Minimally Invasive Adrenal Surgery
Article information
Abstract
Since the introduction of minimally invasive surgery, laparoscopic adrenalectomy has become the main treatment option for adrenal masses. Various studies have reported that laparoscopic adrenalectomy showed fewer postoperative complications and faster recovery than conventional open adrenalectomy. Laparoscopic adrenalectomy can be performed through either the transperitoneal approach or the retroperitoneoscopic approach, which are widely used in most adrenal surgical procedures. Furthermore, with the development of minimally invasive surgery, organ-sparing adrenalectomy has recently emerged as a way to conserve functional adrenal gland tissue. According to recent data, organ-sparing adrenalectomy shows promising surgical, functional, and oncological outcomes including less intraoperative blood loss, maintenance of adrenal function, and low recurrence. Partial adrenalectomy was initially proposed for bilateral adrenal tumors in patients with hereditary disease to avoid chronic adrenal insufficiency. However, it has also gained popularity for the treatment of unilateral adrenal disease involving a small adrenal tumor because even patients with a unilateral adrenal gland may develop adrenal insufficiency in stressful situations. Therefore, partial adrenalectomy has become increasingly common to avoid lifelong steroid replacement and recurrence in most cases, especially in bilateral adrenal disease. This review article evaluates the current evidence on minimally invasive adrenalectomy and organ-preserving partial adrenalectomy.
INTRODUCTION
As a minimally invasive surgical approach to adrenalectomy, laparoscopic adrenalectomy was first described in 1992 by Gagner et al. [1] and Higashihara et al. [2]. Although conventional open adrenalectomy has been the gold standard surgical approach for adrenal disease, after the introduction of laparoscopic adrenal removal, various minimally invasive approaches have been developed and have become popular surgical treatments for adrenal tumors. Another widely used surgical method is the posterior endoscopic approach, which was first demonstrated by Mercan et al. [3] and provides direct access to the adrenal glands via a retroperitoneal approach. Furthermore, after the introduction of robotic surgical systems, robot-assisted adrenalectomy has also been demonstrated [4]. Minimally invasive adrenal surgery has been widely adopted because it has many advantages, including reduced blood loss during the operation, earlier ambulation, shorter length of hospital stay, and faster return to normal activity compared to open adrenalectomy [5]. These advantages suggest that laparoscopic adrenalectomy is the preferable surgical method for removal of most adrenal tumors, but advanced malignancies or large tumors are still considered indications for open adrenalectomy [6].
In addition to minimally invasive surgery, organ-preserving adrenal surgery has gained popularity to prevent adrenal insufficiency. Since there are several side effects of lifelong steroid replacement, for patients with bilateral adrenal tumors or even unilateral adrenal disease, preserving adrenal function is important because complete removal of the adrenal gland may induce adrenal insufficiency [7]. Numerous studies have investigated the suitability of different surgical approaches to adrenal tumors and the extent of resection to compare long-term outcomes. However, it remains unclear which option is optimal. This article reviewed various approaches in adrenalectomy and the optimal extent of resection to incorporate all the accumulated evidence.
SURGICAL APPROACHES
Laparoscopic transperitoneal approach
After the laparoscopic transperitoneal approach (LTA) was introduced, various studies reported experiences and advanced techniques of laparoscopic adrenalectomy. Since it offers the surgeon several advantages in terms of a familiar surgical view and a large working space, this approach is the most widely used technique. Furthermore, in the lateral decubitus position, after mobilization of the surrounding organs, gravity aids in the identification of the adrenal glands from adjacent structures and results in less retraction of surrounding organs [8]. For these reasons, LTA has been considered the gold standard procedure for the surgical treatment of adrenal tumors [9]. However, a drawback of this approach is that the patient’s operative position needs to be changed if the patient has bilateral adrenal tumors [10].
The definitive indications of minimally invasive surgery for adrenalectomy remain unclear. In the first description of laparoscopic adrenalectomy, the patients had 3 cm adrenal Cushing disease or 3.5 cm pheochromocytoma [1]. However, with the accumulation of experience and evidence, its indications expanded to large adrenal tumors and even malignant disease. The current indications are functioning adrenal lesions, including pheochromocytoma, cortisol-producing adenoma, and aldosterone-producing adenoma, as well as malignant adrenal tumors, including adrenal cortical carcinoma and malignant pheochromocytoma [11]. For the removal of benign adrenal tumors, the size criterion is more liberal for the transperitoneal approach [12]. Despite these advantages of the minimally invasive approach, in patients with large or malignant adrenal tumors that have the possibility of infiltration to adjacent organs, conventional open adrenalectomy is still preferred since it offers a wide surgical view and a familiar operative field [13].
It is still controversial whether operating on potentially malignant adrenal tumors with the minimally invasive technique is oncologically safe. Laparoscopic adrenalectomy has traditionally not been indicated for adrenal malignancy because it was associated with a higher incidence of recurrence [5]. However, recent studies demonstrated no significant differences in recurrence or complications between the laparoscopic and open approaches even for large adrenal malignancies [14]. According to the recent European Society of Endocrine Surgeons guidelines, LTA has been verified as safe and oncologically sufficient for adrenocortical carcinomas smaller than 10 cm [15]. In adrenal metastasectomy, the median operating time (159 minutes vs. 195 minutes, P=0.58) and overall complication rate (19% vs. 23.5%, P=0.7) were similar between the laparoscopic and open groups, and LTA was associated with a shorter length of hospital stay (3 days vs. 4 days, P=0.07) [16]. It remains controversial whether the choice of the approach for adrenalectomy affects the oncological outcome; currently, only complete resection of tumor, no presence of tumor rupture, and clear margin control are associated with improved survival [6].
Posterior retroperitoneoscopic approach
The posterior retroperitoneoscopic approach (PRA) was first described by Mercan et al. [3], but was advanced and popularized by Walz et al. [17]. It provides direct access through the retroperitoneal space; therefore, it does not require dissection or mobilization of adjacent organs to approach to the adrenal gland and enter into the peritoneal cavity. After the introduction of PRA, several authors reported that it led to a shorter operation time, less blood loss, less postoperative pain, and a shorter length of hospital stay [18]. PRA has gained popularity after the demonstration of excellent outcomes with this approach [17].
When PRA is used in a patient with bilateral adrenal tumors, the patient does not need to be repositioned to undergo bilateral adrenalectomy, unlike in LTA, because this approach allows the surgeon to directly access the adrenal glands on both sides of the body [19]. Furthermore, PRA was found to reduce the risks of surgical complications, including intestinal injury and postoperative adhesions, while entering the peritoneal cavity. Since patients who have undergone previous abdominal surgery might have adhesions in the peritoneal cavity, a prolonged operation time may be required due to the need to dissect adhesions in the peritoneal cavity [17]. However, PRA provides a unique and unfamiliar posterior anatomic view. Another drawback of PRA is that it does not allow the operator to conduct a laparoscopic exploration of the peritoneal cavity to identify accompanying disease. Regarding CO2 insufflation, the pressure is higher in PRA (18 to 22 mm Hg) than in LTA (12 mm Hg), which allows the creation of a sufficiently wide working space [17]. The high pressure of CO2 insufflation leads to a dry operating field, causing compression of small vessels and thereby preventing severe blood loss. Even under relatively high-pressure CO2 insufflation, gas embolism or instability of hemodynamic status were not found to be clinically significant [17]. However, an intraoperative injury of the inferior vena cava under CO2 insufflation can cause a gas embolism, which is a rare but fatal complication [20].
PREOPERATIVE SETTING AND OPERATIVE PROCEDURES
Laparoscopic transperitoneal approach
Under general anesthesia, the patient is placed into the lateral decubitus position with the affected side upward, and the position is maintained using a vacuum bean bag (Fig. 1A). The surgical bed is flexed just above the level of the iliac crest. Three trocars are used in left adrenalectomy and four in right adrenalectomy for the mobilization and retraction of the liver. Even though the trocars are commonly placed at the subcostal area in the anterior axillary and midclavicular lines, the trocar insertion sites could be modified according to the preference of the surgeon. After the insertion of all trocars, the adrenal gland is identified and exposed by separating surrounding organs. These organs, including the spleen, distal pancreas, and splenic flexure of the colon, are separated from the retroperitoneum for left adrenalectomy, while the liver is mobilized by division of the triangular ligament and moved to the medial side using a retractor for right adrenalectomy (Fig. 1C, E). After identifying the adjacent blood vessels, especially the main adrenal vein, the adrenal gland is retrieved in an endo bag and removed through a trocar insertion site.
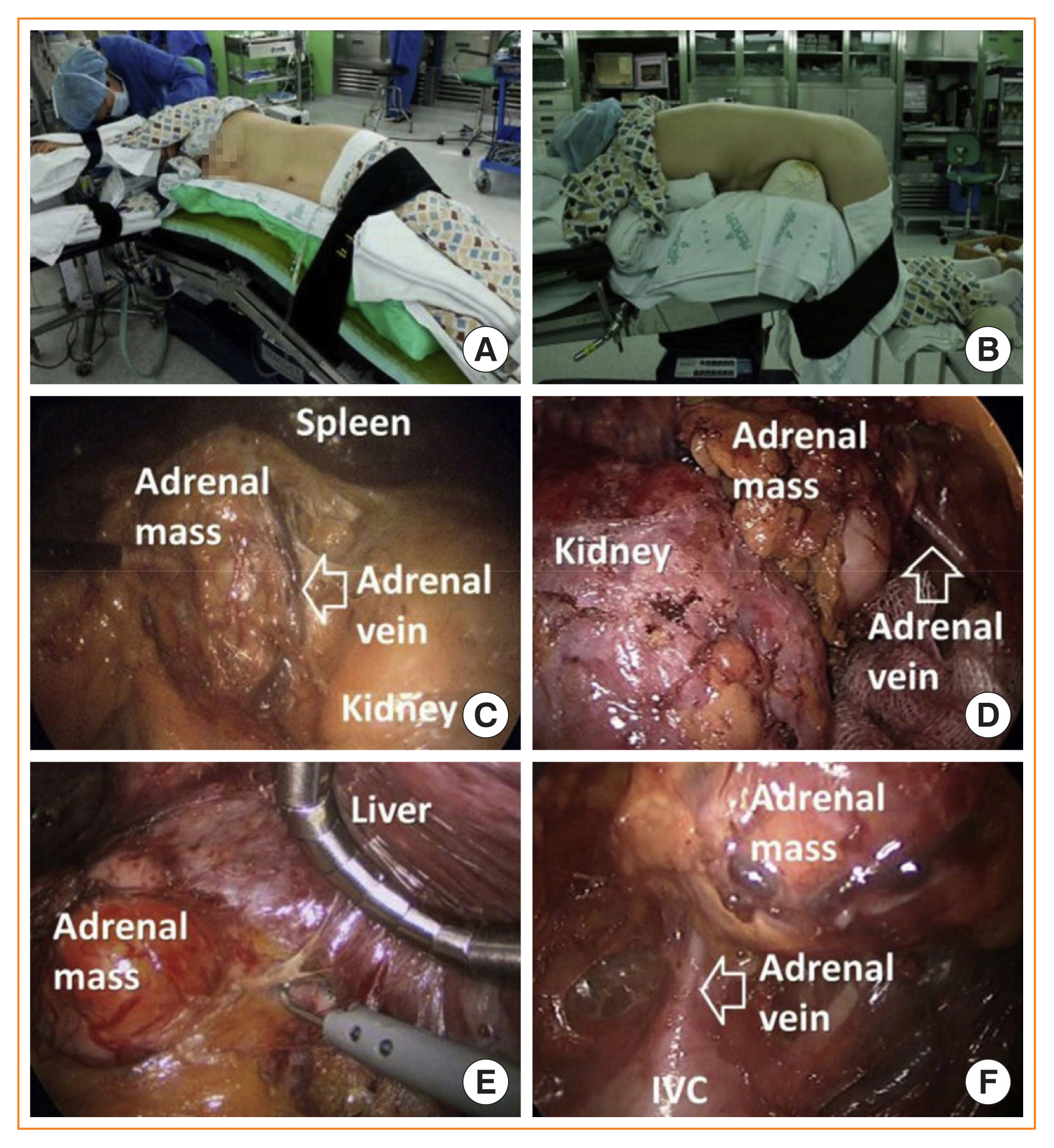
(A) Position for left lateral transperitoneal adrenalectomy. (B) Position for posterior retroperitoneoscopic adrenalectomy. (C) Intraoperative view of left lateral transperitoneal adrenalectomy. (D) Intraoperative view of left posterior retroperitoneoscopic adrenalectomy. (E) Intraoperative view of right lateral transperitoneal adrenalectomy. (F) Intraoperative view of right posterior retroperitoneoscopic adrenalectomy. Adapted from Chai et al. [5]. IVC, inferior vena cava.
Posterior retroperitoneoscopic approach
For PRA, the patient is intubated in a stretcher cart and then turned into the prone position when being transferred onto the operating table. The patient is placed in the prone position, lying on a soft bar below both the anterior and superior iliac spine in the jackknife position, bending the hip and knee joints (Fig. 1B). In general, three trocars are required for this procedure. A 15-mm transverse incision is made just beneath the tip of the 12th rib. With the surgeon’s index finger inside the retroperitoneal space, blunt dissection of the lumbar musculature and Gerota’s fascia is made under direct vision. Through this space, medial (5 mm) and lateral (5 mm) incisions are made just lateral to the erector spinae muscles and just beneath the lower tip of the 11th rib, respectively. Trocars are inserted through these additional incisions with the guidance of the surgeon’s index finger. Then, a 12-mm balloon trocar is inserted via the first incision. The medial port is used as a camera port. After CO2 insufflation (18 to 20 mm Hg), fatty tissues adjacent to the kidney are separated from the posterior part of the kidney, and the superior pole of the kidney is identified. The inferior part of the adrenal gland is separated from the superior pole of the kidney. Complete resection of the adrenal gland is performed after mobilizing the adrenal gland from the surrounding structures and ligating the adrenal vein (Fig. 1D, F). The resected adrenal gland is retrieved in an endo bag and removed through the 15-mm incision site.
COMPARISON OF SURGICAL OUTCOMES BETWEEN LTA AND PRA
Several studies have compared the surgical outcomes of LTA and PRA. These reports demonstrated clinically equivalent outcomes, with a smaller tumor size, less estimated blood loss (EBL), a shorter time to oral intake, and a shorter length of hospital stay in the PRA group (Table 1) [19,21–28]. A recent meta-analysis reported significant differences in demographic characteristics and surgical outcomes between LTA and PRA groups. The PRA group had smaller tumors (0.78 cm, P=0.003), less EBL (18 cc, P=0.006), a shorter time to oral intake (3.4 hours, P=0.009), and a shorter length of hospital stay (0.84 days, P= 0.001) [18]. However, other meta-analyses reported no significant differences in the operation time, blood loss, and length of hospitalization [10,29]. Chai et al. [5] demonstrated that PRA yielded a significant reduction in the operation time and length of hospital stay.
As a result of the relatively short distance between the port insertion site and tumor, the smaller working space in PRA makes it difficult for the surgeon to manipulate large adrenal tumors [30]. Therefore, the treatment of smaller tumors is more likely to be successful with this posterior approach. Several studies have reported that tumors smaller than 8 cm could be safely removed by the PRA technique, so larger tumors (with a size exceeding approximately 8 cm) are more usually removed by LTA [21]. Because of selection bias, tumor size might have played a role as a confounding factor regarding the tendency for a shorter operation time, less EBL, a shorter time to oral intake, and a shorter length of hospital stay in PRA. Surgery for a small tumor also involves less risk of damaging adjacent vessels or organs. This fact may account for the tendency for less EBL and shorter operation and recovery times [11]. However, even in a study that adjusted for tumor size, the operation time, EBL, and length of hospital stay showed significantly lower values in the PRA group than in the LTA group. Constantinides et al. [22] reported surgical outcomes with matched operation model, and found a shorter length of hospital stay (1.63 days vs. 3.5 days, P=0.003) and less analgesia requirements (paracetamol and tramadol, P=0.013 and P=0.011, respectively) in the PRA group than in the LTA group.
In recent years, two prospective randomized controlled trials have compared surgical outcomes between LTA and PRA [19,23]. Barczynski et al. [23] concluded that PRA was associated with a shorter operation time (50.8 minutes vs. 77.3 minutes, P<0.001), less EBL (52.7 cc vs. 97.8 cc, P<0.001), less operative pain (3.0% vs. 37.5%, P<0.001), and improved cost-effectiveness (1,728€ vs. 2,315€, P<0.001). Chai et al. [19] reported that both LTA and PRA were performed safely, showing similar surgical outcomes, including operation time (59.7±18.6 minutes vs. 67.6±28.7 minutes, P=0.139), and no significant difference in secondary outcomes including EBL, intraoperative hemodynamic stability, postoperative pain, time to passing gas, and postoperative complications. According to this recent evidence, both methods show a similarly short operation time even though the difference in surgical positioning tends to make the operation time longer. LTA provides a wide working space and familiar anatomical view, whereas PRA provides direct surgical access for bilateral adrenal tumors without re-positioning and while avoiding bowel adhesions. Therefore, both LTA and PRA can be performed safely with equivalent surgical outcomes.
PARTIAL ADRENALECTOMY
Partial adrenalectomy was first described in 1983 to treat familial bilateral pheochromocytoma by Irvin et al. [31]. In their study, to avoid lifelong steroid replacement, bilateral partial adrenalectomy was done, leaving normal functioning adrenal gland tissue, and the results of bilateral partial adrenalectomy showed normal adrenal function and no recurrence for several years. For patients who undergo bilateral total adrenalectomy, lifelong steroid replacement is mandatory. Therefore, the long-term usage of steroid hormones will expose the patient to the risk of complications related with steroid use. Prior research has indicated that inadequate steroid replacement can lead to Addisonian crisis after bilateral adrenalectomy in up to 25% to 33% of patients, and in 3% of cases, it could be fatal [32].
In light of the promising outcomes of partial adrenalectomy, it has gained wide popularity and has been more fully developed. In 1997, laparoscopic partial adrenalectomy was described by Janetschek et al. [33] for patients who had aldosterone-producing adenoma, resulting in good surgical outcomes and residual adrenocortical function. Therefore, for the treatment of hereditary and sporadic bilateral adrenal tumors, partial adrenalectomy has been performed to preserve adrenal function. Furthermore, patients with a small unilateral adrenal tumor and insufficient function of the contralateral adrenal gland are good candidates for partial adrenalectomy to maintain normal adrenal function. If a patient undergoes unilateral total adrenalectomy and the contralateral adrenal gland does not function well, the patient cannot respond equally to stressful events compared to those with normal bilateral adrenal glands. Therefore, steroid hormone replacement therapy is needed in such cases [34]. Mitchell et al. [35] reported that 22% of patients who did not have evidence of cortisol hypersecretion preoperatively developed adrenal insufficiency after unilateral adrenalectomy. Nagaraja et al. [7] reported in a meta-analysis that the overall steroid-independent rate of patients who underwent partial adrenalectomy was 85% (95% confidence interval [CI], 78% to 90%; P=0.001). The corresponding rates for unilateral and bilateral partial adrenalectomy were 84% (95% CI, 50% to 97%; P=0.001) and 81% (95% CI, 65% to 0.91%; P=0.001), respectively [7].
In patients receiving steroid replacement therapy, maintaining an appropriate steroid dosage is crucial. If the dosage is insufficient, patients are at risk for an Addisonian crisis, while too high of a dosage might lead to obesity, osteoporosis, hypertension, and diabetes. Patients need adequate dosage adjustment during physically or mentally stressful events and they are at risk for over- or under-treatment of steroids, which may induce inappropriate steroid hormone replacement [36].
However, partial adrenalectomy is associated with the risk of local recurrence. A previous study reported an estimated recurrence rate after adrenalectomy of >50% [37]. Nonetheless, advances in minimally invasive techniques seem to have had a positive effects on the outcomes of partial adrenalectomy, including the recurrence rate. In recent studies of laparoscopic or retroperitoneoscopic adrenal surgery, recurrence was found in fewer than 5% of cases after 10 years of follow-up [38]. In a comprehensive literature review, Kaye et al. [39] demonstrated that the rates of recurrence and steroid dependence for partial adrenalectomy were 3% and 5.3%, respectively. A recent systematic review and meta-analysis reviewed 60 relevant articles on recurrence after open, laparoscopic, and retroperitoneoscopic partial adrenalectomy. The overall recurrence rate was 8% (95% CI, 5% to 12%), the highest rate was for open adrenalectomy (15%), and the lowest rate was for retroperitoneoscopic adrenalectomy (1%; 95% CI, 0% to 4%) (Table 2) [7,39–44].
Partial adrenalectomy could obviate the need for steroid replacement in the majority of patients, and does not have a recurrence risk higher than that of total adrenalectomy. According to the 2014 Endocrine Society management guideline, partial adrenalectomy can be considered in patients with bilateral adrenal tumors [45]. Therefore, partial adrenalectomy is a valid and recommended option in patients with hereditary and sporadic bilateral adrenal disease, and even in those with unilateral adrenal disease involving small adrenal masses.
Surgical procedure for partial adrenalectomy
Technically, “cortical-sparing” adrenalectomy has the aim of the complete resection of adrenal medulla tissue while preserving adrenal cortical function. However, even with laparoscopic magnified inspection, there has been debate regarding the identification of a distinct boundary layer between the medulla and cortical tissue, as well as whether it is possible to ensure complete removal of pure medullary tissue while leaving cortical adrenal tissue in situ. Therefore, from the perspective of organ-sparing surgery, the current concept of “partial” adrenalectomy is more widely used than that of “cortical-sparing” adrenalectomy [36].
Partial adrenalectomy is a more difficult procedure than conventional open adrenalectomy. To determine the delineation of the precise surgical plane in partial adrenalectomy, laparoscopic inspection and palpation are crucial steps to identify the tumor location (Fig. 2A). Moreover, the adrenal glands are one of the most vascularized organs in the body, so the bleeding risk from the transected surface of remnant adrenal parenchyma is higher than that in total adrenalectomy, which does not leave residual adrenal gland tissue behind (Fig. 2B) [46]. Progressive advances in minimally invasive surgery techniques have offered magnified endoscopic or robotic views, which help surgeons to perform precise dissection, thereby improving the surgical outcomes of partial adrenalectomy [11].
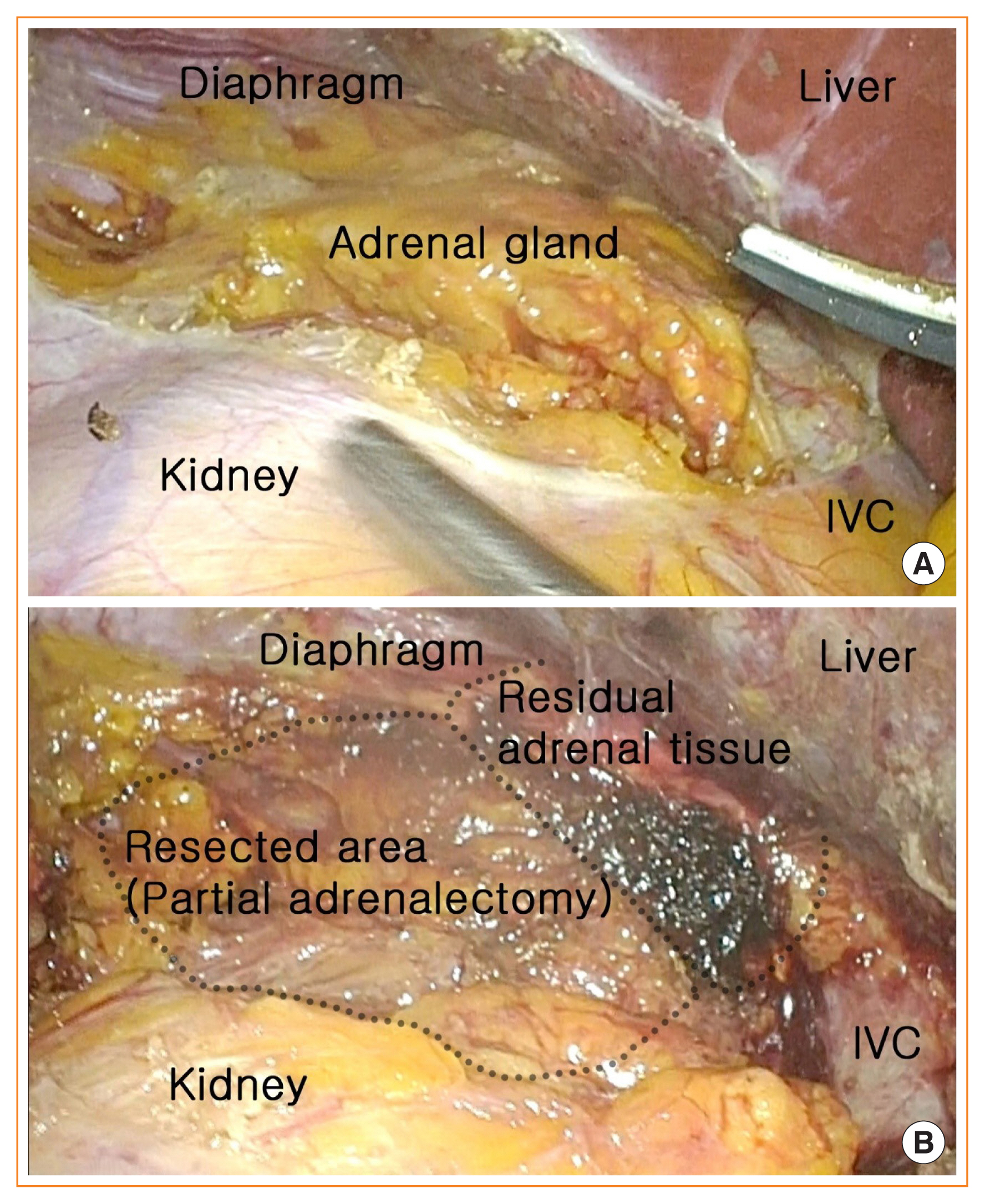
(A) Exposure of left adrenal gland for partial adrenalectomy. (B) Left partial adrenalectomy preserving normal residual adrenal tissue. IVC, inferior vena cava.
Unlike conventional total adrenalectomy, in partial adrenalectomy, the operator leaves a small amount of normal adrenal tissue, which is sufficient to maintain the adequate function of the adrenal gland, thereby minimizing the risk of lifelong steroid hormone supplementation and its complications, including Addisonian crisis [47]. Regarding the amount of residual adrenal tissue needed to maintain adrenal function, prior studies have demonstrated that approximately one-third of a unilateral adrenal gland is sufficient to maintain adrenal function [36]. Another author reported that at least 3 to 5 mm of residual adrenal tissue is required to sustain physiological adrenal function and to avoid steroid replacement. Nonetheless, the amount of adrenal tissue required to preserve adrenal function is still debatable [7].
Another important concern of partial adrenalectomy is preserving the adrenal vein for adequate venous drainage from the residual adrenal gland. Some authors have advocated emphasizing careful preservation of the adrenal vein in partial adrenalectomy. The reasons for this are that it is important for the body to receive hormones from a functional adrenal gland through venous drainage, massive bleeding should be avoided while transecting the adrenal gland, and injury of the adrenal vein could induce adrenal congestion from inadequate venous drainage [40]. However, several comparative studies have shown that the function of the residual adrenal gland does not depend on preservation of the adrenal vein, and there is no difference in steroid dependence between patients with adrenal vein preservation and ligation [48,49]. Therefore, it remains unclear whether preservation of the adrenal vein in partial adrenalectomy is essential. However, if it is possible to save the adrenal vein during complete removal of an adrenal tumor, it is suggested to preserve the adrenal vein [36].
In terms of tumor manipulation, local spillage of the tumor during adrenalectomy increases the risk of metastatic pheochromocytoma and recurrence of malignant adrenal tumors. Therefore, it is important to prevent cell spillage through rupture of the tumor cell capsule or a sudden release of catecholamines [50]. For this reason, these procedures should be performed by experienced surgeons (e.g., in a specialized center with experienced laparoscopic surgeons) with meticulous manipulation of the adrenal gland in cases of pheochromocytoma or malignant adrenal disease.
Treatment strategy: deciding on the extent of adrenalectomy
Despite advances in the surgical management of adrenal tumors, partial adrenalectomy is still not regarded as a gold standard treatment due to recurrence from residual adrenal tissue and surgical complications resulting from the difficulty of the surgical technique. Unlike total adrenalectomy, partial adrenalectomy preserves adrenal tissue through the precise dissection of tumors to reduce the risk of surgical complications, including lifelong steroid replacement therapy, which may cause osteoporosis, hypoandrogenism, and Addisonian crisis [40,41]. However, this strategy should be considered in light of the risk of recurrence of adrenal tumors in residual tissue. Therefore, the treatment strategy should be carefully chosen, with the optimal extent of adrenalectomy determined based on striking an appropriate balance between the preservation of adrenal function and complete removal of the tumor. Thus, there is still a need for studies comparing the surgical and oncological outcomes between total and partial adrenalectomy in terms of long-term efficacy.
CONCLUSIONS
As minimally invasive surgery, laparoscopic or retroperitoneoscopic adrenalectomy is expected to yield excellent surgical outcomes and minor complications regardless of the approach. Moreover, to preserve adrenal function and avoid lifelong steroid dependency, partial adrenalectomy is a safe and feasible option. For patients requiring bilateral adrenalectomy, such as those with sporadic or hereditary bilateral adrenal tumors, as well as unilateral adrenal tumors if the function of the contralateral adrenal gland may be insufficient in stressful situations, laparoscopic or retroperitoneoscopic partial surgery may be the treatment of choice. Additional evidence may be required to demonstrate a clear difference in the long-term outcomes of adrenalectomy according to the approach and extent of surgery.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.