Articles
- Page Path
- HOME > Endocrinol Metab > Volume 31(4); 2016 > Article
-
Review ArticleBrain Regulation of Energy Metabolism
-
Eun Roh1,2
 , Min-Seon Kim2
, Min-Seon Kim2
-
Endocrinology and Metabolism 2016;31(4):519-524.
DOI: https://doi.org/10.3803/EnM.2016.31.4.519
Published online: December 20, 2016
1Department of Biomedical Science, University of Ulsan College of Medicine, Seoul, Korea.
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding author: Min-Seon Kim. Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. Tel: +82-2-3010-3245, Fax: +82-2-3010-6962, mskim@amc.seoul.kr
• Received: September 8, 2016 • Revised: September 20, 2016 • Accepted: September 30, 2016
Copyright © 2016 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- In healthy individuals, energy intake is in balance with energy expenditure, which helps to maintain a normal body weight. The brain's inability to control energy homeostasis underlies the pathology of hyperphagia and obesity. The brain detects body energy excess and deficit by sensing the levels of circulating metabolic hormones and nutrients and by receiving metabolic information from the periphery via the autonomic nervous system. A specialized neuronal network coordinates energy intake behavior and the metabolic processes affecting energy expenditure. Here, we briefly review neuronal mechanisms by which our body maintains energy balance.
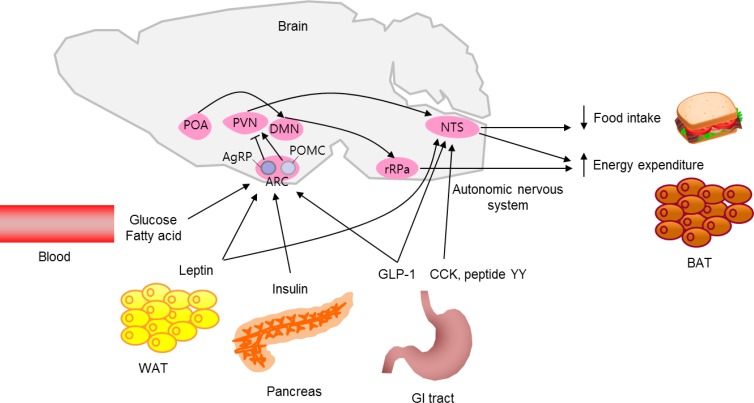
- Over the past decades, our knowledge of the neuronal regulation of energy homeostasis has dramatically expanded. Substantial evidence indicates that the brain plays a central role in the homeostatic regulation of energy metabolism. The brain integrates multiple peripheral metabolic inputs, such as nutrients, gut-derived hormones, and adiposity-related signals. This information on energy intake and body energy stores is transferred to specialized neurons in the hypothalamus and brainstem. In order to maintain energy homeostasis, the brain regulates diverse aspects of body metabolism, such as food-seeking behavior; gastric emptying; nutrient uptake in the gut; thermogenesis; pancreatic insulin secretion; and the effects of insulin in the liver, adipose tissue, and skeletal muscle. In this review, we describe the brain's regulatory mechanisms of food intake and energy expenditure (Fig. 1).
INTRODUCTION
- The hypothalamus is the region of the brain that controls food intake and body weight. The hypothalamic arcuate nucleus (ARC) is ideally situated near the third ventricle and the median eminence, which is an area with a relatively porous blood-brain barrier. This provides the ARC free access to circulating nutrients and hormones, making it the primary nutrient-sensing center of the hypothalamus. There are two distinct neuronal populations in the ARC, orexigenic neurons that express both neuropeptide Y (NPY) and agouti-related peptide (AgRP) and anorexigenic neurons that express proopiomelanocortin (POMC). These neurons are first-order neurons that respond to peripheral metabolic signals and project to second-order neurons of the paraventricular nucleus (PVN), the perifornical area adjacent to the fornix and the lateral hypothalamus (LH), and to autonomic preganglionic neurons in the brain stem and spinal cord.
