Articles
- Page Path
- HOME > Endocrinol Metab > Volume 32(3); 2017 > Article
-
Review ArticleMetabolic Surgery in Korea: What to Consider before Surgery
-
Mi-Kyung Kim1
 , Yoonseok Heo2
, Yoonseok Heo2
-
Endocrinology and Metabolism 2017;32(3):307-315.
DOI: https://doi.org/10.3803/EnM.2017.32.3.307
Published online: September 18, 2017
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
2Department of Surgery, Inha University School of Medicine, Incheon, Korea.
- Corresponding author: Yoonseok Heo. Department of Surgery, Inha University School of Medicine, 27 Inhang-ro, Jung-gu, Incheon 22332, Korea. Tel: +82-32-890-3431, Fax: +82-32-890-3097, gshur@inha.ac.kr
Copyright © 2017 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Obesity is increasing globally and represents a significant global health problem because it predisposes towards various diseases, such as type 2 diabetes mellitus, cardiovascular disease, degenerative joint disease, and certain types of cancer. Numerous studies have shown that bariatric surgery reduces body mass and ameliorates obesity-related complications, such as hypertension and hyperglycemia, suggesting that surgery is the most effective therapeutic option for severely obese and obese diabetic patients. Recent international guidelines recommend surgical treatment for diabetic patients with class III obesity (body mass index [BMI] >40 kg/m2), regardless of their level of glycemic control or the complexity of their glucose-lowering regimens, and for patients with class II obesity (BMI 35.0 to 39.9 kg/m2) and hyperglycemia that is poorly controlled despite appropriate lifestyle and pharmacological therapy. The most popular procedures are Roux-en-Y gastric bypass and sleeve gastrectomy, but new procedures with better outcomes have been reported. For optimal surgical outcome, comprehensive management including assessments of a medical condition, nutrition, mental health, and social support is needed before and after surgery. However, there is still a lack of understanding regarding metabolic surgery in Korea. Therefore, this article reviews indications for metabolic surgery in patients with a specific focus on the situation in Korea.
- The prevalence of obesity is increasing globally and this condition predisposes towards diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease, degenerative joint disease, and certain types of cancer. Therefore, it is considered to be one of the principal global health problems. These worldwide trends are also reflected in Korea. According to the Organization for Economic Co-operation and Development (OECD) Health Data (2014) [1], the prevalence of obesity in Korea is lower than in other OECD countries, but it has substantially increased in recent years. According to a 2016 obesity fact sheet published by the Korean Society for the Study of Obesity [2], the prevalence of obesity increased from 28.7% in 2006 to 32.4% in 2015, and the prevalence of class II obesity was 4.8% in 2015, although the criteria used are different from those used in the Western world. The prevalence of obesity has increased particularly among people aged between 20 and 40 years.
- Obesity is a major risk factor for T2DM, so an increase in the prevalence of T2DM can also be predicted. In addition, patients with diabetes have a higher prevalence of obesity than the non-diabetic population, so treatment of overweight and obesity is a key therapeutic aim for patients with T2DM [3]. Recently, the 2nd Diabetes Surgery Summit (DSS-II) and leading diabetes organizations issued a joint statement on metabolic surgery as part of the treatment algorithm for T2DM. This suggested that metabolic surgery should be recommended as an option for the treatment of T2DM in appropriate surgical candidates with class III obesity (body mass index [BMI] >40 kg/m2 in Caucasian, >37.5 kg/m2 in Asian people), regardless of their level of glycemic control or the complexity of the glucose-lowering regimens being used, and in patients with class II obesity (BMI 35.0 to 39.9 kg/m2 in Caucasian, 32.5 to 37.59 kg/m2 in Asian people) with inadequately controlled hyperglycemia despite appropriate lifestyle and pharmacological therapy [4]. In the Korean Diabetes Association (KDA) clinical practice guidelines of 2015 [5], patients of BMI >35 kg/m2 are recommended to undergo surgery. However, although many guidelines now recommend surgical treatment for obese patients with T2DM, additional factors must be taken into account before pursuing this strategy. Therefore, this article reviews the indications for metabolic surgery and specific considerations for individual patients, with a specific focus on Korea.
INTRODUCTION
- Although bariatric surgery was developed as a treatment strategy for morbid obesity, many studies have shown that it can improve glycemic control and other metabolic parameters. The first report that surgery could ameliorate hyperglycemia was published in 1955 by Friedman [6]. This showed that T2DM was brought under control after subtotal gastrectomy for the treatment of peptic ulcer disease. In 1984, bariatric surgery was reported to improve glucose tolerance in insulin-treated patients [7], and then in 1995 Pories et al. [8] reported that it improved glucose homeostasis and promoted T2DM remission, suggesting that surgery could be a very effective therapy for adult-onset T2DM. Since then, many studies have demonstrated improvements in glycemia and other metabolic parameters after surgery, and this has led to the issue of therapeutic guidelines, such as those from the American Diabetes Association (ADA) [45], which suggest that surgery is an option for obese patients with T2DM. In addition, the recommended name for this intervention has been changed from bariatric to metabolic surgery [9].
- Bariatric surgery was first introduced in Korea in 2003 and it led to weight loss and improvement in obesity-related metabolic comorbidities, including T2DM [10]. Because of a high incidence of gastric cancer in Korea, a large number of gastrectomies are performed here annually, and therefore re-analysis of existing data was used to evaluate the effect of gastrectomy on glycemic control in patients with T2DM. Kang et al. [11] analyzed the effect of gastrectomy on glycemic control using data collected from gastric cancer patients who also had T2DM. These patients achieved BMIs of <30 kg/m2 and less severe diabetes after gastrectomy. Roux-en-Y (RY) esophagojejunostomy and Billroth-II procedures resulted in greater improvements than Billroth-I, and RY esophagojejunostomy resulted in the greatest improvements, suggesting that the specific procedure determines how effective surgery is for the treatment of T2DM. Other studies of gastrectomy patients with T2DM also yielded similar results [12].
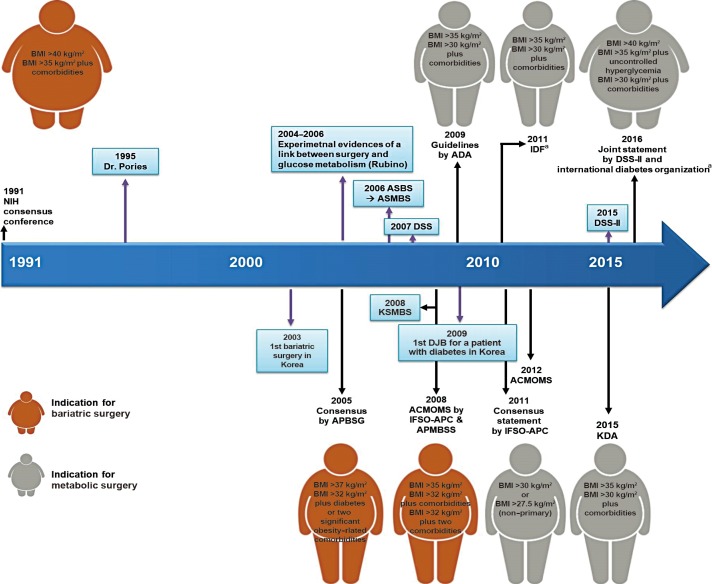
- Duodenojejunal bypass (DJB) was first performed on non-obese diabetic patients in Korea in 2009 by Hong et al. [13]. His group performed DJB on 25 patients with T2DM and BMI <25 kg/m2. One week after surgery, postoperative 75 g oral glucose tolerance testing suggested significantly improved glycemic control [13], and 1 year later ~40% of patients showed improved glycemic control, but just 9% of patients were in remission [14]. Paik et al. [15] also reported the clinical effects of laparoscopic DJB in non-morbidly obese patients with T2DM, after which ~60% of the patients showed better glycemic control. DJB has been abandoned as a metabolic surgery because of the low remission and improvement rates achieved compared to other procedures, despite its advantages from a nutritional respect. Subsequently, RY gastric bypass and sleeve gastrectomy (SG) were performed in T2DM patients, resulting in acceptable remission and improvement rates. There are summaries of the published studies of metabolic surgery in Tables 1, 2 [11121516171819202122232425262728293031 32]. Although there are limitations to these, such as short durations of follow-up, the small size of the studies, varying definitions of remission, and various types of surgery, these all resulted in improvements in glycemic control and suggested mechanisms for these effects. The milestones in the development of bariatric/metabolic surgery are depicted in Fig. 1.
BARIATRIC AND METABOLIC SURGERY FOR PATIENTS WITH T2DM IN KOREA
- Should patients be selected for metabolic surgery based on their BMI?
- In 1991, the National Institutes of Health (NIH) released its recommendations for bariatric surgery. This statement recommended that patients undergo bariatric surgery if they had BMI >40 or >35 kg/m2 if accompanied by serious comorbidities [33], and many subsequent guidelines adhered to these recommendations. In 2005, the Asia-Pacific Bariatric Consensus Meeting suggested that the NIH BMI guidelines be lowered to 37 and 32 kg/m2 for Asians [34]. However, many studies have since shown that bariatric surgery not only improves body mass, but also the severity of T2DM and its complications [35]. In addition, some studies have elucidated mechanisms; whereby, T2DM can be ameliorated beyond those that are consequences of weight reduction.
- In 2011, the International Diabetes Federation included bariatric surgery in its list of approved treatments for T2DM and recommended it for patients with BMI >35 kg/m2 for Caucasians and >32.5 kg/m2 for Asians [36]. Based on these developments, many societies have changed the name used for such interventions from bariatric to metabolic surgery [9]. Recently, DSS-II and six leading international diabetes organizations, namely the ADA, International Diabetes Federation, Chinese Diabetes Society, Diabetes India, European Association for the Study of Diabetes, and Diabetes UK, issued a joint statement on metabolic surgery that recommended it should be an option for the treatment of T2DM in appropriate surgical candidates with class III obesity (BMI ≥40 kg/m2), regardless of their glycemic control or the complexity of their glucose-lowering regimens, and also in patients with class II obesity (BMI 35.0 to 39.9 kg/m2) and poorly controlled hyperglycemia, even if receiving appropriate lifestyle and medical therapy. Furthermore, it stated that metabolic surgery should also be considered as a therapeutic option for T2DM patients with class I obesity (BMI 30.0 to 34.9 kg/m2) and poorly controlled hyperglycemia, if the use of oral or injectable medication had been ineffective. They also recommended that the BMI threshold be 2.5 kg/m2 lower for Asian patients [4]. The Clinical Practice Guidelines (2011) issued by the KDA suggested that bariatric surgery be performed on patients with T2DM and BMI >35 kg/m2. However, since 2015 the Clinical Practice Guidelines of the KDA have recommended surgical treatment for patients with T2DM and BMI >35 kg/m2 [5].
- Thus, the BMI thresholds for metabolic surgery, the principal factor used to select surgical candidates, have evolved alongside the publication of numerous studies, but there is still concern regarding whether the consideration of BMI alone is sufficient for patient selection. In part, this is because the metabolic outcome varies according to fat distribution [37]. Visceral adiposity is a well-known risk factor for metabolic syndrome. Kim et al. [18] reported that after Roux-en-Y gastric bypass (RYGB) surgery, despite there being no difference in preoperative BMI between patients who went into remission and those who did not, the visceral-to-subcutaneous adipose volume ratio was greater in patients who did not go into remission, suggesting that visceral adiposity is a negative predictor of diabetic remission after RYGB. Importantly, the Asian population has a higher percentage of body fat for a given body mass and is predisposed to abdominal adiposity [38]. Moreover, recent studies have reported that gluteal subcutaneous adipose tissue has a protective effect against cardiometabolic risk factors [39]. These reports thus demonstrate the importance of fat distribution, which BMI does not reflect.
- Many studies have shown that the hyperglycemia of obese patients with T2DM was ameliorated even before significant weight loss had occurred [840], suggesting that there is a mechanism independent of weight loss. If so, metabolic surgery could also have benefits for non-obese patients. Patients with diabetes who underwent gastrectomy for gastric cancer and who had a BMI of ~25 kg/m2 showed improved hyperglycemia [12]. In addition, there have been a small number of studies on the effect of bariatric surgery in non-obese diabetic patients. These studies reported amelioration of hyperglycemia, but the remission rate was lower than for obese patients [1416]. Therefore, other factors should be considered for patients with lower BMI. Impaired insulin secretion is also an important pathological feature of T2DM. A study of a large cohort in Chicago showed that 13% of patients with diabetes had a BMI ≤25 kg/m2. Compared with their obese counterparts, they were more likely to use insulin and have a history of pancreatitis, suggesting that the lean group had more advanced β-cell failure [41]. Studies of Asian subjects have shown that β-cell function is worse than that of otherwise comparable Caucasian subjects [42]. Indeed, a study of the insulin secretory function of Korean patients with diabetes showed that it was impaired early in the pathogenesis of T2DM [43]. Moreover, cohort studies have shown that progressors to diabetes have more pronounced impairment of β-cell function than non-progressors in the Korean population [44]. Preservation of β-cell function is one of the predictive factors for the remission of diabetes [45]. Therefore, given that Korean patients with T2DM are more likely to have worse β-cell function than severe insulin resistance, it would be sensible to check β-cell function in addition to measuring BMI during surgical candidate selection, especially with regard to the proper timing of the surgery. However, to further assess this suggestion, long-term studies are required. Nevertheless, improvement in β-cell function after metabolic surgery is reported to be the most important factor in achieving remission in T2DM patients with low BMI in Korea. The preoperative C-peptide level does not affect much surgical outcomes, although it tends to be higher in the target-achieved group than in the target non-achieved group; thus, recovery of β-cell function is a more important predictive factor than the preoperative C-peptide level. These findings suggest that metabolic surgery could improve β-cell secretory function [32].
- Which procedure is most effective for patients with T2DM?
- There are four accepted procedures for metabolic surgery: RYGB, adjustable gastric banding (AGB), SG, and biliopancreatic division. The joint statement by the International Diabetes Organization recommends that the choice of procedure is based on the evaluation of the risk-benefit ratio for individual patients because of the lack of randomized controlled trials that have compared surgical outcomes [4]. Among these procedures, RYGB and SG are commonly used in patients with diabetes [46]. However, according to a nationwide survey of bariatric and metabolic surgery, gastric banding is the most common pro-cedure undertaken in Korea. Nevertheless, if patients with diabetes are considered alone, RYGB, mini gastric bypass, and SG were performed much more frequently than AGB [47].
- Many studies have shown that RYGB is beneficial and it is a widely accepted procedure. There is a great deal of evidence that RYGB has beneficial effects through restriction of food and calorie intake, altered incretin hormone secretion, altered gut microflora, and bile acid metabolism [4849]. However, because of the high incidence of gastric cancer in Korea, there is concern about its effects on cancer screening. A nationwide survey showed that the number of SG procedures being undertaken has been increasing because they have the advantages of efficacy, relative simplicity, and fewer problems in the long term with malnutrition and other complications, as well as that of avoiding problems with surveillance for gastric cancer in the remaining portion of the stomach. In addition, some studies have reported that SG has direct effects on glucose metabolism that are not secondary to weight loss [5051]. However, there are few data from long-term studies to date.
- Recently, new procedures have been introduced for the Asian population. After Rubino and Marescaux [52] reported the use of DJB, other studies reported that its effects did not represent an improvement over conventional surgery [53]. Lee et al. [54] reported the results of the use of a combined SG/DJB procedure. This was similarly or more efficacious than conventional procedures and was accompanied by less malnutrition. Another advantage is the avoidance of the risk of gastric cancer arising from the remaining portion of the stomach [54]. Because a similar situation exists in the Korean population, this approach could be effective here, but the results of further studies are awaited.
FACTORS TO CONSIDER WHEN SELECTING PATIENTS FOR METABOLIC SURGERY
- A recent report showed that the stigma of obesity might result in negative health outcomes, such as weight regain and depression. Indeed, many patients undergoing bariatric surgery might have a long history of weight stigma. Weight stigma has been observed in a healthcare setting, in which it resulted in impaired postsurgery dietary adherence [55]. In addition, candidates for bariatric surgery have similarly high levels of depression to psychotherapy inpatients, but they are perceived as having better mental well-being [56]. According to the report by Brewis et al. [57] on the 2014 Korean National Health and Nutritional Survey (KNHANES) cohort, people with higher body mass in Korea report more symptoms of depression and women, in particular, are at more risk. Such symptoms reduce the quality of life for obese people. However, a number of reports have shown that surgery improved the quality of life and alleviated mental health problems [5658]. Therefore, mental health should be evaluated before and after surgery, considering whether barriers such as weight stigma are present.
- Metabolic surgery is a cost-effective treatment for obese people, especially for those with T2DM [59]. According to the KNHANES, many obese people are not financially well off. In the case of the United States, most insurance companies covered bariatric surgery when medical treatment for obesity failed for a certain period. The American Society for Metabolic and Bariatric Surgery recommend [60] that patients seeking surgical treatment should be evaluated based on their BMI and comorbid conditions because the discriminatory, arbitrary practice of insurance-mandated preoperative weight loss causes an unnecessary delay of life-saving treatment and leads to the progression of life-threatening comorbid conditions. However, unlike in Western countries, both surgical treatment and anti-obesity drugs are not covered by insurance in Korea. Thus, a system for the support of obese people, especially those with diabetes, is required.
PSYCHOLOGICAL AND SOCIAL SUPPORT REQUIREMENTS FOR PATIENTS UNDERGOING METABOLIC SURGERY
- There is a consensus that obesity is a significant risk factor for a number of diseases. Many obesity-related diseases, especially T2DM, decrease the quality of life and increase medical costs, resulting not only in health but also in social problems. Therefore, a comprehensive approach is needed to select candidates for metabolic surgery. A number of problems remain to be solved, e.g., despite its potential benefits for obese patients with T2DM, there is still a lack of understanding of metabolic surgery in Korea, which could delay its implementation unnecessarily. Moreover, for the success of metabolic surgery, further studies are needed to establish: (1) the criteria, besides BMI, for patients with T2DM, (2) the effect of metabolic surgery on micro- and macro-complications in the long-term, (3) the timing of surgery, (4) the best procedure for each individual, (5) its long-term effect on nutrition, and (6) its long term safety.
CONCLUSIONS
-
Acknowledgements
- This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1C1A2A01052054) and by the Korea Government (MSIP) (No. 2014R1A5A2010008) and by Korean Health Technology R&D Project (HC15C1322), Ministry of Health and Welfare, Republic of Korea.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Organization for Economic Co-operation and Development. OECD obesity update 2014 [Internet]; Paris: OECD; c2017. cited 2017 Jun 19. Available from: http://www.oecd.org/health/obesity-update.htm.
- 2. Korean Society for the Study of Obesity. 2016 Obesity fact sheet [Internet]; Seoul: Korean Society for the Study of Obesity; 2017. cited 2017 Jun 19. Available from: http://www.kosso.or.kr.
- 3. Task Force Team for Basic Statistical Study of Korean Diabetes Mellitus from the Korean Diabetes Association. Diabetes fact sheet in Korea 2015 [Internet]; Seoul: Korean Diabetes Association; c2011. cited 2017 Jun 19. Available from: http://www.diabetes.or.kr.
- 4. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis 2016;12:1144–1162. ArticlePubMed
- 5. Korean Diabetes Association. Treatment guideline for diabetes 2015 [Internet]; Seoul: Korean Diabetes Association; c2011. cited 2017 Jun 19. Available from: http://www.diabetes.or.kr.
- 6. Friedman MN, Sancetta AJ, Magovern GJ. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet 1955;100:201–204. PubMed
- 7. Herbst CA, Hughes TA, Gwynne JT, Buckwalter JA. Gastric bariatric operation in insulin-treated adults. Surgery 1984;95:209–214. PubMed
- 8. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350. ArticlePubMedPMC
- 9. Cummings DE, Cohen RV. Beyond BMI: the need for new guidelines governing the use of bariatric and metabolic surgery. Lancet Diabetes Endocrinol 2014;2:175–181. ArticlePubMedPMC
- 10. Lee H, Kim D, Lee S, Nam K, Kim E. Initial evaluation of laparoscopic Roux-en-Y gastric bypass and adjustable gastric banding in Korea: a single institution study. Obes Surg 2010;20:1096–1101. ArticlePubMedPDF
- 11. Kang KC, Shin SH, Lee YJ, Heo YS. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J Korean Surg Soc 2012;82:347–355. ArticlePubMedPMC
- 12. Lee W, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes Surg 2012;22:1238–1243. ArticlePubMedPDF
- 13. Hong IK, Kim JY, Lee YJ, Choe YM, Choi SK, Lee KY, et al. The effect of duodenojejunal bypass for T2DM patients below BMI 25 kg/m2 in early postoperative period. J Korean Surg Soc 2011;80:103–110.Article
- 14. Heo Y, Ahn JH, Shin SH, Lee YJ. The effect of duodenojejunal bypass for type 2 diabetes mellitus patients below body mass index 25 kg/m(2): one year follow-up. J Korean Surg Soc 2013;85:109–115. ArticlePubMedPMC
- 15. Paik KY, Kim W, Song KH, Kwon HS, Kim MK, Kim E. The preliminary clinical experience with laparoscopic duodenojejunal bypass for treatment of type 2 diabetes mellitus in non-morbidly obese patients: the 1-year result in a single institute. Surg Endosc 2012;26:3287–3292. ArticlePubMedPDF
- 16. Kim Z, Hur KY. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg 2011;35:631–636. ArticlePubMedPDF
- 17. Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol 2012;18:49–54. ArticlePubMedPMC
- 18. Kim MK, Lee HC, Kwon HS, Baek KH, Kim EK, Lee KW, et al. Visceral obesity is a negative predictor of remission of diabetes 1 year after bariatric surgery. Obesity (Silver Spring) 2011;19:1835–1839. ArticlePubMed
- 19. An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol 2013;19:9410–9417. ArticlePubMedPMC
- 20. Kim WS, Kim JW, Ahn CW, Choi SH. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: a prospective pilot study. J Korean Surg Soc 2013;84:88–93. ArticlePubMedPMC
- 21. Kwon Y, Abdemur A, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg Obes Relat Dis 2014;10:235–242. ArticlePubMed
- 22. Pak J, Kwon Y, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg Obes Relat Dis 2015;11:1266–1272. ArticlePubMed
- 23. Kim JW, Kim KY, Lee SC, Yang DH, Kim BC. The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index <35 kg/m(2): preliminary results. Ann Surg Treat Res 2015;88:215–221. ArticlePubMedPMC
- 24. Kim S, Richards WO. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann Surg 2010;251:1049–1055. ArticlePubMed
- 25. Kim MK, Lee HC, Lee SH, Kwon HS, Baek KH, Kim EK, et al. The difference of glucostatic parameters according to the remission of diabetes after Roux-en-Y gastric bypass. Diabetes Metab Res Rev 2012;28:439–446. ArticlePubMed
- 26. Kim MK, Jang EH, Hong OK, Chun HJ, Yoo SJ, Baek KH, et al. Changes in serum levels of bone morphogenic protein 4 and inflammatory cytokines after bariatric surgery in severely obese korean patients with type 2 diabetes. Int J Endocrinol 2013;2013:681205ArticlePubMedPMCPDF
- 27. Dixon JB, Hur KY, Lee WJ, Kim MJ, Chong K, Chen SC, et al. Gastric bypass in type 2 diabetes with BMI <30: weight and weight loss have a major influence on outcomes. Diabet Med 2013;30:e127–e134. ArticlePubMed
- 28. Kwon HN, Lee YJ, Kang JH, Choi JH, An YJ, Kang S, et al. Prediction of glycated hemoglobin levels at 3 months after metabolic surgery based on the 7-day plasma metabolic profile. PLoS One 2014;9:e109609. ArticlePubMedPMC
- 29. Kim MK, Kim W, Kwon HS, Baek KH, Kim EK, Song KH. Effects of bariatric surgery on metabolic and nutritional parameters in severely obese Korean patients with type 2 diabetes: a prospective 2-year follow up. J Diabetes Investig 2014;5:221–227.ArticlePubMed
- 30. Kim MJ, Hur KY. Short-term outcomes of laparoscopic single anastomosis gastric bypass (LSAGB) for the treatment of type 2 diabetes in lower BMI (<30 kg/m(2)) patients. Obes Surg 2014;24:1044–1051. ArticlePubMedPDF
- 31. Kim MJ, Park HK, Byun DW, Suh KI, Hur KY. Incretin levels 1 month after laparoscopic single anastomosis gastric bypass surgery in non-morbid obese type 2 diabetes patients. Asian J Surg 2014;37:130–137. ArticlePubMed
- 32. Kwon O, Lee YJ, Yu JH, Kim MS, Heo Y. The recovery of beta-cell function is critical for antidiabetic outcomes of gastric bypass in Asian subjects with type 2 diabetes and a body mass index below 30. Obes Surg 2017;27:541–544. ArticlePubMedPDF
- 33. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956–961. ArticlePubMed
- 34. Lee WJ, Wang W. Bariatric surgery: Asia-Pacific perspective. Obes Surg 2005;15:751–757. ArticlePubMed
- 35. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752. ArticlePubMed
- 36. Dixon JB, Zimmet P, Alberti KG, Rubino F. International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011;28:628–642. ArticlePubMedPMC
- 37. Goyal A, Nimmakayala KR, Zonszein J. Is there a paradox in obesity? Cardiol Rev 2014;22:163–170. ArticlePubMedPMC
- 38. Lakdawala M, Bhasker A. Asian Consensus Meeting on Metabolic Surgery (ACMOMS). Report: Asian Consensus Meeting on Metabolic Surgery. Recommendations for the use of bariatric and gastrointestinal metabolic surgery for treatment of obesity and type II diabetes mellitus in the Asian population: August 9th and 10th, 2008, Trivandrum, India. Obes Surg 2010;20:929–936. ArticlePubMedPDF
- 39. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. ArticlePubMedPDF
- 40. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481. ArticlePubMed
- 41. Coleman NJ, Miernik J, Philipson L, Fogelfeld L. Lean versus obese diabetes mellitus patients in the United States minority population. J Diabetes Complications 2014;28:500–505. ArticlePubMed
- 42. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–1688. ArticlePubMed
- 43. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism 2001;50:590–593. ArticlePubMed
- 44. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-Year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol 2016;4:27–34. ArticlePubMed
- 45. Wang GF, Yan YX, Xu N, Yin D, Hui Y, Zhang JP, et al. Predictive factors of type 2 diabetes mellitus remission following bariatric surgery: a meta-analysis. Obes Surg 2015;25:199–208. ArticlePubMedPDF
- 46. Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822–1832. ArticlePubMedPDF
- 47. Lee HJ, Ahn HS, Choi YB, Han SM, Han SU, Heo YS, et al. Nationwide survey on bariatric and metabolic surgery in Korea: 2003-2013 results. Obes Surg 2016;26:691–695. ArticlePubMedPDF
- 48. Hutch CR, Sandoval DA. Physiological and molecular responses to bariatric surgery: markers or mechanisms underlying T2DM resolution? Ann N Y Acad Sci 2017;1391:5–19. ArticlePubMed
- 49. Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet 2012;379:2300–2311. ArticlePubMed
- 50. Zhu Z, Yang X, Wang K, Wang Z, Zhao Y, Yu M. The effects of sleeve gastrectomy on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Eur Surg 2014;46:189–196. ArticlePubMedPMC
- 51. Perugini RA, Malkani S. Remission of type 2 diabetes mellitus following bariatric surgery: review of mechanisms and presentation of the concept of ‘reversibility’. Curr Opin Endocrinol Diabetes Obes 2011;18:119–128. ArticlePubMed
- 52. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004;239:1–11. ArticlePubMedPMC
- 53. Lee HC, Kim MK, Kwon HS, Kim E, Song KH. Early changes in incretin secretion after laparoscopic duodenal-jejunal bypass surgery in type 2 diabetic patients. Obes Surg 2010;20:1530–1535. ArticlePubMedPDF
- 54. Lee WJ, Lee KT, Kasama K, Seiki Y, Ser KH, Chun SC, et al. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg 2014;24:109–113. ArticlePubMedPDF
- 55. Raves DM, Brewis A, Trainer S, Han SY, Wutich A. Bariatric surgery patients' perceptions of weight-related stigma in healthcare settings impair post-surgery dietary adherence. Front Psychol 2016;7:1497ArticlePubMedPMC
- 56. Osterhues A, von Lengerke T, Mall JW, de Zwaan M, Muller A. Health-related quality of life, anxiety, and depression in bariatric surgery candidates compared to patients from a psychosomatic inpatient hospital. Obes Surg 2017;27:2378–2387.ArticlePubMedPDF
- 57. Brewis AA, Han SY, SturtzSreetharan CL. Weight, gender, and depressive symptoms in South Korea. Am J Hum Biol 2017;29:e22972.ArticlePubMedPMC
- 58. Oh SH, Song HJ, Kwon JW, Park DJ, Lee YJ, Chun H, et al. The improvement of quality of life in patients treated with bariatric surgery in Korea. J Korean Surg Soc 2013;84:131–139. ArticlePubMedPMC
- 59. Keating C, Neovius M, Sjoholm K, Peltonen M, Narbro K, Eriksson JK, et al. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2015;3:855–865. ArticlePubMedPMC
- 60. Kim JJ, Rogers AM, Ballem N, Schirmer B. American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS updated position statement on insurance mandated preoperative weight loss requirements. Surg Obes Relat Dis 2016;12:955–959. ArticlePubMed
References
A history of bariatric and metabolic surgery. In 1991, the National Institutes of Health (NIH) released the guidelines for bariatric surgery. Since then, based on numerous studies, the guidelines concerning the use of the body mass index (BMI) as an indication for bariatric surgery and metabolic surgery have undergone numerous changes. The important events are highlighted in the blue-colored boxes. ASBS, American Society for Bariatric Surgery; ASMBS, American Society for Metabolic and Bariatric Surgery; DSS, Diabetes Surgery Summit; ADA, American Diabetes Association; IDF, International Diabetes Federation; KSMBS, Korean Society of Metabolic and Bariatric Surgery; DJB, duodenojejunal bypass; APBSG, Asia-Pacific Bariatric Surgery Group; ACMOMS, Asian Consensus Meeting on Metabolic Surgery; IFSO, International Federation for the Surgery of Obesity and Metabolic Disorders; APC, Asia-Pacific Chapter; APMBSS, Asia Pacific Metabolic and Bariatric Surgery Society; KDA, Korean Diabetes Association. aBMI threshold was reduced to 2.5 kg/m2 for Asians.
A Summary of Previous Publications Regarding the Effect of Gastrectomy on Glycemic Control in Patients with Gastric Cancer
| Study | No. | Type of surgery | BMI, kg/m2 | Follow- up, mo | Definition of remission | Remission rate, % |
|---|---|---|---|---|---|---|
| Kim et al. (2012) [17] | 403 | B-I, B-II, RY | 24.7±3.0 | 33.7±20.6 | Euglycemia or HbA1c <6.0% without medication | 15.1 |
| Kang et al. (2012) [11] | 75 | B-I, B-II, RY | 23.8±2.9 | 35.0±25.9 | No medication, Normal FBS, HbA1c <6.0% |
B-I: 0 B-II: 22.2 RY:23.5 |
| Lee et al. (2012) [12] | 0229 | B-I, B-II, RYGJ, RYEJ | 23.9±3 | 12 | FBS must be <100 mg/dL and the patient no longer taking antidiabetic medication or insulin for at least 1 year |
B-I: 15.1 B-II: 20.3 RYGJ: 20 RYEJ: 50 |
| An et al. (2013) [19] | 64 | B-I, B-II, TG with RY | 24.7±3.4 | 12 | No medication and FBS <126 mg/dL and HbA1c <6.0% |
B-I: 0 B-II: 6.2 TG: 8.3 |
| Kim et al. (2013) [20] | 15 | RYGJ, RYEJ | 25.2±3.4 | 12.5±5.5 | HbA1c <6.0% without medication | 78.6 |
| Kwon et al. (2014) [21] | 49 | B-I, B-II | <30 | 24 | FBS <126 mg/dL and HbA1c of <6.5% for at least 1 year without pharmacotherapy |
B-I: 39.1 B-II: 47.9 |
| Park et al. (2015) [22] | 90 | B-I, B-II, RY | 24.8±3.4 | 24 | Normal HbA1c and FBS <100 mg/dL for at least 1 year in the absence of pharmacotherapy or ongoing procedures | NA |
| Kim et al. (2015) [23] | 30 | Long RYGJ | 26.8±3.5 | 12 | HbA1c <6.5% without antidiabetic medication | 30.0 |
A Summary of the Studies Published on Metabolic Surgery in Korea
| Study | No. | Type of surgery | BMI, kg/m2 | Follow-up, mo | Definition of remission | Remission rate, % |
|---|---|---|---|---|---|---|
| Kim et al. (2010) [24] | 0219 | LRYGBP | 50.4±8.7 | 12–48 | HbA1c <7% without medication | 71.1 |
| Kim et al. (2011) [18] | 50 | RYGB | 34.5±2.5 | 12 | HbA1c <6.5% and FBS <126 mg/dL for 1 year or more without medication | 68.0 |
| Kim et al. (2011) [16] | 10 | LMGB | 27.2 | 6 | HbA1c <7% | 70.0 |
| Paik et al. (2012) [15] | 12 | LDJB | 27.9±0.37 | 12 | NA | NA |
| Kim et al. (2012) [25] | 22 | RYGB | 32.6±3.3 | 12 |
HbA1c <6.5% and FBS <126 mg/dL for 1 year or more without medication |
73.0 |
| Kim et al. (2013) [26] | 57 | RYGB | 34.2±4.1 | 12 |
HbA1c <6.5% and FBS <126 mg/dL for 1 year or more without medication |
70.0 |
| Dixon et al. (2013) [27]a | RYGB MGP | 26±3.0 | 12 | HbA1c <6.0% without medication | 30.1 | |
| Heo et al. (2013) [14] | 31 | DJB | 23.1±1.3 | 12 | HbA1c <6.0% without medication | 13.3 |
| Kwon et al. (2014) [28] | 22 | RYGB DJB | 27.4±5.3 | 3 | Improved: HbA1c <7.0% without medicationb | 45.5 |
| Kim et al. (2014) [29] | 33 | RYGB | 32.9±4.3 | 24 |
HbA1c <6.5% and FBS <126 mg/dL for 1 year or more without medication |
55.0 |
| Kim et al. (2014) [30] | 0107 | LSAGB | 25.3±3.2 | 12–36 | Therapeutic target: HbA1c <7% |
1-Year: 53 2-Year: 63 3-Year: 90 |
| Kim et al. (2014) [31] | 12 | Laparoscopic single anastomosis GB | 25.3±5.2 | 1 | NA | NA |
| Kwon et al. (2017) [32] | 15 | RYGB | 26.1 | 24 |
Target-achieved: HbA1c <6.5% without medication |
47 |
Values are expressed as mean±SD.
BMI, body mass index; LRYGBP, laparoscopic Roux-en-Y gastric bypass; HbA1c, glycated hemoglobin; RYGB, Roux-en-Y gastric bypass; FBS, fasting blood sugar; LMGB, laparoscopic mini gastric bypass; LDJB, laparoscopic duodenojejunal bypass; NA, not applicable; MGP, mini-gastric bypass; DJB, duodenojejunal bypass; LSAGB, laparoscopic single anastomosis gastric bypass; GB, gastric bypass.
aThis study included participants in Korea and Taiwan; bDifferent definition of outcome
Figure & Data
References
Citations

- Relationship between peak expiratory flow and impaired functional capacity in obese individuals
Graziele Mayra Santos Moreira, Angela Maria Ribeiro, Patrícia Maria de Melo Carvalho, Pedro Augusto de Carvalho Mira, Isabelle Magalhães Guedes Freitas
Fisioterapia em Movimento.2021;[Epub] CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite


