Metabolically Healthy and Unhealthy Normal Weight and Obesity
Article information
Abstract
Increased fat mass is an established risk factor for the cardiometabolic diseases type 2 diabetes and cardiovascular disease (CVD) and is associated with increased risk of all-cause and CVD mortality. However, also very low fat mass associates with such an increased risk. Whether impaired metabolic health, characterized by hypertension, dyslipidemia, hyperglycemia, insulin resistance, and subclinical inflammation, may explain part of the elevated risk of cardiometabolic diseases that is found in many subjects with very low fat mass, as it does in many obese subjects, is unknown. An important pathomechanism of impaired metabolic health is disproportionate fat distribution. In this article the risk of cardiometabolic diseases and mortality in subjects with metabolically healthy and unhealthy normal weight and obesity is summarized. Furthermore, the change of metabolic health during a longer period of follow-up and its impact on cardiometabolic diseases is being discussed. Finally, the implementation of the concept of metabolic health in daily clinical practice is being highlighted.
INTRODUCTION
Worldwide there is an increase in the prevalence of overweight and obesity [1]. Both, overweight and particularly obesity are associated with an increased risk of the cardiometabolic diseases type 2 diabetes and cardiovascular disease (CVD), certain types of cancer and mortality [2-5]. In most studies that investigated the relationships of fat mass with all cause and cause-specific mortality, fat mass has been estimated by the calculation of the body mass index (BMI) [2-4]. In this respect the nadir of allcause mortality risk was found to be 23 kg/m2 at age younger than 70 years, rising to 25 kg/m2 at age 70 years and older, while a BMI in the range of 21 to 25 kg/m2 was associated with the lowest risk of cancer, cardiovascular, and respiratory mortality [4]. In all of these studies a J-shaped relationship of BMI with all-cause and disease-specific mortality was found [2-4]. The increased mortality, that has been observed at a BMI <21 kg/m2, and particularly in the range of underweight (<18.5 kg/m2), may be largely attributed to increased mortality from mental and behavioural, neurological, and external causes [4,6]. However, as patients with lipodystrophy [7] or a lipodystrophy-like phenotype [8,9] have an increased cardiometabolic risk, it cannot be excluded that particularly a low amount of fat mass in the subcutaneous region may also increase mortality in the general population with a low BMI. Recent analyses from the Women’s Health Initiative study in postmenopausal women with normal BMI (18.5 to <25kg/m2) showed that lower gluteofemoral fat mass, estimated using dual-energy X-ray absorptiometry (DEXA), was associated with a higher incidence of CVD and that this increased risk was independent of increased trunk fat mass [10]. Furthermore, there is emerging evidence from precise phenotyping studies and from genetic studies showing that increased gluteofemoral and leg fat mass is protective of cardiometabolic diseases [11-13].
METABOLICALLY HEALTHY OBESITY
Because it is often not feasible to precisely measure fat distribution using DEXA, computed tomography or magnetic resonance imaging, during the last decade estimation of the cardiometabolic risk using insulin resistance and/or parameters of the metabolic syndrome has been the focus of intense research. Stefan et al. [14] found in 2008 that insulin sensitive obese subjects have a very characteristic body fat distribution that is characterized by a low amount of visceral fat and, more so, by a lower amount of liver fat content. In a back-to-back publication with our study, Wildman et al. [15] found in the National Health and Nutrition Examination Surveys 1999 to 2004 a similar prevalence of metabolically healthy obese subjects, when using parameters of the metabolic syndrome, a high homeostasis model assessment of insulin resistance (HOMA-IR) and an elevated high-sensitivity C-reactive protein (hs-CRP) level. Importantly, assuming that subjects cannot be considered ‘metabolically healthy’ when setting the cut-off for ‘health’ <3 parameters of the metabolic syndrome, they considered individuals being metabolically healthy when <2 parameters of the metabolic syndrome, except waist circumference, and high HOMA-IR and hs-CRP levels were present. Thus, while currently there is no broad agreement among the researchers and clinicians on how to define metabolic health (MH) [16-21], the definition proposed by Wildman et al. [15] appears to be most useful (Table 1). Nevertheless, many studies having investigated the relationship of metabolic healthy obesity (MHO) with cardiometabolic risk or cardiometabolic mortality used the definition of the metabolic syndrome. In meta-analyses of these studies subjects with MHO were found to have a moderately increased (~30%) risk of CVD and CVD mortality, when compared to subjects with metabolically healthy normal weight (MHNW). However, for a comparable BMI, the same risk was much higher (~150%) in subjects with metabolically unhealthy obesity (MUHO) [22-25]. While these studies mostly included Caucasians, similar relationships were also found in Asian populations. For example in an analysis of 323,175 adults from the large Korean National Health Insurance System (NHIS) database, who were followedup for a median of 96 months, subjects with MHO even had a lower all-cause (–19%) and cardiovascular (–27%) mortality risk, when compared to non-obese and metabolically healthy subjects. Of note, in that study the authors used a very strict definition of MH, in that subjects with MH were required to have none of three metabolic disease components (hypertension, diabetes, dyslipidemia) [26]. Thus, in general, subjects with MHO cannot be considered being truly healthy, but of having a much lower cardiometabolic risk, than most of the obese individuals.
METABOLICALLY UNHEALTHY NORMAL WEIGHT
In the course of the investigation of the cardiometabolic risk of subjects with MHO, for a long time the unexpected finding of very high risk (~120%) of CVD and CVD mortality of subjects with metabolically unhealthy normal weight (MUHNW), compared to subjects with MHNW, was not well recognized [22- 25]. When investigating the body fat distribution phenotypes of subjects with MUHNW Stefan et al. [8] found that it somewhat differed from the one observed in subjects with MUHO. While subjects with MUHO mostly have a very high visceral fat mass and an elevated liver fat content, subjects with MUHNW are predominantly characterized by a low amount of gluteofemoral fat mass [8]. Recently data from the Women’s Health Initiative Study support that a low gluteofemoral fat mass, measured by DEXA, associates with a high risk of CVD in lean women, and that this risk is independent of elevated trunk fat mass, which was also measured using DEXA [10]. It is widely accepted that adipose tissue in the gluteofemoral region serves as a healthy sink to store excess fat. This is mostly due to the fact that under excess energy intake this fat compartment responds predominantly by hyperplasia, which is accompanied by lower lipolytic activity. Thus, there is less spill-over of fatty acids into the circulation and less storage of fat ectopically in metabolic relevant organs, such as the liver and the pancreas [11]. Genetic studies further suggest that while the MUHO phenotype is predominantly characterized by variability in genes regulating food intake, the MUHNW phenotype is strongly characterized by variability in genes regulating adipocyte differentiation, lipogenesis and lipolysis. Furthermore, genes regulating hepatic de novo lipogenesis and lipid release from the liver and lifestyle parameters of the individual and its parents, impact on the pathogenesis of fatty liver (Fig. 1) [8,27,28].
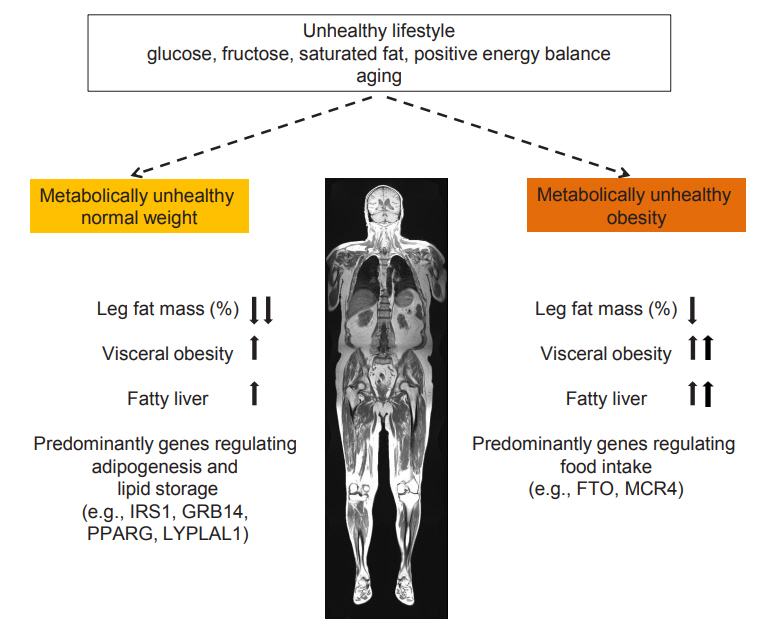
Fat distribution, fatty liver and main lifestyle and genetic determinants of metabolically unhealthy normal weight and obesity. The arrows indicate whether the prevalence of fat distribution and fatty liver is increased or decreased in the metabolically unhealthy condition. Modified from Stefan et al. [8]. IRS1, insulin receptor substrate 1; GRB14, growth factor receptor-bound protein 14; PPARG, peroxisome proliferator activated receptor gamma; LYPLAL1, lysophospholipase like 1; FTO, fat mass and obesity-associated; MC4R, melanocortin-4 receptor.
METABOLIC HEALTH AS A TRANSIENT STATE
During ageing there is body fat redistribution, predominantly from the gluteofemoral area to the upper body and, in most cases, a decrease in physical activity. This may result in a decline of the prevalence of MH. In agreement, in the Whitehall II study, about 50% of initially healthy obese individuals converted to an unhealthy phenotype over 20 years of follow-up [29]. In the largest study, also with the longest period of follow-up, that investigated the change of MH over time, the Nurses’ Health Study, among the initially metabolically healthy obese women only 16% remained metabolically healthy over 20 years of follow-up. This number decreased to 6% after 30 years of followup. However, also in initially metabolically healthy women with normal weight MH declined and after 30 years of follow-up only 15% of the women remained metabolically healthy [30].
CARDIOMETABOLIC RISK IN PERISTENT METABOLIC HEALTH
An important question is whether and to what extent the cardiometabolic risk differs in obese subjects who can retain MH over a longer period of follow-up. Data from the North West Adelaide Health Study suggest that risk of diabetes, CVD, or stroke is not increased in people with MHO compared to people with MHNW, if the metabolically healthy obesity phenotype is maintained during a natural follow-up over 5.5 to 10.3 years [31]. In the Nurses’ Health Study, women who maintained the MHO phenotype over 20 years still had a higher (34%) risk of CVD over the following 10 years of follow-up, when compared with normal weight women who were metabolically healthy over the same time period. However, this risk was much higher (120%) in obese women who could not retain MH (Fig. 2) [30]. These relationships were found in studies that predominantly included Caucasians. However, they may also be present in Asian populations. For example in a community-based population in Shanghai, China, 46.8% of individuals with MHO developed a metabolically unhealthy status during a follow-up period of 4.4 years. While subjects with transient MHO had an increased risk (152%) of a composite subclinical atherosclerosis endpoint, the risk was statistically not different (8%) in subjects with stable MHO, when compared to subjects who were metabolically healthy and non-obese (MHNO) [32].

Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease (CVD) risk across body mass index categories in 90,257 women of the Nurses’ Health Study. Risk of CVD in women with normal weight and obesity, stratified by metabolic health status. Hazard ratios (HRs) are adjusted for age, race, highest degree, alcohol consumption, postmenopausal status, physical examinations for screening purposes, family history of myocardial infarction and diabetes, aspirin use, smoking status change, physical activity. The data are from Eckel et al. [30], with permission from Elsevier. CI, confidence interval.
Of interest, persistent MH in obesity does not protect from heart failure as was most recently shown in an analysis of data from the Korean NHIS datasets from 2002 to 2017 [33]. When compared to stable MHNO individuals, subjects who retained MHO during a mean follow-up of 3.70 years, had a 17.3% increased hazard of hospitalization for heart failure (hHF). Furthermore, individuals who shifted from MHO to MHNO had a 34.3% lower hazard of hHF [33]. This is in agreement with previous observations showing that subjects with MHO may have no or only a moderately increased risk of myocardial infarction, but are not protected from heart failure [34], which is also strongly driven by obesity [35,36].
PREDICTORS OF THE CHANGE OF METABOLIC HEALTH
An important question related to the concept of MH is how can a metabolically healthy state be retained or achieved in subjects with impaired MH. In this respect weight loss that is brought about by a lifestyle intervention, may be very effective. In support of this hypothesis in the Tübingen Lifestyle Intervention Program, in subjects with MUHO, a median weight loss of 9.2 kg over a median period of 9 months was associated with the conversion to MH. Furthermore, among several of the baseline parameters tested (age, sex, BMI, waist circumference, systolic and diastolic blood pressure, fasting glucose, high density lipoprotein cholesterol and triglycerides, and MRI- and 1 HMRspectroscopy-derived measurements of visceral fat mass and liver fat content), only BMI and liver fat content remained independent predictors of the conversion from MUHO to MH [37]. In agreement with an important role of fatty liver in the pathogenesis of cardiometabolic diseases, involving insulin resistance, subclinical inflammation, increased hepatic glucose production, dyslipidemia and dysregulated hepatokine production [38-43], fatty liver was also found to be a stronger determinant of subclinical atherosclerosis than visceral fat mass and hyperglycemia [44].
However, can such a beneficial effect also be observed with a lifestyle intervention that is not accompanied by weight loss? In this respect data from the Prevención con Dieta Mediterránea (PREDIMED) study show that even in the absence of a large amount of weight loss, adherence to a Mediterranean diet can promote the transition to MH in subjects with MUHO and protect against deterioration of MH in subjects with MHO [45]. Similar results were also found in other studies involving healthy diets [18].
THE CONCEPT OF METABOLIC HEALTH IN CLINICAL PRACTICE
It is undisputed that all obese individuals, independent of the status of MH, should aim at achieving normal weight. For this several weight-loss programs have been recommended by the medical guidelines focusing on diet, exercise, pharmacological therapy and bariatric surgery [46]. However, based on the limited resources that are available for weight-loss programs, it may be reasonable to allocate resources predominantly to the MUHO people. In support of this assumption a study investigating the effect of phentermine/topiramate-induced weight loss compared to placebo on the prevention of diabetes in subjects who were stratified by the Cardiometabolic Disease Staging (CMDS) score in those with a high or a low cardiometabolic risk, provided interesting information. This score incorporates the parameters of the metabolic syndrome and, thus, is very similar to the MHO/MUHO concept. In that study the baseline mean BMI ranged from 34.2 to 41.2 kg/m2 among the subgroups and the percentage of weight loss was with around 10% almost identical in the verum groups. Although the treatment reduced diabetes risk compared to placebo in the high-risk group, in the latter group a risk as low as baseline in the low-risk was not achieved. Thus, substantially larger weight loss may be necessary to achieve a similarly low diabetes risk as the low-risk group. Second, still, the numbers needed to treat to prevent one case of diabetes over about 1 year were 120 in the low-risk group but only 24 in the high risk group. Thus, this study provided support that targeting high-risk patients for specific weight-loss strategies may improve the cost-benefit ratio of such interventions. Furthermore, while during the COVID-19 pandemic obesity emerged as an important and independent risk factor for a more severe course of the disease [47-49], a metabolically unhealthy condition is still considered to substantially increase this risk [49,50].
CONCLUSIONS
MH is not an entirely new concept. The parameters that are being used for its definition are well-known parameters of the metabolic syndrome [51]. However, there is agreement among the researchers and health care providers that a more stringent exclusion of risk parameters, such as is being done when defining MH, may improve the cardiometabolic risk prediction [11,18,20]. Furthermore, while intense research was done to understand the risk of diseases in subjects with MHO compared to people with MUHO, the widely neglected increased risk of cardiometabolic diseases in some of the lean people, became a major focus of recent cardiometabolic studies. Finally, as for many overweight and obese people MH may be an interesting and easily reachable ‘low hanging fruit’ on the path of weight-loss [37], implementation of the concept of MH in the communication of the health care providers with their patients may be of great motivational value.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was funded by the German Research Foundation (STE 1096/1-3) and the German Federal Ministry of Education and Research to the German Centre of Diabetes Research.