- POMC neurons produce the anorectic peptide α-melanocyte stimulating hormone (α-MSH) by posttranscriptional processing of POMC. α-MSH binds to the melanocortin receptors 3 and 4 (MC3R and MC4R) on second-order neurons and activates catabolic pathways, leading to reduced food intake and increased energy expenditure [1]. On the other hand, central administration of NPY increases food intake via Y1 or Y5 receptors, which are highly expressed in the ARC, PVN, and ventromedial hypothalamus (VMH) [2]. Likewise, central administration of AgRP induces hyperphagia and weight gain by inhibiting the binding of α-MSH to MC3R/MC4R [3]. Selective ablation of NPY/AgRP neurons in adult mice results in anorexia and weight loss [4], demonstrating a critical role of these neurons in the regulation of energy homeostasis.
- Both POMC and NPY/AgRP neurons in the ARC alter their activity in response to blood glucose level [5]. Elevated extracellular glucose level activates POMC neurons, whereas NPY/AgRP neurons are activated in glucose-deprived conditions. Hypothalamic neuronal glucose deprivation induced by administration of 2-deoxy-D-glucose potentially increases food intake [6]. Circulating long-chain fatty acids (LCFAs) also act as nutrient abundance signals in the hypothalamus. Intracerebroventricular administration of LCFAs, specifically oleic acid, inhibits food intake by decreasing hypothalamic AgRP and NPY expression [7]. Increased levels of lipid metabolites such as malonyl CoA and LCFA-CoA in hypothalamic neurons are indicative of nutrient excess and lead to signals for food intake reduction [78]. The hypothalamic ARC is critical for sensing adiposity signals such as leptin and insulin. Leptin and insulin signal the status of body energy stores to the hypothalamus. Both leptin and insulin activate POMC neurons, while inhibiting NPY/AgRP neurons [910]. Ghrelin is a gut hormone secreted from the stomach during fasting, signaling the need for increased food intake. The orexigenic action of ghrelin is mediated by NPY/AgRP neurons in the ARC [11].
- The PVN is an important brain region that regulates neuroendocrine function by releasing critical neuropeptides, including oxytocin, thyrotropin-releasing hormone, and corticotrophin-releasing hormone. The PVN is also important for regulating energy balance. The PVN neurons are densely innervated by POMC and NPY/AgRP neurons [12], and they serve as secondorder neurons in the melanocortinergic neuronal circuit. For example, PVN oxytocin neurons mediate melanocortinergic control of food intake by innervating and regulating neurons in the nucleus of the solitary tract (NTS) [13]. Moreover, the PVN is a key brain region that mediates the actions of glucagon-like peptide-1 (GLP-1), an important gut-derived satiety signal [14].
- The VMH neurons mainly receive neuronal inputs from the ARC and then project their axons to the ARC, PVN, LH, dorsomedial nucleus (DMN), and the NTS. Most VMH neurons express steroidogenic factor 1 (SF-1) [15], and those VMH neurons that express SF-1 release brain-derived neurotrophic factor (BDNF). Selective deletion of BDNF in the VMH results in hyperphagia and obesity in mice [16]. Moreover, loss of function mutations in both BDNF and the BDNF receptor tropomyosin receptor kinase B cause hyperphagia and severe obesity in humans and in rodents, indicating that BDNF is an important satiety factor [1718].
- The LH is in the hypothalamic region where metabolic and reward-related information is integrated. This information is transferred to various brain areas such as the hindbrain, cortex, limbic system, thalamus, and spinal cord, allowing for a complex modulation of both behavioral and autonomic outflow. The LH contains two distinct neuronal populations that produce melaninconcentrating hormone (MCH) and orexin, respectively. Orexin demonstrates appetite-enhancing actions of directly activating NPY/AgRP neurons and indirectly inhibiting POMC neurons in the ARC [1920]. MCH also exerts orexigenic effects by modulating the ARC melanocortin system [21]. Genetic overexpression of MCH in mice leads to hyperphagia and obesity [22].
- The brainstem is another major brain area involved in the control of food intake. Meal-elicited gastrointestinal signals induce neuronal activation in the caudal brainstem, where vagal afferents terminate. Given that chemical and surgical vagal denervation is known to decrease meal size and duration, it is thought that meal-related signals are transferred to the brain via the vagal afferent [23]. The NTS is a major neuronal connection between the gut and brain. Like the ARC, the NTS is anatomically close to the area postrema. Thus, the NTS is specialized for receiving both humoral and neural signals from the periphery. Extensive reciprocal neuronal connections exist between the hypothalamus and the brainstem, and the amount of food intake is determined based on metabolic information delivered to both brain regions [24]. Metabolic signals from gut hormones are transferred to the brainstem through the vagal nerve. Cholecystokinin, GLP-1, and peptide YY are released from the enteroendocrine cells upon food intake, and they bind their receptors on the vagus nerve terminals. These food intake signals are delivered to the hypothalamus via the NTS; thereby, inducing satiety [2526]. Like hypothalamic neurons, NTS neurons produce POMC, NPY, and GLP-1. POMC-producing NTS neurons are activated upon food intake. These neurons also exhibit signal transducer and activator of transcription 3 (STAT3) activation in response to exogenous leptin [27], suggesting a role of brainstem neurons in sensing peripheral metabolic signals.
BRAIN REGULATION OF FOOD INTAKE
- Energy is consumed in the processes of physical activity, basal metabolism, and adaptive thermogenesis, all of which are modulated by the brain. The hypothalamic ARC is considered a key site for mediating leptin's effect on locomotor activity, since selective restoration of leptin signaling in the ARC, especially in POMC neurons, normalized locomotor activity in leptin receptor-null mice [28]. Meanwhile, NPY, AgRP, and orexin promote food-seeking behavior [2930].
- Thermogenesis refers to heat that is generated in order to maintain body temperature or in order to dissipate excess energy upon food intake. Brown adipose tissue (BAT), located in the interscapular area of rodents, plays a major role in thermogenesis [31]. Thermogenesis is a critical component of energy expenditure, especially in rodents. Central regulation of BAT thermogenesis is dependent on sympathetic outflow to BAT. Norepinephrine released from sympathetic nerve terminals binds to β3-adrenergic receptors on adipocytes in BAT and inguinal fat pads. Activated adrenergic receptors trigger cyclic-adenosine monophosphate signaling, which activates mitochondrial uncoupling protein-1 (UCP-1) and promotes enhanced thermogenesis.
- The preoptic area (POA) has been identified as the neural circuit that regulates sympathetic outflow to BAT by directly projecting to sympathetic premotor neurons in the rostral raphe pallidus [32]. Alternatively, POA neurons project to DMN neurons, which mediate POA-evoked thermoregulatory responses [33]. In addition, many hormonal and nutrient signals, such as glucose, insulin, leptin, and GLP-1, can influence sympathetic outflow to BAT [3435]. Central administration of an MC3R/MC4R agonist stimulates BAT activity through sympathetic outflow, suggesting a regulatory role of the hypothalamic melanocortin system in BAT thermogenesis [36].
- Brown-like fat adipocytes, so-called “beige” or “brite” adipocytes, are found in the inguinal subcutaneous area of rodents and in the supraclavicular, suprarenal, pericardial, and para-aortic areas and around the pancreas, kidney, and trachea in humans [37]. UCP-1 expression in beige adipocytes is low under basal conditions, but its expression is induced under certain circumstances, such as exposure to cold temperatures. Induction of white adipose tissue (WAT) browning in rodents increases energy expenditure and attenuates diet-induced obesity [38]. Conversely, blockade of WAT browning through deletion of Prdm16, a transcriptional coregulator that controls the development of brown adipocytes, promotes obesity [39]. Interestingly, insulin and leptin act synergistically on POMC neurons to promote both WAT browning and energy expenditure. These mechanisms seem to be important for resistance against the development of diet-induced obesity [40]. Therefore, it is thought that POMC neurons convey leptin and insulin signaling to drive WAT browning and to enhance energy expenditure.
BRAIN REGULATION OF ENERGY EXPENDITURE
- Obesity has reached epidemic levels worldwide, accompanied by the increased prevalence of multiple comorbidities. Considerable attention is now being paid to understand how our body maintains energy balance under healthy conditions and why these mechanisms become defective in obesity and cachexia. Expanding our knowledge of the brain's regulation of food intake and energy expenditure will lead us to effective therapeutic strategies for combating obesity and related metabolic disorders.
CONCLUSIONS
-
Acknowledgements
- This study was supported by grants from the National Research Foundation of Korea (NRF), funded by Ministry of Science, ICT, and Future Planning (2013M3C7A1056024 and 2015M3A9E 7029172).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 1999;24:155–163. ArticlePubMed
- 2. Raposinho PD, Pierroz DD, Broqua P, White RB, Pedrazzini T, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol Cell Endocrinol 2001;185:195–204. ArticlePubMed
- 3. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671. ArticlePubMedPDF
- 4. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005;310:683–685. ArticlePubMed
- 5. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 2003;144:1331–1340. ArticlePubMed
- 6. Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat. Am J Physiol 1975;229:1438–1447. ArticlePubMed
- 7. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–275. ArticlePubMed
- 8. Hu Z, Cha SH, Chohnan S, Lane MD. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc Natl Acad Sci U S A 2003;100:12624–12629. ArticlePubMedPMC
- 9. Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999;23:775–786. ArticlePubMed
- 10. Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 1999;848:114–123. ArticlePubMed
- 11. Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 2008;454:846–851. ArticlePubMedPMCPDF
- 12. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 2005;8:571–578. ArticlePubMedPDF
- 13. Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 2009;10:355–365. ArticlePubMed
- 14. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72. ArticlePubMedPDF
- 15. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 1997;18:361–377. ArticlePubMedPDF
- 16. Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of BDNF in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci 2007;27:14265–14274. ArticlePubMedPMC
- 17. Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 2004;7:1187–1189. ArticlePubMedPDF
- 18. Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 2006;55:3366–3371. ArticlePubMedPMC
- 19. van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 2004;7:493–494. ArticlePubMedPDF
- 20. Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci 2007;27:1529–1533. ArticlePubMedPMC
- 21. Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A 2003;100:10085–10090. ArticlePubMedPMC
- 22. Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest 2001;107:379–386. ArticlePubMedPMC
- 23. Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 2000;16:866–873. ArticlePubMed
- 24. van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 1984;224:1–24. ArticlePubMed
- 25. Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J 2012;36:391–398. ArticlePubMedPMC
- 26. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagalbrainstem-hypothalamic pathway. Brain Res 2005;1044:127–131. ArticlePubMed
- 27. Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 2006;147:3190–3195. ArticlePubMedPDF
- 28. Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72. ArticlePubMed
- 29. Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res 2000;873:181–187. ArticlePubMed
- 30. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011;14:351–355. ArticlePubMedPDF
- 31. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359. ArticlePubMed
- 32. Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 2004;19:67–74. ArticlePubMed
- 33. Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci 2009;29:11954–11964. ArticlePubMedPMC
- 34. Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 2004;114:652–658. ArticlePubMedPMC
- 35. Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012;61:2753–2762. ArticlePubMedPMC
- 36. Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 2007;148:5339–5347. ArticlePubMedPDF
- 37. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Coldactivated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–1508. ArticlePubMed
- 38. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105. ArticlePubMed
- 39. Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–316. ArticlePubMedPMC
- 40. Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015;160:88–104. ArticlePubMedPMC
References
Fig. 1
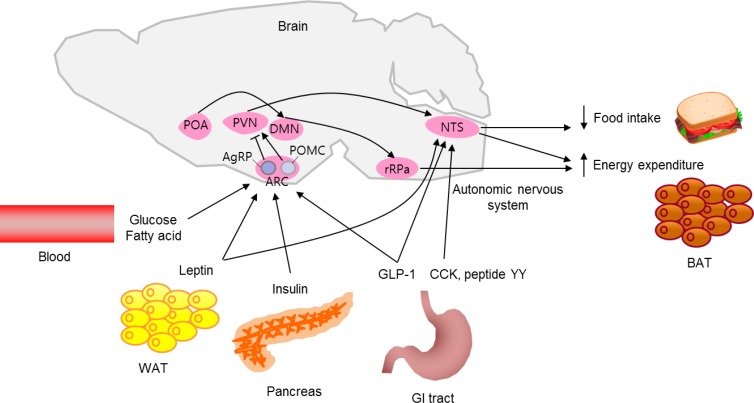
Model of brain regulation of energy metabolism. The brain integrates multiple, peripheral metabolic inputs, such as nutrients, gut-derived hormones (glucagon-like peptide-1 [GLP-1], cholecystokinin [CCK], and peptide YY), and adiposity-related signals (leptin and insulin) to regulate food intake and energy expenditure. Proopiomelanocortin (POMC)-producing and neuropeptide Y/agouti-related peptide (AgRP)-producing neurons in the hypothalamic arcuate nucleus (ARC) primarily sense the body's energy state and project to other hypothalamic nuclei, including the paraventricular nucleus (PVN) and lateral hypothalamus (not shown), which, in turn, project to the nucleus of the solitary tract (NTS) in the brainstem. The NTS responds to satiety signals via direct inputs to the NTS and indirect inputs to the hypothalamus and activates vagal afferents to reduce food intake. The preoptic area (POA) in the hypothalamus receives thermal sensory signals from cold exposure and activates the POA-dorsomedial hypothalamus (DMN)-rostral raphe pallidus (rRPa) pathway to promote brown adipose tissue (BAT) thermogenesis. The rRPa contains sympathetic premotor neurons that convey thermal signals from the POA and DMN to influence sympathetic outflow to the BAT in order to produce heat. The hypothalamic melanocortin system is also involved in thermoregulation. WAT, white adipose tissue; GI, gastrointestinal.

Figure & Data
References
Citations
Citations to this article as recorded by 

- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
Endocrinology and Metabolism.2024; 39(1): 1. CrossRef - Central inhibition of stearoyl-CoA desaturase has minimal effects on the peripheral metabolic symptoms of the 3xTg Alzheimer’s disease mouse model
Laura K. Hamilton, Paule E. H. M’Bra, Sophia Mailloux, Manon Galoppin, Anne Aumont, Karl J. L. Fernandes
Scientific Reports.2024;[Epub] CrossRef - Adipokines from white adipose tissue in regulation of whole body energy homeostasis
Bijayashree Sahu, Naresh C. Bal
Biochimie.2023; 204: 92. CrossRef - Growth hormone receptor (GHR) in AgRP neurons regulates thermogenesis in a sex-specific manner
Lukas Stilgenbauer, Juliana Bezerra Medeiros de Lima, Lucas Kniess Debarba, Manal Khan, Lisa Koshko, John J. Kopchick, Andrzej Bartke, Augusto Schneider, Marianna Sadagurski
GeroScience.2023; 45(3): 1745. CrossRef - Living high - training low model applied to C57BL/6J mice: Effects on physiological parameters related to aerobic fitness and acid-base balance
Pedro Paulo Menezes Scariot, Marcelo Papoti, Emanuel Elias Camolese Polisel, Juan Bordon Orsi, Paul R. Van Ginkel, Tomas A. Prolla, Fúlvia Barros Manchado-Gobatto, Claudio Alexandre Gobatto
Life Sciences.2023; 317: 121443. CrossRef - Whole Transcriptome Analysis of Hypothalamus in Mice during Short-Term Starvation
Eun-Young Oh, Byong Seo Park, Hye Rim Yang, Ho Gyun Lee, Thai Hien Tu, Sunggu Yang, Mi-Ryung Han, Jae Geun Kim
International Journal of Molecular Sciences.2023; 24(4): 3204. CrossRef - Hormonal Gut–Brain Signaling for the Treatment of Obesity
Eun Roh, Kyung Mook Choi
International Journal of Molecular Sciences.2023; 24(4): 3384. CrossRef - Neuronal Blockade of Thyroid Hormone Signaling Increases Sensitivity to Diet-Induced Obesity in Adult Male Mice
Eva Rial-Pensado, Laurence Canaple, Romain Guyot, Christoffer Clemmensen, Joëlle Wiersema, Shijia Wu, Sabine Richard, Anita Boelen, Timo D Müller, Miguel López, Frédéric Flamant, Karine Gauthier
Endocrinology.2023;[Epub] CrossRef - Genetic Contributors to Obesity
Ramya Sivasubramanian, Sonali Malhotra
Gastroenterology Clinics of North America.2023; 52(2): 323. CrossRef - Neurocomputational mechanisms of food and physical activity decision-making in male adolescents
Seung-Lark Lim, Amanda S. Bruce, Robin P. Shook
Scientific Reports.2023;[Epub] CrossRef - Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound
Yaoheng Yang, Jinyun Yuan, Rachael L. Field, Dezhuang Ye, Zhongtao Hu, Kevin Xu, Lu Xu, Yan Gong, Yimei Yue, Alexxai V. Kravitz, Michael R. Bruchas, Jianmin Cui, Jonathan R. Brestoff, Hong Chen
Nature Metabolism.2023; 5(5): 789. CrossRef - Changes in hypothalamic mu-opioid receptor expression following acute olanzapine treatment in female rats: Implications for feeding behavior
Maiken Krogsbaek, Nick Yao Larsen, Anne M. Landau, Connie Sanchez, Jens Randel Nyengaard
Journal of Chemical Neuroanatomy.2023; 132: 102324. CrossRef - Insulin Resistance and Glucose Metabolism during Infection
Borros Arneth
Endocrines.2023; 4(4): 685. CrossRef - The PACAP Paradox: Dynamic and Surprisingly Pleiotropic Actions in the Central Regulation of Energy Homeostasis
Nikki Le, Sarah Sayers, Veronica Mata-Pacheco, Edward J. Wagner
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Link Between Energy-Related Sensations and Metabolism: Implications for Treating Fatigue
Marco Filippi, Rainer Krähenmann, Patrick Fissler
Frontiers in Psychology.2022;[Epub] CrossRef - Unaltered Tonic Inhibition in the Arcuate Nucleus of Diet-induced Obese Mice
Moonsun Sa, Jung Moo Lee, Mingu Gordon Park, Jiwoon Lim, Jong Min Joseph Kim, Wuhyun Koh, Bo-Eun Yoon, C. Justin Lee
Experimental Neurobiology.2022; 31(3): 147. CrossRef - Hypothalamus–Muscle Parallel Induction of Metabolic Pathways Following Physical Exercise
Almog Katz, Meital Gonen, Yael Shahar, Asael Roichman, Batia Lerrer, Haim Yosef Cohen
Frontiers in Neuroscience.2022;[Epub] CrossRef - Monocarboxylate transporters (MCTs) in skeletal muscle and hypothalamus of less or more physically active mice exposed to aerobic training
P.P.M. Scariot, F.B. Manchado-Gobatto, W.R. Beck, M. Papoti, P.R. Van Ginkel, C.A. Gobatto
Life Sciences.2022; 307: 120872. CrossRef - Obesity-Related Genes Expression in Testes and Sperm Parameters Respond to GLP-1 and Caloric Restriction
Ana S. Correia, Sara C. Pereira, Tiago Morais, Ana D. Martins, Mariana P. Monteiro, Marco G. Alves, Pedro F. Oliveira
Biomedicines.2022; 10(10): 2609. CrossRef - A pilot study of contrast-enhanced electrical impedance tomography for real-time imaging of cerebral perfusion
Yuyan Zhang, Jian’an Ye, Yang Jiao, Weirui Zhang, Tao Zhang, Xiang Tian, Xuetao Shi, Feng Fu, Liang Wang, Canhua Xu
Frontiers in Neuroscience.2022;[Epub] CrossRef - Repercussions of maternal exposure to high-fat diet on offspring feeding behavior and body composition: a systematic review
Wenicios Ferreira Chaves, Isabeli Lins Pinheiro, Jacqueline Maria da Silva, Raul Manhães-de-Castro, Raquel da Silva Aragão
Journal of Developmental Origins of Health and Disease.2021; 12(2): 220. CrossRef - Obesity-associated Pathways of Anthocyanins
Elif YILDIZ, Metin GULDAS, Pinar ELLERGEZEN, Asli Gul ACAR, Ozan GURBUZ
Food Science and Technology.2021; 41( suppl 1): 1. CrossRef - Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism
Ming-Liang Lee, Hirokazu Matsunaga, Yuki Sugiura, Takahiro Hayasaka, Izumi Yamamoto, Taiga Ishimoto, Daigo Imoto, Makoto Suematsu, Norifumi Iijima, Kazuhiro Kimura, Sabrina Diano, Chitoku Toda
Nature Communications.2021;[Epub] CrossRef - Sleep and Cardiovascular Risk
Lyudmila Korostovtseva, Mikhail Bochkarev, Yurii Sviryaev
Sleep Medicine Clinics.2021; 16(3): 485. CrossRef - Evaluation and Management of Early Onset Genetic Obesity in Childhood
Sonali Malhotra, Ramya Sivasubramanian, Gitanjali Srivastava
Journal of Pediatric Genetics.2021; 10(03): 194. CrossRef - Gene expression atlas of energy balance brain regions
Maria Caterina De Rosa, Hannah J. Glover, George Stratigopoulos, Charles A. LeDuc, Qi Su, Yufeng Shen, Mark W. Sleeman, Wendy K. Chung, Rudolph L. Leibel, Judith Y. Altarejos, Claudia A. Doege
JCI Insight.2021;[Epub] CrossRef - New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases
Magdalena Czerwińska, Katarzyna Czarzasta, Agnieszka Cudnoch-Jędrzejewska
Frontiers in Physiology.2021;[Epub] CrossRef - A putative role for lncRNAs in epigenetic regulation of memory
Ashleigh B. Irwin, Rudhab Bahabry, Farah D. Lubin
Neurochemistry International.2021; 150: 105184. CrossRef - Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis
Kirill V. Zhukov, Alexandre A. Vetcher, Bagrat A. Gasparuan, Alexander Y. Shishonin
Polymers.2021; 13(23): 4248. CrossRef - Placental NEGR1 DNA methylation is associated with BMI and neurodevelopment in preschool-age children
E Breton, V Gagné-Ouellet, K Thibeault, R Guérin, Rj Van Lieshout, P Perron, Mf Hivert, L Bouchard
Epigenetics.2020; 15(3): 323. CrossRef - The dorsomedial hypothalamus and nucleus of the solitary tract as key regulators in a rat model of chronic obesity
Chen Zhang, Pernille Barkholt, Jens Christian Nielsen, Ditte Dencker Thorbek, Kristoffer Rigbolt, Niels Vrang, David Paul Drucker Woldbye, Jacob Jelsing
Brain Research.2020; 1727: 146538. CrossRef - Hypothalamic NAD+-Sirtuin Axis: Function and Regulation
Eun Roh, Min-Seon Kim
Biomolecules.2020; 10(3): 396. CrossRef - The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain
Jaewoo Hong, Yurim Kim, Sudhirkumar Yanpallewar, P. Charles Lin
International Journal of Molecular Sciences.2020; 21(4): 1341. CrossRef - Sirtuin (SIRT)-1: At the crossroads of puberty and metabolism
Carlos F. Aylwin, Alejandro Lomniczi
Current Opinion in Endocrine and Metabolic Research.2020; 14: 65. CrossRef - Metabolomics Reveals the Alteration of Metabolic Pathway by Alpha-Melanocyte-Stimulating Hormone in B16F10 Melanoma Cells
Seung-Ho Seo, Jae Kwon Jo, Eun-Ju Kim, Seong-Eun Park, Seo Yeon Shin, Kyung Mok Park, Hong-Seok Son
Molecules.2020; 25(15): 3384. CrossRef - Noninvasive real-time detection of cerebral blood perfusion in hemorrhagic shock rabbits based on whole-brain magnetic induction phase shift: an experimental study
Wencai Pan, Wei Zhuang, Yinbao Chong, Mingxin Qin, Yang Li, Jingjing Xiao, Qing Wang, Shihui Zhang, Shuanglin Zhao, Peng Zhao
Physiological Measurement.2020; 41(9): 095004. CrossRef - Neurochemical regulators of food behavior for pharmacological treatment of obesity: current status and future prospects
Gayane Sargis Vardanyan, Hasmik Samvel Harutyunyan, Michail Iosif Aghajanov, Ruben Sargis Vardanyan
Future Medicinal Chemistry.2020; 12(20): 1865. CrossRef - The Co-occurrence of Pediatric Obesity and ADHD: an Understanding of Shared Pathophysiology and Implications for Collaborative Management
Valerie M. O’Hara, Jennifer L. Curran, Nancy T. Browne
Current Obesity Reports.2020; 9(4): 451. CrossRef - Constitutive Androstane Receptor: A Peripheral and a Neurovascular Stress or Environmental Sensor
Fabiana Oliviero, Céline Lukowicz, Badreddine Boussadia, Isabel Forner-Piquer, Jean-Marc Pascussi, Nicola Marchi, Laila Mselli-Lakhal
Cells.2020; 9(11): 2426. CrossRef - Automated diffusion-based parcellation of the hypothalamus reveals subunit-specific associations with obesity
Melanie Spindler, Jale Özyurt, Christiane M. Thiel
Scientific Reports.2020;[Epub] CrossRef - SIRT1 in Astrocytes Regulates Glucose Metabolism and Reproductive Function
Irene Choi, Emily Rickert, Marina Fernandez, Nicholas J G Webster
Endocrinology.2019; 160(6): 1547. CrossRef - Hypothalamic mechanisms associated with corticotropin-releasing factor-induced anorexia in chicks
Jinxin Wang, Justin Matias, Elizabeth R. Gilbert, Tetsuya Tachibana, Mark A. Cline
Neuropeptides.2019; 74: 95. CrossRef - HMG-CoA synthase 2 drives brain metabolic reprogramming in cocaine exposure
Xue Shao, Yunxuan Tang, Hailei Long, Hui Gu, Jie Zhang, Pengchi Deng, Yinglan Zhao, Xiaobo Cen
Neuropharmacology.2019; 148: 377. CrossRef - The Effect of Feeding Behavior on Hypothalamus in Obese Type 2 Diabetic Rats with Glucagon-like Peptide-1 Receptor Agonist Intervention
Ke Lu, Xiaoyan Chen, Jianhua Yan, Xinchun Li, Chen Huang, Qi Wan, Xuelian Deng, Qiao Zou
Obesity Facts.2018; 11(3): 181. CrossRef - The Long-Term Impact of High Levels of Alpha-Melanocyte-Stimulating Hormone in Energy Balance Among Obese Adolescents
Ana Claudia Pelissari Kravchychyn, Raquel Munhoz da Silveira Campos, Flávia Campos Corgosinho, Deborah Cristina Landi Masquio, Sofia Emanuelle de Castro Ferreira Vicente, Yasmin Alaby Martins Ferreira, Patrícia Leão Silva, Aline de Piano Ganen, Lila Missa
Annals of Nutrition and Metabolism.2018; 72(4): 279. CrossRef - Psychopharmacological advances in eating disorders
Hubertus Himmerich, Janet Treasure
Expert Review of Clinical Pharmacology.2018; 11(1): 95. CrossRef - Food engineering into the XXI century
José Miguel Aguilera
AIChE Journal.2018; 64(1): 2. CrossRef - The Role of Glucagon-Like Peptide 1 (GLP1) in Type 3 Diabetes: GLP-1 Controls Insulin Resistance, Neuroinflammation and Neurogenesis in the Brain
Choon Bae, Juhyun Song
International Journal of Molecular Sciences.2017; 18(11): 2493. CrossRef - “I Am I and My Bacterial Circumstances”: Linking Gut Microbiome, Neurodevelopment, and Depression
Juan M. Lima-Ojeda, Rainer Rupprecht, Thomas C. Baghai
Frontiers in Psychiatry.2017;[Epub] CrossRef - Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity
Katharina Timper, Jens C. Brüning
Disease Models & Mechanisms.2017; 10(6): 679. CrossRef - Brain glucose metabolism: Role of Wnt signaling in the metabolic impairment in Alzheimer’s disease
Pedro Cisternas, Nibaldo C. Inestrosa
Neuroscience & Biobehavioral Reviews.2017; 80: 316. CrossRef - Astrocyte-Specific Deletion of Peroxisome-Proliferator Activated Receptor-γ Impairs Glucose Metabolism and Estrous Cycling in Female Mice
Marina O Fernandez, Katherine Hsueh, Hyun Tae Park, Consuelo Sauceda, Vicky Hwang, Deepak Kumar, Sun Kim, Emily Rickert, Sumana Mahata, Nicholas J G Webster
Journal of the Endocrine Society.2017; 1(11): 1332. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite