 11695. CrossRef - Voluntary wheel running behaviour as a tool to assess the severity in a mouse pancreatic cancer model
Nora Weegh, Eva Zentrich, Dietmar Zechner, Birgitta Struve, Laura Wassermann, Steven Roger Talbot, Simone Kumstel, Miriam Heider, Brigitte Vollmar, André Bleich, Christine Häger, Dragan Hrncic
PLOS ONE.2021; 16(12): e0261662. CrossRef - Chronic Restraint Stress Decreases the Excitability of Hypothalamic POMC Neuron and Increases Food Intake
Go Eun Ha, Eunji Cheong
Experimental Neurobiology.2021; 30(6): 375. CrossRef - Protective Effect of Sphaerococcus coronopifolius Crude Extract in Combination with Bacillus Calmette-Guerin on Ligature-Induced Depression in Female Wistar Rats
Fahima Fellah, Rédha Djenidi, Imen Chebout
Psychiatry Investigation.2020; 17(2): 130. CrossRef - Physical and Physiological Indicators of Welfare in Guinea Pigs (Cavia porcellus) Serving as Ambassador Animals
David M. Powell, Corinne P. Kozlowski, John Clark, Alice Seyfried, Eli Baskir, Ashley D. Franklin
Animals.2020; 10(5): 815. CrossRef - 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside Restores BDNF-TrkB and FGF2-Akt Signaling Axis to Attenuate Stress-induced Depression
Xi-Xi Li, Yun Yu, Xiu-Yuan Lang, Cheng-Yong Jiang, Rongfeng Lan, Xiao-Yan Qin
Neuroscience.2020; 430: 25. CrossRef - Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters
Swati Sahoo, S. Brijesh
Journal of Functional Foods.2020; 68: 103884. CrossRef - Vitamin C supplementation during chronic variable stress exposure modulates contractile functions of testicular artery and sperm parameters in male Wistar rats
Shakiru Ademola Salami, Hussein Mofomosara Salahdeen, Oyinlola Toluwani Moronkola, Babatunde Adekunle Murtala, Yinusa Raji
Middle East Fertility Society Journal.2020;[Epub] CrossRef - A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia
Na Qu, Yanlin He, Chunmei Wang, Pingwen Xu, Yongjie Yang, Xing Cai, Hesong Liu, Kaifan Yu, Zhou Pei, Ilirjana Hyseni, Zheng Sun, Makoto Fukuda, Yi Li, Qing Tian, Yong Xu
Molecular Psychiatry.2020; 25(5): 1006. CrossRef - Mirogabalin prevents repeated restraint stress-induced dysfunction in mice
Takashi Iwai, Akinori Kikuchi, Misa Oyama, Shun Watanabe, Mitsuo Tanabe
Behavioural Brain Research.2020; 383: 112506. CrossRef - A Systematic Review of In Vitro and In Vivo Radio Frequency Exposure Methods
Jared W. Hansen, Ellen M. Swartz, Jerika D. Cleveland, Sajid M. Asif, Benjamin Brooks, Benjamin D. Braaten, Daniel L. Ewert
IEEE Reviews in Biomedical Engineering.2020; 13: 340. CrossRef - Diminished ovarian reserve induced by chronic unpredictable stress in C57BL/6 mice
Lingyun Gao, Fangui Zhao, Yang Zhang, Wenjun Wang, Qi Cao
Gynecological Endocrinology.2020; 36(1): 49. CrossRef - Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways
Menizibeya O. Welcome, Nikos E. Mastorakis
Pharmacological Research.2020; 157: 104769. CrossRef - Anaesthetic-dependent changes in gene expression following acute and chronic exposure in the rodent brain
Dannielle H. Upton, Kata Popovic, Roger Fulton, Michael Kassiou
Scientific Reports.2020;[Epub] CrossRef - Comparative analysis of acute and chronic stress-induced neurobehavioral alteration and liver injury in mice
Tae Woo Oh, Kwang-Youn Kim, Hyun Ju Do, Young-Woo Kim, Kwang-Il Park
Molecular & Cellular Toxicology.2020; 16(4): 367. CrossRef - The impact of chronic stress on energy metabolism
Michael A. van der Kooij
Molecular and Cellular Neuroscience.2020; 107: 103525. CrossRef - In vitro and in vivo studies of nanoparticles of chitosan-Pandanus tectorius fruit extract as new alternative treatment for hypercholesterolemia via Scavenger Receptor Class B type 1 pathway
Efriyana Oksal, Inten Pangestika, Tengku Sifzizul Tengku Muhammad, Habsah Mohamad, Hermansyah Amir, Murni Nur Islamiah Kassim, Yosie Andriani
Saudi Pharmaceutical Journal.2020; 28(10): 1263. CrossRef - Behavioral, metabolic, and renal outcomes of 1-month isolation in adolescent male Dahl salt-sensitive rats
Oksana Nikolaienko, Elena Isaeva, Vladislav Levchenko, Oleg Palygin, Alexander Staruschenko
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2020; 319(6): R684. CrossRef - The Good, the Bad and the Unknown Aspects of Ghrelin in Stress Coping and Stress-Related Psychiatric Disorders
Eva Maria Fritz, Nicolas Singewald, Dimitri De Bundel
Frontiers in Synaptic Neuroscience.2020;[Epub] CrossRef Therapeutic Effects of Modified Gengnianchun Formula on Stress-Induced Diminished Ovarian Reserve Based on Experimental Approaches and Network Pharmacology
Lingyun Gao, Yang Zhang, Huangfang Xu, Fangui Zhao, Wenjun Wang
Drug Design, Development and Therapy.2020; Volume 14: 4975. CrossRef- Vitamin C protects against chronic social isolation stress-induced weight gain and depressive-like behavior in adult male rats
Alireza Najaf Dulabi, Zeinab Shakerin, Nasrin Mehranfard, Maedeh Ghasemi
Endocrine Regulations.2020; 54(4): 266. CrossRef - Intermittent restraint-induced sympathetic activation attenuates hepatic steatosis and inflammation in a high-fat diet-fed mouse model
Sung Bae Lee, Hyeong Geug Kim, Jin Seok Lee, Won Yong Kim, Myong Min Lee, Yun Hee Kim, Jung Ok Lee, Hyeon Soo Kim, Chang Gue Son
American Journal of Physiology-Gastrointestinal and Liver Physiology.2019; 317(6): G811. CrossRef - Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats
Qiuxia Pan, Jiajia Wu, Yueyun Liu, Xiaojuan Li, Jiaxu Chen
Molecules.2019; 24(3): 480. CrossRef - miR-132/212 is induced by stress and its dysregulation triggers anxiety-related behavior
Sydney Aten, Chloe E. Page, Anisha Kalidindi, Kelin Wheaton, Anzela Niraula, Jon P. Godbout, Kari R. Hoyt, Karl Obrietan
Neuropharmacology.2019; 144: 256. CrossRef - Oxytocin involves in chronic stress-evoked melanoma metastasis via β-arrestin 2-mediated ERK signaling pathway
Haoyi Ji, Na Liu, Jing Li, Dawei Chen, Dan Luo, Qian Sun, Yingchun Yin, Yanli Liu, Bing Bu, Xiaoyang Chen, Jingxin Li
Carcinogenesis.2019; 40(11): 1395. CrossRef - Anxiolytic effects of γ-oryzanol in chronically- stressed mice are related to monoamine levels in the brain
Salina Akter, Hiroyuki Sasaki, Kazi Rasel Uddin, Yuko Ikeda, Hiroki Miyakawa, Shigenobu Shibata
Life Sciences.2019; 216: 119. CrossRef - Resveratrol and dimethyl fumarate ameliorate testicular dysfunction caused by chronic unpredictable mild stress-induced depression in rats
Atef Tadros Fahim, Amal Ahmed Abd El-Fattah, Nermin Abdel Hamid Sadik, Bassam Mohamed Ali
Archives of Biochemistry and Biophysics.2019; 665: 152. CrossRef - Centella asiaticaPrevents Increase of Hippocampal Tumor Necrosis Factor-αIndependently of Its Effect on Brain-Derived Neurotrophic Factor in Rat Model of Chronic Stress
Mawaddah Ar Rochmah, Ika Murti Harini, Dian Eurike Septyaningtrias, Dwi Cahyani Ratna Sari, Rina Susilowati
BioMed Research International.2019; 2019: 1. CrossRef - Stress-related over-enhancement of the hypothalamic-pituitary-adrenal axis causes experimental neurolathyrism in rats
Kimino Minagawa, Shin-ichi Yamada, Ayano Suzuki, Saeko Ta, Toshio Kumai, Fernand Lambein, Kuniko Kusama-Eguchi
Environmental Toxicology and Pharmacology.2019; 72: 103245. CrossRef - Chronic Stress Combined with a Fructose Diet Reduces Hypothalamic Insulin Signaling and Antioxidative Defense in Female Rats
Sanja Kovačević, Jelena Nestorov, Gordana Matić, Ivana Elaković
Neuroendocrinology.2019; 108(4): 278. CrossRef - Gut-Brain Neuroendocrine Signaling Under Conditions of Stress—Focus on Food Intake-Regulatory Mediators
Andreas Stengel, Yvette Taché
Frontiers in Endocrinology.2018;[Epub] CrossRef - Chronic unpredictable mild stress-induced depressive-like behavior and dysregulation of brain levels of biogenic amines in Drosophila melanogaster
Stífani Machado Araujo, Marcia Rósula Poetini, Vandreza Cardoso Bortolotto, Shanda de Freitas Couto, Franciane Cabral Pinheiro, Luana Barreto Meichtry, Francielli Polet de Almeida, Elize Aparecida Santos Musachio, Mariane Trindade de Paula, Marina Prigol
Behavioural Brain Research.2018; 351: 104. CrossRef - Melatonin attenuates chronic immobilization stress-induced muscle atrophy in rats: Influence on lactate-to-pyruvate ratios and Na+/K+ ATPase activity
Eman S.H. Abd Allah, Ahmed M. Mahmoud
Pathophysiology.2018; 25(4): 353. CrossRef - Chronic stress effects and their reversibility on the Fallopian tubes and uterus in rats
S. Divyashree, H. N. Yajurvedi
Reproduction, Fertility and Development.2018; 30(2): 380. CrossRef - Evidence-based severity assessment: Impact of repeated versus single open-field testing on welfare in C57BL/6J mice
Carina Bodden, Sophie Siestrup, Rupert Palme, Sylvia Kaiser, Norbert Sachser, S. Helene Richter
Behavioural Brain Research.2018; 336: 261. CrossRef - Repeated Neck Restraint Stress Bidirectionally Modulates Excitatory Transmission in the Dentate Gyrus and Performance in a Hippocampus-dependent Memory Task
Jadwiga Spyrka, Grzegorz Hess
Neuroscience.2018; 379: 32. CrossRef - Stress-induced strain and brain region-specific activation of LINE-1 transposons in adult mice
Ugo Cappucci, Giulia Torromino, Assunta Maria Casale, Jeremy Camon, Fabrizio Capitano, Maria Berloco, Andrea Mele, Sergio Pimpinelli, Arianna Rinaldi, Lucia Piacentini
Stress.2018; 21(6): 575. CrossRef - The involvement of Rhopr-CRF/DH in feeding and reproduction in the blood-gorging insect Rhodnius prolixus
Shirin Mollayeva, Ian Orchard, Angela B. Lange
General and Comparative Endocrinology.2018; 258: 79. CrossRef - Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans
Muhammad I. ul Akbar Yousufzai, Elia S. Harmatz, Mohsin Shah, Muhammad O. Malik, Ki A. Goosens
Translational Psychiatry.2018;[Epub] CrossRef - Sub-Chronic Stress Exacerbates the Pro-Thrombotic Phenotype in BDNFVal/Met Mice: Gene-Environment Interaction in the Modulation of Arterial Thrombosis
Leonardo Sandrini, Alessandro Ieraci, Patrizia Amadio, Fabrizio Veglia, Maurizio Popoli, Francis Lee, Elena Tremoli, Silvia Barbieri
International Journal of Molecular Sciences.2018; 19(10): 3235. CrossRef - Juvenile stress induces behavioral change and affects perineuronal net formation in juvenile mice
Hiroshi Ueno, Shunsuke Suemitsu, Shinji Murakami, Naoya Kitamura, Kenta Wani, Yosuke Matsumoto, Motoi Okamoto, Shozo Aoki, Takeshi Ishihara
BMC Neuroscience.2018;[Epub] CrossRef - Chronic stress induced duration dependent alterations in immune system and their reversibility in rats
H.N. Sarjan, H.N. Yajurvedi
Immunology Letters.2018; 197: 31. CrossRef - Chronic stress alters adrenal clock function in a sexually dimorphic manner
Matthew Stagl, Mary Bozsik, Christopher Karow, David Wertz, Ian Kloehn, Savin Pillai, Paul J Gasser, Marieke R Gilmartin, Jennifer A Evans
Journal of Molecular Endocrinology.2018; 60(2): 55. CrossRef - Impaired Growth Performance and Testicular Cells Apoptosis Following Restraint Stress in Adult Hypothyroid Mice
Asif Mehfooz, Quanwei Wei, Mohamed Babo Fadlalla, Farman Ali Siyal, Kuldeep Dhama, Dagan Mao, Fangxiong Shi
International Journal of Pharmacology.2017; 13(6): 541. CrossRef - Chronic stress induced disruption of the peri-infarct neurovascular unit following experimentally induced photothrombotic stroke
Zidan Zhao, Lin Kooi Ong, Sarah Johnson, Michael Nilsson, Frederick R Walker
Journal of Cerebral Blood Flow & Metabolism.2017; 37(12): 3709. CrossRef - Anticoagulation with warfarin and rivaroxaban ameliorates experimental autoimmune encephalomyelitis
Leonie Stolz, Amin Derouiche, Kavi Devraj, Frank Weber, Robert Brunkhorst, Christian Foerch
Journal of Neuroinflammation.2017;[Epub] CrossRef - Maternal high-fat diet intensifies the metabolic response to stress in male rat offspring
Roxana Karbaschi, Homeira Zardooz, Fariba Khodagholi, Leila Dargahi, Mina Salimi, FatemehSadat Rashidi
Nutrition & Metabolism.2017;[Epub] CrossRef - The effect of chronic noise stress on serum levels of cortisol, gonadotropins, and sexual hormones at implantation time of mice
Alireza Shafiei, Hassan Ehteram, Hossein Akbari, Masoud Motalebi Kashani, Mandana Beigi, Javad Amini Mahabadi, Tahere Mazoochi
Comparative Clinical Pathology.2017; 26(4): 779. CrossRef - Aging rather than stress strongly influences amino acid metabolisms in the brain and genital organs of female mice
Momoko Kodaira, Mao Nagasawa, Takeshi Yamaguchi, Hiromi Ikeda, Kimie Minaminaka, Vishwajit S. Chowdhury, Shinobu Yasuo, Mitsuhiro Furuse
Mechanisms of Ageing and Development.2017; 162: 72. CrossRef - Histone deacetylase-2 is involved in stress-induced cognitive impairment via histone deacetylation and PI3K/AKT signaling pathway modification
Jie Wu, Cui Liu, Ling Zhang, Chun-Hui Qu, Xiao-Long Sui, Hua Zhu, Lan Huang, Yan-Feng Xu, Yun-Lin Han, Chuan Qin
Molecular Medicine Reports.2017; 16(2): 1846. CrossRef - High Hydrostatic Pressure Extract of Ginger Exerts Antistress Effects in Immobilization-Stressed Rats
Sohee Moon, Mak-Soon Lee, Sunyoon Jung, Bori Kang, Seog-Young Kim, Seonyoung Park, Hye-Yeon Son, Chong-Tai Kim, Young-Hee Jo, In-Hwan Kim, Young Soon Kim, Yangha Kim
Journal of Medicinal Food.2017; 20(9): 864. CrossRef - Insulin, not glutamine dipeptide, reduces lipases expression and prevents fat wasting and weight loss in Walker 256 tumor-bearing rats
Hely de Morais, Flaviane de Fatima Silva, Francemilson Goulart da Silva, Milene Ortiz Silva, Maria Fernanda Rodrigues Graciano, Maria Isabel Lovo Martins, Ângelo Rafael Carpinelli, Tânia Longo Mazucco, Roberto Barbosa Bazotte, Helenir Medri de Souza
European Journal of Pharmacology.2017; 806: 67. CrossRef - HBK-15 protects mice from stress-induced behavioral disturbances and changes in corticosterone, BDNF, and NGF levels
Karolina Pytka, Monika Głuch-Lutwin, Magdalena Kotańska, Elżbieta Żmudzka, Magdalena Jakubczyk, Anna Waszkielewicz, Paulina Janiszewska, Maria Walczak
Behavioural Brain Research.2017; 333: 54. CrossRef - The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels
Chong Chen, Shin Nakagawa, Yan An, Koki Ito, Yuji Kitaichi, Ichiro Kusumi
Frontiers in Neuroendocrinology.2017; 44: 83. CrossRef - Long-term chronic stress exposure induces PCO phenotype in rat
S Divyashree, H N Yajurvedi
Reproduction.2016; 152(6): 765. CrossRef - Vasculoprotective Effects of 3-Hydroxybenzaldehyde against VSMCs Proliferation and ECs Inflammation
Byung Soo Kong, Soo Jung Im, Yang Jong Lee, Yoon Hee Cho, Yu Ri Do, Jung Woo Byun, Cheol Ryong Ku, Eun Jig Lee, Maria Cristina Vinci
PLOS ONE.2016; 11(3): e0149394. CrossRef - Effects of long-term chronic stress on the lymphoid organs and blood leukocytes of the rat (Rattus norvegicus)
S. Divyashree, H.N. Sarjan, H.N. Yajurvedi
Canadian Journal of Zoology.2016; 94(2): 137. CrossRef - Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe
Keitaro Yoshida, Yu Mimura, Ryosuke Ishihara, Hiroshi Nishida, Yuji Komaki, Tomohito Minakuchi, Tomokazu Tsurugizawa, Masaru Mimura, Hideyuki Okano, Kenji F. Tanaka, Norio Takata
Journal of Neuroscience Methods.2016; 274: 38. CrossRef - Integrated circuits and molecular components for stress and feeding: implications for eating disorders
J. A. Hardaway, N. A. Crowley, C. M. Bulik, T. L. Kash
Genes, Brain and Behavior.2015; 14(1): 85. CrossRef - The Use of Animal Models to Decipher Physiological and Neurobiological Alterations of Anorexia Nervosa Patients
Mathieu Méquinion, Christophe Chauveau, Odile Viltart
Frontiers in Endocrinology.2015;[Epub] CrossRef - Kososan, but not milnacipran, elicits antidepressant‐like effects in a novel psychological stress‐induced mouse model of depression
Atsushi Hori, Naoki Ito, Tetsuro Oikawa, Toshihiko Hanawa
Traditional & Kampo Medicine.2015; 2(1): 1. CrossRef - Electroconvulsive stimulation, but not chronic restraint stress, causes structural alterations in adult rat hippocampus—A stereological study
Mikkel V. Olesen, Gitta Wörtwein, Bente Pakkenberg
Hippocampus.2015; 25(1): 72. CrossRef - Dopamine release in the lateral hypothalamus is stimulated by α-MSH in both the anticipatory and consummatory phases of feeding
Romain Legrand, Nicolas Lucas, Jonathan Breton, Pierre Déchelotte, Sergueï O. Fetissov
Psychoneuroendocrinology.2015; 56: 79. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef - Contrasting mechanisms by which social isolation and restraint impair healing in male mice
Leah M. Pyter, Linglan Yang, Cassandra McKenzie, José M. da Rocha, C. Sue Carter, Bin Cheng, Christopher G. Engeland
Stress.2014; 17(3): 256. CrossRef - G Protein-Coupled Estrogen Receptor-1 Is Involved in the Protective Effect of Protocatechuic Aldehyde against Endothelial Dysfunction
Byung Soo Kong, Yoon Hee Cho, Eun Jig Lee, Rajesh Mohanraj
PLoS ONE.2014; 9(11): e113242. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite