Articles
- Page Path
- HOME > Endocrinol Metab > Volume 33(4); 2018 > Article
-
Original ArticleClinical Outcomes of Differentiated Thyroid Cancer Patients with Local Recurrence or Distant Metastasis Detected in Old Age
 Audioslide
Audioslide -
Ji Min Han1, Ji Cheol Bae1, Hye In Kim1, Sam Kwon1, Min Ji Jeon2, Won Gu Kim2, Tae Yong Kim2, Young Kee Shong2, Won Bae Kim2

-
Endocrinology and Metabolism 2018;33(4):459-465.
DOI: https://doi.org/10.3803/EnM.2018.33.4.459
Published online: November 30, 2018
1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding author: Won Bae Kim. Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. Tel: +82-2-3010-3913, Fax: +82-2-3010-6962, kimwb@amc.seoul.kr
• Received: July 24, 2018 • Revised: September 13, 2018 • Accepted: September 19, 2018
Copyright © 2018 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Differentiated thyroid carcinoma (DTC) shows a very good prognosis, but older patients have a higher recurrence rate and those show poor prognosis than younger patients. The aim of this study was to determine the clinical outcomes of thyroid cancer patients who experienced recurrence in old age according to the treatment strategy used.
-
Methods
- This retrospective observational cohort study was conducted at Asan Medical Center, Seoul, Korea. Among DTC patients with no evidence of disease after initial treatment, we enrolled 86 patients who experienced recurrence at an age >65 years from 1994 to 2012. Sixty-nine patients had local recurrence and 17 patients showed distant metastasis.
-
Results
- The mean age of patients at recurrence was 72 years. Patients were followed up for a median of 4.1 years after recurrence. Sixty-three of the 69 patients with local recurrence received additional treatment, while the other six received conservative care. The cancer-specific mortality rate was 15.5% in the local recurrence group. Airway problems were the main cause of death in patients who did not receive further treatment for local recurrence. Among the 17 patients with distant metastasis, 10 underwent specific treatment for metastasis and seven received only supportive management. Seven of those 17 patients died, and the cancer-specific mortality rate was 35% in the distant metastasis group.
-
Conclusion
- The overall cancer-specific mortality rate was 20% in DTC patients in whom recurrence was first detected at an age >65 years. Mortality due to uncontrolled local disease occurred frequently in patients who did not receive definitive management for recurrence.
- Differentiated thyroid carcinoma (DTC) has a very good prognosis, and the overall long-term survival rate is from 80% to 95% [12]. Even patients with distant metastasis show a 10-year disease-specific survival rate of 45% [3]. Despite the excellent prognosis, up to 35% of DTC patients experience recurrence of the disease over the course of 40 years of follow-up [4]. Mazzaferri and Kloos [4] suggested that the recurrence rate increases with age and that older patients with recurrence have a poorer prognosis than young patients. Cases of distant recurrence increase in the eighth decade of life, and all types of recurrence, including neck lesions, become more common in the seventh decade of life. The cancer-related mortality rate is approximately 25% in patients in their 60s, and reaches 30% in patients over 70 [4].
- Treatment options for recurrent DTC include surgical excision or experimental therapy (such as ethanol or radiofrequency ablation [RFA]) for local recurrence, radioactive iodine (RAI) adjuvant therapy for RAI-avid lesions, external beam radiation therapy (EBRTx), and systemic chemotherapy for distant metastasis [5]. The relative safety and efficacy of these approaches are unclear. It can be difficult to determine the proper treatment for recurrence in older patients, because elderly patients are at a high risk for medical comorbidities and hazards from additional treatment. Therefore, the treatment for recurrent thyroid cancer could be more conservative in elderly patients.
- The incidence of thyroid cancer in elderly patients has significantly increased in recent decades [6]. However, few studies have evaluated the clinical outcomes and treatment plans for elderly patients with recurrent or persistent disease. The aim of this study was to determine the clinical outcomes and treatment strategies of thyroid cancer patients who first experienced recurrence in old age.
INTRODUCTION
- Study population
- This retrospective observational cohort study was conducted from 1994 to 2012 at Asan Medical Center (Seoul, Korea). Among DTC patients with no clinical evidence of disease after initial treatment, we enrolled patients who experienced recurrence at an age of over 65 years regardless of initial cancer status and age at initial diagnosis. Patients who experienced recurrence within 1 year after initial treatment were excluded due to the possibility of persistent disease. Eighty-six patients were ultimately enrolled. Of the 86 patients, 75 had received initial treatment at Asan Medical Center, and 11 were referred from other hospitals after initial treatment. Sixty-nine patients (80%) had local recurrence and 17 patients (20%) showed distant metastasis. Patients with both local recurrence and distant metastasis were included in the group with distant metastasis. This study was approved by the Institutional Review Board of Asan Medical Center (2013-0473). It is not necessary to have informed consent due to retrospective design.
- Definition of local recurrence and distant metastasis
- No clinical evidence of disease was defined as: (1) no evidence of disease based on a physical examination and neck ultrasonography (US) and (2) no evidence of distant metastasis on a whole body scan (WBS) at the time of ablation or from other imaging studies (neck/chest computed tomography [CT], F-18-fluorodeoxyglucose positron emission tomography [FDG-PET], or brain magnetic resonance imaging). Local recurrence was defined as the detection of cytologically or pathologically proven malignant thyroid tissue in the cervical lymph nodes or thyroid bed. Distant metastasis was defined as the detection of malignant tissue in metastatic lesions (lung, bone, and/or brain) and/or distant metastatic lesions on images obtained using any imaging modality.
- Follow-up strategy
- A history was taken and a physical examination, including neck palpation, was performed for all patients at each visit. For patients who underwent total thyroidectomy and RAI ablation therapy, a diagnostic WBS, neck US, serum stimulated thyroglobulin (Tg) level, and anti-thyroglobulin antibody (TgAb) level were usually obtained at 6 to 12 months after RAI therapy. Subsequently, neck US was performed and unstimulated Tg measurements were made at follow-up visits every 1 to 2 years. In patients who did not undergo RAI therapy, serum unstimulated Tg and TgAb levels were usually checked every 6 to 12 months, and neck US was performed annually or biannually. If there was clinical suspicion of recurrence in a patient, neck/chest CT and/or FDG-PET was performed. In patients with a neck US abnormality, we performed fine needle aspiration cytology.
- Statistical analysis
- Categorical variables are presented as numbers and percentages, and continuous variables are expressed as mean±standard deviation or median (minimum to maximum). The Kaplan-Meier method with the log-rank test was used to compare cancer-specific survival between groups. R version 2.13 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) was used for all statistical analyses. All P values were 2-sided, with P<0.05 considered to indicate statistical significance.
METHODS
- Baseline clinicopathological characteristics
- The mean age of patients when recurrence was first detected was 72 years, and 65 patients (76%) were female. Almost all had papillary thyroid cancer (PTC), except one patient. Eighty-three patients had undergone total thyroidectomy and 78 patients had undergone RAI remnant ablation. The frequencies of a tumour size larger than 4 cm, central neck lymph node metastasis (N1a), and lateral neck lymph node metastasis (N1b) at the time of initial treatment were 22.0%, 28%, and 38%, respectively (Table 1).
- Clinical outcomes and treatment of study population
- The median duration from initial treatment to the detection of recurrence was 2.95 years (range, 1.0 to 19.8). Patients were followed up for a median of 4.1 years (range, 0.3 to 13.3) after the detection of recurrence, and 25 patients died during the follow-up period. Among them, 16 patients died of thyroid cancer. The all-cause mortality rate was 29.07%, and the cancer-specific mortality rate was 18.6%. Cancer-specific survival graphs of study subjects are shown in Fig. 1. The overall cancer-specific survival rate of all patients was about 80%. Patients with local recurrence showed better outcomes than those with distant metastasis, although this tendency did not reach statistical significance (P=0.066).
- Among the enrolled patients, 69 (80%) had local recurrence and 17 (20%) had distant metastasis. Of the 69 patients with local recurrence group, 10 (14.5%) had newly developed distant metastasis during the follow-up period after the initial detection of local recurrence. Four patients had only lung metastasis, two had both lung and bone metastasis, two had bone metastasis, and two had brain metastasis.
- We evaluated clinical outcomes according to the treatment for recurrence in each group (Fig. 2). Forty-one of the patients with local recurrence underwent re-operation with or without RAI adjuvant therapy, and three patients received RAI therapy only. Fourteen patients underwent RFA and five received EBRTx. Six patients only received thyroid-stimulating hormone suppression therapy. Of the 69 patients with local recurrence, 18 died during follow-up: six from airway problems due to uncontrolled local recurrence, four from uncontrolled distant metastasis, and eight from unknown causes or other comorbidities. Among the 18 patients who died, 10 died due to thyroid cancer, and the cancer-specific mortality rate was 15.5% in this group. It is noteworthy that the death rate from airway problems was much higher in patients who did not receive treatment for local disease (two of three, 67%) than in patients who received additional treatment (four of 15, 27%). One of the three patients who died without treatment had anaplastic transformation of the neck lesion.
- Among the 17 patients with distant metastasis, 10 underwent specific treatment for metastasis and seven received only supportive management. Seven of the 17 patients died: six from aggravation of distant metastasis and one from a myocardial infarction. The cancer-specific mortality rate was 35%.
RESULTS
- As the incidence of thyroid cancer has increased in recent decades [67], the prevalence of geriatric patients with thyroid cancer has risen gradually. In this study, we characterized the clinical outcomes and treatment of DTC patients who experienced recurrence in old age. We found that approximately 20% of patients with recurrence or metastasis died of thyroid cancer during a median of 4 years of follow-up. Although it is widely known that local recurrence has a generally good prognosis, the mortality rate, especially for airway-related death, was markedly higher in patients who did not receive further treatment than in patients who did receive treatment.
- When geriatric patients with newly detected local recurrence or distant metastasis visit the clinic, clinicians must consider how to evaluate their disease status, how to conduct follow-up, and whether to treat their disease. Special considerations, such as comorbidities, the will to live, or economic support, play a larger role in decisions about treatment in elderly patients than in young patients. Age is a significant poor prognostic factor and is associated with a higher recurrence rate of DTC [8910]. A study conducted in Taiwan reported the results of multivariate analyses for overall survival and disease-specific survival in PTC patients. Older age predicted worse overall survival (hazard ratio [HR] for patients 40 to 60 years vs. <40 years: 5.44; and HR for patients >60 years vs. <40 years: 24.52) and disease-specific survival (HR for patients 40 to 60 years vs. <40 years: 9.11; and HR for patients >60 years vs. <40 years: 46.12) [11].
- Approximately 10% to 30% of DTC patients who are in a disease-free state after initial treatment will experience local recurrence and/or distant metastasis during the follow-up period. Of these patients, about 80% present with neck recurrence alone [8]. DTC patients over 45 years of age with neck recurrence are more likely to die of thyroid cancer than those under 45 years of age [12]. The 5-year survival rate after neck recurrence was 100% in patients under 45 years of age and 61% in patients over 45 years of age. In our study, the most common cause of death in patients with local recurrence was airway problems due to uncontrolled local disease (six of 18 deaths). Airway-related mortality was more common in patients who did not receive additional treatment than in those who did receive additional treatment (67% vs. 27%). One of the three patients who died without treatment died due to anaplastic transformation. Although the pathogenesis of anaplastic thyroid cancer is still unclear, anaplastic transformation arising from DTC has become a well-known phenomenon. Because most cases of anaplastic cancer occur in the thyroid gland and neck lymph nodes [13], active management is needed for patients with local recurrent disease, even in older patients. Surgery with or without RAI therapy is useful in the management of cases of local recurrence [14]. Approximately 50% of patients who undergo a second operation for recurrent DTC can be rendered free of disease [15]. Furthermore, roughly 15% of patients with local recurrence showed newly developed distant metastasis after the initial detection of recurrence in our study. This finding means that a systemic evaluation and close follow-up are required for those patients.
- The prevalence of distant metastasis also increased from 2% in patients under 60 years of age to more than 5% in those over 60 years of age [16]. The extent of disease beyond the thyroid is a crucial factor contributing to a poor prognosis in older patients, whereas it did not affect the good prognosis of younger patients [917]. Distant metastasis is more threatening in elderly patients with DTC. The 5-year disease-specific survival rate of DTC patients with distant metastasis has been reported to range from 35% to 85%, depending on the clinical characteristics of the study subjects [181920]. In our study, the cancer-specific mortality rate was roughly 35%, and the main cause of death was aggravation of distant metastasis. Patients with distant metastasis showed poor outcomes compared with patients with local recurrence, but the difference was not statistically significant (Fig. 1B). The relatively small number of enrolled patients could explain the absence of a significant difference between the two groups. The other possible explanation for this finding is related to the study population. Follicular thyroid cancer with distant metastasis was found to have a 2.6-fold risk of disease specific survival compared to PTC [18]. Ninety-nine percent of our study patients had PTC, which could explain the relatively favourable outcomes among patients with distant metastasis.
- The present study had several limitations. First, the number of enrolled patients was not sufficiently high to show a statistically significant difference in outcomes according to treatment modality. We simply enumerated the results of treatment, the number of deaths, and the causes of death. Our findings cannot be interpreted as suggesting which therapy is superior for patients who experience recurrence, and the evidence likewise does not support decisions about treatment strategies. Second, the characteristics of the enrolled patients were heterogeneous. Although all patients had no clinical evidence of disease after initial treatment, clinical characteristics, such as initial tumor, node, metastasis (TNM) staging, type of surgery, and whether RAI treatment was performed, varied among patients. Therefore, the median time from initial treatment to recurrence was only 2.95 years and the periods ranged widely. Each patient had an individually unique risk of recurrence and mortality. Third, serum Tg and TgAb levels are important markers of disease status in DTC patients. We did not have information on serum Tg and TgAb levels at the time of initial treatment in patients who were referred from other hospitals. For that reason, we could not consider the influence of serum Tg and TgAb levels in our assessment of remission and recurrence. To exclude patients with persistent disease after initial treatment, we enrolled only patients who experienced recurrence more than 1 year after initial treatment. Fourth, selection bias may have occurred in this retrospective setting. In our study, patients with aggressive disease, such as anaplastic transformation, were more likely not to have received additional treatment for recurrent disease.
- Nevertheless, this study suggested specific considerations relevant for the treatment of elderly patients with recurrence. Most previous studies on elderly patients included patients who were initially diagnosed with DTC in old age. In contrast, our study was conducted among a special subgroup in whom recurrent or metastatic DTC was detected in old age, regardless of age at initial diagnosis.
- In conclusion, the overall cancer-specific mortality rate was 20% in DTC patients with recurrence first detected at an age of >65 years. Mortality due to uncontrolled local disease occurred frequently in patients who did not receive definitive management for recurrence around the airway. Systematic evaluation and careful follow-up of such patients are needed, and administering proper treatment for local recurrence might be helpful to improve disease-related mortality in elderly patients.
DISCUSSION
-
Acknowledgements
- This work was supported by the National Research Foundation of Korea Research Grant (NRF-2015R1D1A1A09057966), Seoul, Korea.
- This study was presented at the 15th International Thyroid Congress, Orlando, FL, USA.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS:
Article information
- 1. Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 1997;79:564–573. ArticlePubMed
- 2. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med 1998;338:297–306. ArticlePubMed
- 3. Sugitani I, Fujimoto Y, Yamamoto N. Papillary thyroid carcinoma with distant metastases: survival predictors and the importance of local control. Surgery 2008;143:35–42. ArticlePubMed
- 4. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447–1463. ArticlePubMed
- 5. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214. ArticlePubMed
- 6. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009;115:3801–3807. ArticlePubMed
- 7. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–2167. ArticlePubMed
- 8. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–428. ArticlePubMed
- 9. Cady B, Sedgwick CE, Meissner WA, Wool MS, Salzman FA, Werber J. Risk factor analysis in differentiated thyroid cancer. Cancer 1979;43:810–820. ArticlePubMed
- 10. Jeon MJ, Kim WG, Kim TH, Kim HK, Kim BH, Yi HS, et al. Disease-specific mortality of differentiated thyroid cancer patients in Korea: a multicenter cohort study. Endocrinol Metab (Seoul) 2017;32:434–441. ArticlePubMedPMC
- 11. Su DH, Chang SH, Chang TC. The impact of locoregional recurrences and distant metastases on the survival of patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2015;82:286–294. ArticlePubMed
- 12. Voutilainen PE, Multanen MM, Leppaniemi AK, Haglund CH, Haapiainen RK, Franssila KO. Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid 2001;11:953–957. ArticlePubMed
- 13. Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr, Douglas WG, Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck 2003;25:662–670. ArticlePubMed
- 14. Pak H, Gourgiotis L, Chang WI, Guthrie LC, Skarulis MC, Reynolds JC, et al. Role of metastasectomy in the management of thyroid carcinoma: the NIH experience. J Surg Oncol 2003;82:10–18. ArticlePubMed
- 15. Goretzki PE, Simon D, Frilling A, Witte J, Reiners C, Grussendorf M, et al. Surgical reintervention for differentiated thyroid cancer. Br J Surg 1993;80:1009–1012. ArticlePubMed
- 16. Ito Y, Miyauchi A, Kihara M, Takamura Y, Kobayashi K, Miya A. Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J 2012;59:399–405. ArticlePubMed
- 17. Ito Y, Kudo T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Prognostic factors of papillary thyroid carcinoma vary according to sex and patient age. World J Surg 2011;35:2684–2690. ArticlePubMedPDF
- 18. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 2012;22:884–889. ArticlePubMedPMC
- 19. Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab 1988;67:501–508. ArticlePubMedPDF
- 20. Kim H, Kim HI, Kim SW, Jung J, Jeon MJ, Kim WG, et al. Prognosis of differentiated thyroid carcinoma with initial distant metastasis: a multicenter study in Korea. Endocrinol Metab (Seoul) 2018;33:287–295. ArticlePubMedPMC
References
Fig. 1

Cancer-specific survival of patients after recurrence. (A) Survival of all enrolled patients. (B) Survival according to the type of recurrence.

Fig. 2
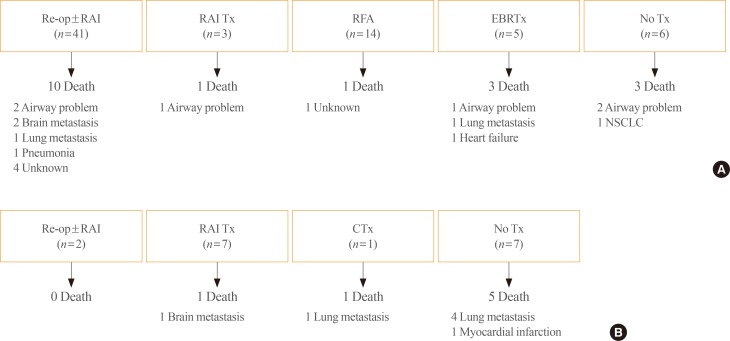
Clinical outcomes of patients according to treatment strategy. (A) Patients with isolated local recurrence (n=69). (B) Patients with distant metastasis (n=17). Re-op, re-operation; RAI, radioactive iodine; Tx, treatment; RFA, radiofrequency ablation; EBRTx, external beam radiation therapy; NSCLC, non-small cell lung cancer; CTx, chemotherapy.

Table 1
![enm-33-459-i001.jpg]()
Baseline Characteristics of Patients
Figure & Data
References
Citations
Citations to this article as recorded by 

- Identification of Circulating Tumor Cell Phenotype in Differentiated Thyroid Carcinoma
Huiling Wang, Mian Lv, Yonghong Huang, Xiaoming Pan, Changyuan Wei
Journal of Biomaterials and Tissue Engineering.2022; 12(4): 813. CrossRef - Long-Term Outcomes and Prognoses of Elderly Patients (≥65-Years-Old) With Distant Metastases From Well-Differentiated Thyroid Cancer During Radioiodine Therapy and Follow-Up
Zhong-Ling Qiu, Chen-Tian Shen, Zhen-Kui Sun, Hong-Jun Song, Chuang Xi, Guo-Qiang Zhang, Yang Wang, Quan-Yong Luo
Frontiers in Endocrinology.2021;[Epub] CrossRef - Head-to-Head Comparison of Neck 18F-FDG PET/MR and PET/CT in the Diagnosis of Differentiated Thyroid Carcinoma Patients after Comprehensive Treatment
Yangmeihui Song, Fang Liu, Weiwei Ruan, Fan Hu, Muhsin H. Younis, Zairong Gao, Jie Ming, Tao Huang, Weibo Cai, Xiaoli Lan
Cancers.2021; 13(14): 3436. CrossRef - Highly sensitive electrochemical immunosensor using a protein-polyvinylidene fluoride nanocomposite for human thyroglobulin
Maria Oneide Silva de Moraes, João de Deus Pereira de Moraes Segundo, Marcos Marques da Silva Paula, Maria Goreti Ferreira Sales, Walter Ricardo Brito
Bioelectrochemistry.2021; 142: 107888. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite



