Effectiveness of Injecting Cold 5% Dextrose into Patients with Nerve Damage Symptoms during Thyroid Radiofrequency Ablation
Article information
Abstract
Background
Although radiofrequency ablation (RFA) is a safe treatment for thyroid tumors, nerve damage is a frequent complication. A previous retrospective study suggested that an injection of cold 5% dextrose in water (5% DW) can reduce nerve damage during RFA. This study validated the efficacy of injecting cold 5% DW for management of nerve damage during RFA.
Methods
Between November 2017 and December 2018, 242 patients underwent 291 RFA sessions for treatment of benign thyroid nodules or recurrent thyroid cancers. Using a standardized technique, cold (0°C to 4°C) 5% DW was immediately injected around the damaged nerve into patients with any symptoms suggesting nerve damage. The incidence of nerve damage, the volume of 5% DW injected, symptom recovery time and the incidence of permanent nerve damage were evaluated.
Results
Nineteen patients experienced nerve damage symptoms related to 21 RFA sessions, including 17 patients during 19 sessions and two patients on the day after two sessions. Patients with nerve damage symptoms detected during RFA were treated by injection of a mean 41 mL (range, 3 to 260) cold 5% DW, but the two patients who experienced symptoms the next day did not receive cold 5% DW injections. Immediate recovery was observed after 15 RFA sessions in 14 patients. No patient experienced permanent nerve damage.
Conclusion
Injection of cold 5% DW is effective in managing nerve damage during RFA of thyroid lesions.
INTRODUCTION
Ultrasonography (US)-guided radiofrequency ablation (RFA) is an alternative treatment for benign thyroid nodules and recurrent thyroid cancers [1,2]. Previous studies [3–11], including meta-analyses, showed that RFA is safe, with a low incidence of complications. Among patients undergoing RFA for benign thyroid nodules and recurrent thyroid cancers, the incidences of overall complications have been reported to be 2.1% and 11.0%, respectively, and the incidences of major complications 1.3% and 6.7%, respectively [12]. Complications can include nerve damage symptoms, such as voice changes, Horner syndrome, limitations of shoulder movement, and palpitations, which must be carefully managed to avoid permanent sequelae [7,9].
Complications during RFA can be minimized by better understanding of nerve anatomy and by methods such as the moving-shot technique, hydrodissection and using a small-sized electrode tip (0.38 to 0.5 cm) [1,13–15]. However, no standard management method has yet been introduced for complications (e.g., voice changes). Although one previous retrospective study suggested that injection of 5% cold dextrose in water (DW) solution around the damaged nerve can mitigate nerve damage during RFA [16], this has not been validated. The present study evaluated the efficacy of injecting 5% cold DW using a standardized technique [16] into patients with symptoms of nerve damage during RFA.
METHODS
This retrospective study was approved by the Institutional Review Boards (2019–0783) of Asan Medical Center. Written informed consent by the patients was waived due to a retrospective nature of our study.
Study population
Between November 2017 and December 2018, 242 patients, including 48 men and 194 women, of mean age 50.9±15.7 years, underwent RFA for US-guided biopsy-proven benign thyroid nodules causing cosmetic or symptomatic problems [1] or biopsy-proven recurrent thyroid cancers. The study population consisted of the subset of these patients who experienced nerve-related complications.
RFA technique
RFA was performed according to the guidelines of the Korean Society of Thyroid Radiology [1]. US-guided biopsy samples were obtained by radiologists under the supervision of three staff radiologists who had 10 years (Y.J.C.), 21 years (J.H.L.), and 25 years (J.H.B.) of experience with thyroid US. US-guided RFA was performed by three staff radiologists. RFA was performed using an RF generator (VIVA RF generator, STARmed, Goyang, Korea; and M-2004, RF Medical, Seoul, Korea) and 18-gauge thyroid dedicated modified internally cooled electrodes (VIVA, STARmed; and RFT-0710, RF Medical) with multiple active tips (3.8, 5, 7, and 10 mm). The size of the active tip selected was dependent on the size of the thyroid lesion [2].
To reduce pain, 2% lidocaine was injected into the perithyroidal or perilesional area and at the skin puncture site [1,2]. RFA of benign nodules was performed using the trans-isthmic approach and the moving-shot technique, whereas RFA of recurrent cancers was performed using the moving-shot and hydrodissection techniques [17–20]. Adjacent structures were carefully monitored and hydrodissection was performed to minimize nerve damage in patients undergoing RFA for recurrent cancers. Hydrodissection consisted of a continuous injection of cold 5% DW to separate critical structures, including the nerve and esophagus, from the thyroid tumors [2,21,22]. Safety margins during RFA were preserved by continuous fluid injection, as injected fluid spreads along and is absorbed by the muscle plane. RFA was terminated when the entire targeted lesion became transiently hyperechoic [1]. Patient symptoms, especially nerve damage symptoms, were monitored during RFA. Patients were evaluated in the hospital for 30 minutes after RFA.
Definition of complications and side effects
Major and minor complications, as well as side effects, were classified according to the guidelines of the Society of Interventional Radiology [7,9,23]. Major complications were those that led to substantial morbidity or disability, requiring hospital admission or increased hospital stay. Major complications in this study included voice changes and shoulder weakness lasting over 1 month. All other complications, including voice changes lasting less than 1 month and hematoma that induced an acute symptom or required medication, were defined as minor complications. Side effects were defined as signs and symptoms not requiring therapy or prescription medications, as well as any undesired consequences of RFA. Following the reporting in previous studies [7,9], transient voice change due to lidocaine injection was regarded as neither complications nor side effects.
Management of suspected nerve damage
Nerve damage symptoms evaluated during RFA included voice changes, Horner syndrome, palpitations, limitations of shoulder movement, and paresthesia. Because the recurrent laryngeal nerve is the nerve most frequently affected during RFA [3,6,8,9, 16], voice changes during RFA were monitored regularly. Voice changes can also be induced by injury to the vagus nerve. Horner syndrome can be induced by injury to the cervical sympathetic ganglion, and limitations to shoulder movement can be induced by injury to the spinal accessory nerve [3,9,16]. We regularly monitored voice changes, symptoms of Horner syndrome (i.e., injection of conjunctiva), and shoulder movement during RFA, especially during ablation near critical structures (e.g., nerves, trachea, and esophagus). Patients’ vital signs, including heart rate, blood pressure, and oxygen saturation, were also monitored. Cold 5% DW was prepared before ablation, allowing immediate injection when nerve symptoms appeared. Ablation was stopped immediately in patients with nerve damage symptoms detected during RFA. To manage nerve damage and reduce its progression, cold (0°C to 4°C) 5% DW was injected into the area around the damaged nerve (Fig. 1) [16]. Cold 5% DW at 0°C to 4°C was chosen because it was isotonic and did not conduct electricity [24]. Using a 21 G spinal needle, cold 5% DW was injected continuously into the tracheoesophageal groove, the most common course of the recurrent laryngeal nerve [25], and the site of suspected nerve injury, until patient recovery from voice changes. Additional fluid was injected after recovery from voice changes to reduce thermal injury caused by residual heat. We did not include this extra volume of additionally injected cold 5% DW in the measurement of the injected volume. Nerve symptoms were monitored the next day by telephone, with follow-up calls repeated until complete symptom relief. Oral corticosteroids were prescribed for patients who did not recover from voice changes or other nerve damage symptoms after injection of cold 5% DW, but were not prescribed for patients who experienced immediate symptom relief after injection of cold 5% DW. Parameters evaluated included the incidence of nerve damage, the volume of cold 5% DW injected, symptom recovery time, and the incidence of permanent nerve damage. We defined immediate recovery of a nerve symptom as symptom relief a few minutes after starting cold 5% DW injection.
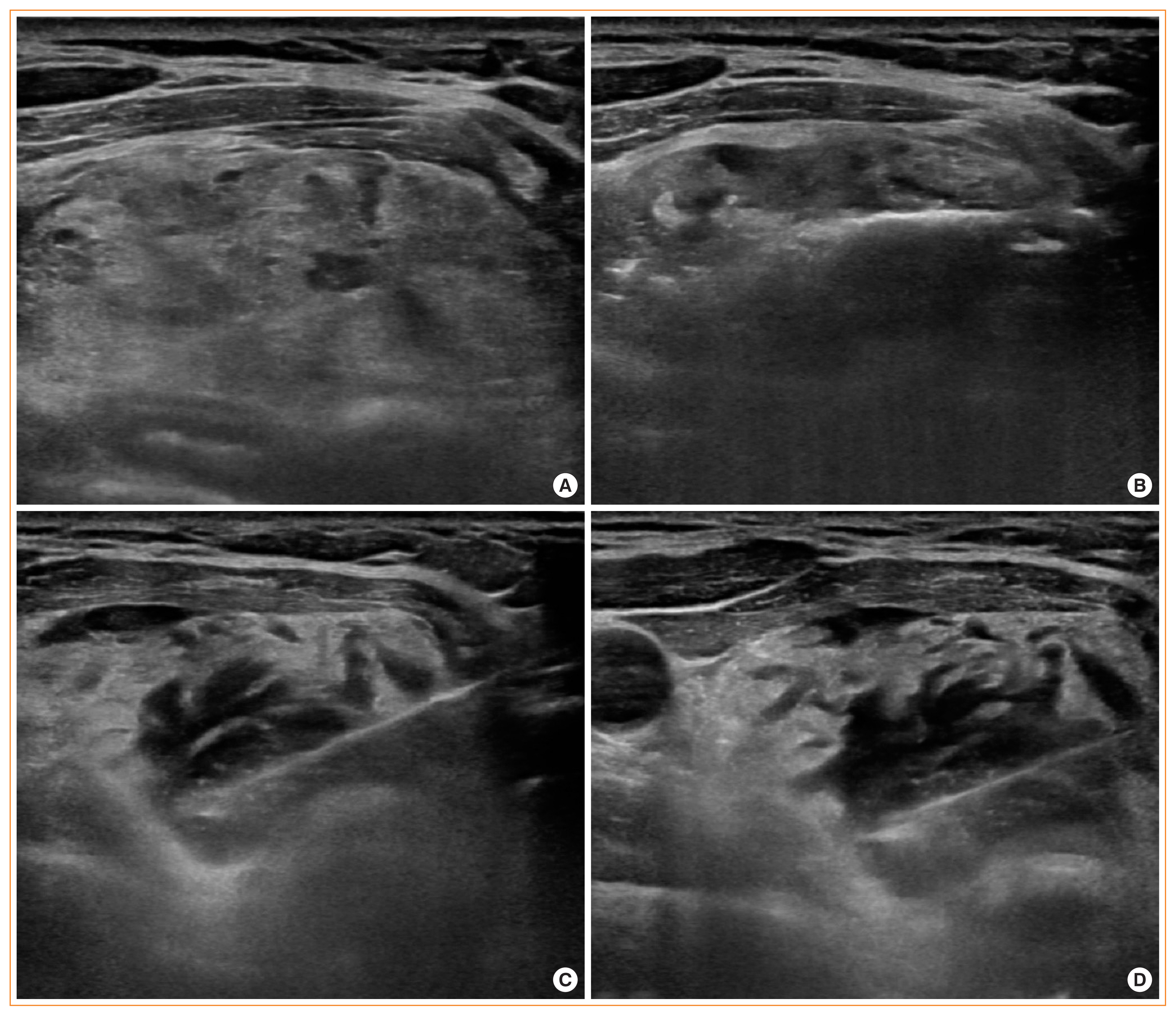
(A) Ultrasonography showing a benign thyroid nodule (1.7×3.8×4.5 cm) in the left isthmic thyroid gland. (B) Radiofrequency electrode with a 1 cm sized active tip located within the nodule for ablation. (C) During ablation, the patient complained of voice changes; ablation was immediately stopped and cold 5% dextrose water was injected into the left tracheoesophageal groove. (D) After injection of 20 mL cold 5% dextrose water, the voice change was completely relieved.
RESULTS
The 242 patients, including 177 with benign nodules and 65 with recurrent thyroid cancers (papillary thyroid cancer, 62 patients; medullary thyroid carcinoma, three patients), underwent a total of 291 RFA sessions, including 212 (72.9%) single sessions. Table 1 shows the demographic and clinical characteristics of these patients. Mean thyroid nodule volume was 15.5±46.8 mL (range, 0.1 to 178), and mean total RF energy was 27.5±17.4 J (range, 5 to 90).
Complications and side effects of RFA are summarized in Table 2. The incidences of major and minor complications were 2.1% and 6.5%, respectively. Voice change was the most common major complication (50%, 3/6). Table 3 shows cases of suspected nerve damage during and after RFA. Nineteen patients experienced nerve damage during and after 21 sessions of RFA (20 voice changes and one limitation of arm elevation). Two patients with recurrent tumors in the operation bed each experienced voice problems on two occasions.
Fig. 2 shows a flowchart of the patients included in this study. Of the 19 patients who experienced nerve damage symptoms during and after 21 RFA sessions, 17 patients experienced nerve damage symptoms during 19 RFA sessions and two patients experienced nerve damage symptoms the day after two RFA sessions. Cold 5% DW solution was injected into all patients with nerve damage symptoms during RFA, but not in the two patients with symptoms the next day. The mean volume of cold 5% DW solution injected during the 19 sessions was 41 mL (range, 3 to 260). Injection of cold 5% DW resulted in immediate recovery during 15 of the 19 sessions. The two patients with delayed detection of nerve damage symptoms recovered after 1 and 3 months later. No patient experience permanent nerve damage.
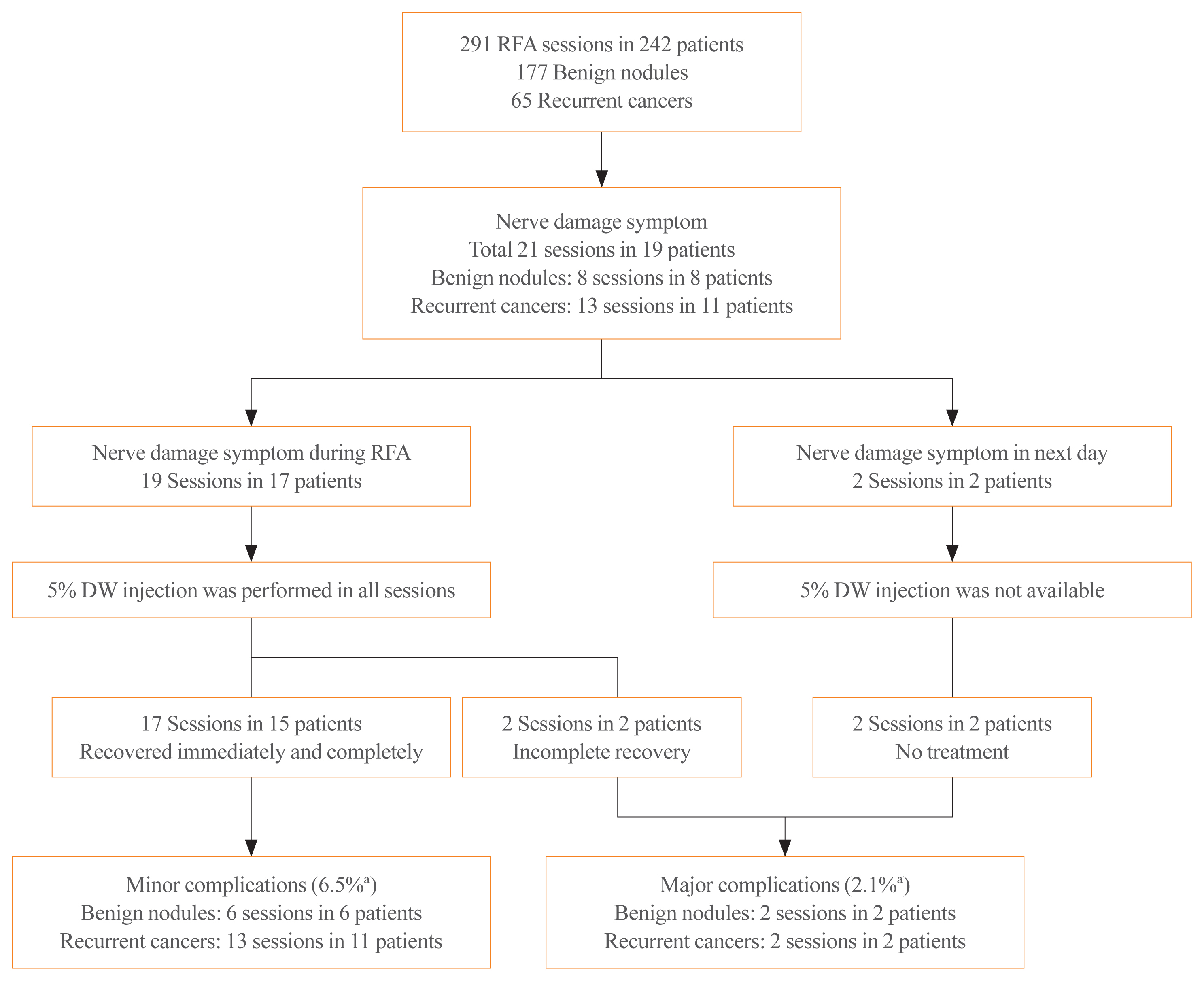
Study flow diagram. There are four major complications, including three in the recurrent laryngeal nerves and one in the spinal accessory nerve. RFA, radiofrequency ablation; DW, dextrose water. aSession based percentage.
The left operation bed (6/21 sessions) was the most frequent location of nerve damage, followed by the left thyroid gland (5/21 sessions) and the right operation bed (5/21 sessions). The injured nerves included the recurrent laryngeal nerve (n=19, 90%), the vagus nerve (n=1, 5%), and the spinal accessory nerve (n=1, 5%). Of the 19 patients with suspected nerve damage, 11 had recurrent cancers, and eight had benign nodules.
DISCUSSION
This study demonstrated that patients with nerve damage during RFA were managed successfully by injection of cold 5% DW. Nineteen patients (7.9%) experienced nerve damage symptoms (voice change or shoulder weakness) during 21 sessions (7.2%) of RFA. Cold 5% DW was injected into 17 patients during 19 RFA sessions, with immediate complete recovery observed in 14 patients (82.4%) during 15 RFA sessions (78.9%). By contrast, the two patients who did not receive injections of cold 5% DW recovered after 1 and 3 months. The immediate symptom relief resulting from cold 5% DW injection may have been due to its initial cooling of the damaged nerve and its reduction of additional conductive heat transfer to the nerve. The injection of cold 5% DW into peritumoral soft tissue may result in complete treatment of thyroid masses during RFA, as it is effective in treating thermal nerve damage.
A previous study, the first to assess the efficacy of cold 5% DW for treatment of thermal nerve damage, also found that this solution provided rapid symptom relief [16]. Although that study assessed the effectiveness of cold 5% DW injection, the standardized method of injection was not evaluated. By contrast, the present study evaluated the injection of cold 5% DW using a standardized technique [16]. Nerve symptoms were regularly evaluated during RFA, including voice changes, conjunctival injection, ptosis, palpitations, and shoulder movement. Because immediate injection is important for the relief of thermal nerve injury, cold 5% DW was prepared before RFA was started. As soon as any suspicious nerve symptom appeared, ablation was stopped and cold 5% DW was injected at the site of suspected nerve injury. Most injections were into the tracheoesophageal groove, because the most frequently involved nerve was the recurrent laryngeal nerve (90%). The standardized injection was effective in treating nerve damage. The mean volume of injected cold 5% DW was 41 mL, with an additional 10 to 20 mL [16] provided to the targeted site, as the injected fluid spread along the muscle plane and was rapidly absorbed into the surrounding space [21]. Strong compression of the RFA site after RFA is not recommended as it enhances conductive heat transfer. Rather, we routinely performed applying smooth pressure to the needle puncture site. Corticosteroids have been shown to reduce perineural edema and inflammatory changes after thyroid operations, because the recurrent laryngeal nerve may be mechanically injured and cause dissection during thyroid operations [26,27]. Oral corticosteroids were therefore prescribed to patients who did not recover from voice changes or other nerve damage symptoms after cold 5% DW injection, but not to patients who experienced immediate symptom relief after injection of cold 5% DW.
US-guided RFA is an effective and safe treatment for benign thyroid nodules and recurrent thyroid cancers [18,21]. Despite its many advantages, this technique has several important complications [2]. The most serious complication is thermal nerve injury, as it may cause permanent sequelae after RFA, especially voice changes [7,9]. Recurrent thyroid cancers are more difficult to ablate than benign thyroid nodules, because the former are smaller and have no safety margins of normal thyroid tissue [2,3]. The rate of nerve damage was higher in patients undergoing RFA for recurrent thyroid cancer than for benign thyroid nodules [7,9]. This study showed that injection of cold 5% DW was effective for rapid improvement of nerve damage and that it reduced the rate of permanent sequelae after RFA. Effective control of nerve damage using cold 5% DW can result in higher rates of safe and curative ablation for recurrent thyroid cancer.
Reduced rates of nerve damage and effective treatment of nerve damage are important for successful ablation of thyroid nodules. Nerve damage during RFA may be reduced by early detection of symptoms and skilled injection of cold 5% DW. We found that delayed detection of nerve damage may cause long standing symptoms and permanent sequelae. For early detection of nerve damage, we routinely performed repeated monitoring of patient symptoms. The most common symptom of nerve damage is voice change, usually caused by injury to the recurrent laryngeal and vagus nerves [3,7,9,13]. Anatomic determination of nerve structure is recommended for ablation near the nerves and skilled injection of cold 5% DW. The recurrent laryngeal nerve is usually located in the tracheoesophageal groove and passes along the posteromedial sides of both thyroid glands [13]. The vagus nerve is located within the carotid sheath, passing to the posterolateral side of the common carotid artery and the posteromedial side of the internal jugular vein [14,15]. Anatomic variations of the vagus nerve, including anterior, medial, and posterior types, bring the nerve closer to the thyroid gland, possibly increasing the risk of thermal nerve damage during RFA [9]. Careful control of the electrode tip for ablation of tumors near the tracheoesophageal groove and carotid sheath is recommended, as is detailed understanding of the anatomic locations of perithyroidal structures.
This study had several limitations. First, complications and side effects were evaluated symptomatically, based on subjective complaints by patients, not on objective and quantitative criteria. The objective evaluation of symptoms such as voice change during RFA is limited, because methods such as vocal cord examination are difficult to perform during RFA. Evaluation of the vocal cords on US may help to detect vocal cord palsy, and can therefore be useful for evaluating symptom relief after cold DW injection. We will update our technique to incorporate US evaluation of the vocal cords. Second, RFA of thyroid tumors was performed by three skilled technicians. Nerve damage may be reduced by RFA being performed by more experienced individuals with better understanding of perithyroidal anatomic structures. Third, the number of patients with nerve damage was small, making it difficult to determine whether our technique should be adapted as standard treatment. Large-sized prospective studies are required to determine a standard treatment strategy for patients experiencing thermal nerve injury during RFA.
In conclusion, this study showed that injection of cold 5% DW was effective in managing suspected nerve damage during RFA. This technique is effective in controlling thermal damage to nerves, and may encourage curative ablation for recurrent thyroid cancer.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.K.L., J.H.B., S.R.C., Y.J.C., J.H.L. Acquisition, analysis, or interpretation of data: M.K.L., J.H.B., S.R.C., Y.J.C., Y.M.L., T.Y.K., J.H.L. Drafting the work or revising: M.K.L., J.H.B. Final approval of the manuscript: M.K.L., J.H.B.