Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(6); 2022 > Article
-
Original ArticleThyroid Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
 Keypoint
Keypoint
An excess of thyroid hormones in Graves’ disease (GD) has profound effects on systemic energy metabolism. This study provides an overview of alterations of plasma metabolites in 18 patients with GD according to age during 12 weeks of methimazole treatment. Significant variations were detected in plasma metabolomic patterns during the transition from hyperthyroidism to euthyroidism in patients with GD. The results are beneficial for understanding the clinical features and management of patients with GD. -
Ho Yeop Lee1,2*
 , Byeong Chang Sim1,2*
, Byeong Chang Sim1,2* , Ha Thi Nga1,2*
, Ha Thi Nga1,2* , Ji Sun Moon1, Jingwen Tian1,2, Nguyen Thi Linh1,2, Sang Hyeon Ju3, Dong Wook Choi4, Daiki Setoyama5
, Ji Sun Moon1, Jingwen Tian1,2, Nguyen Thi Linh1,2, Sang Hyeon Ju3, Dong Wook Choi4, Daiki Setoyama5 , Hyon-Seung Yi1,2,3
, Hyon-Seung Yi1,2,3
-
Endocrinology and Metabolism 2022;37(6):891-900.
DOI: https://doi.org/10.3803/EnM.2022.1590
Published online: December 26, 2022
1Laboratory of Endocrinology and Immune System, Chungnam National University College of Medicine, Daejeon, Korea
2Department of Medical Science, Chungnam National University College of Medicine, Daejeon, Korea
3Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
4Department of Biochemistry, Chungnam National University College of Natural Sciences, Daejeon, Korea
5Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, Fukuoka, Japan
- Corresponding authors: Hyon-Seung Yi. Laboratory of Endocrinology and Immune System, Chungnam National University College of Medicine, 282 Munhwa-ro, Jung-gu, Daejeon 35015, Korea Tel: +82-42-280-6994, Fax: +82-42-280-7995, E-mail: jmpbooks@cnu.ac.kr
- Daiki Setoyama. Department of Clinical Chemistry and Laboratory Medicine, Kyushu University Hospital, 3-1-1 Maidashi, Fukuoka 812-8582, Japan Tel: +81-92-642-5752, Fax: +81-92-642-5752, E-mail: setoyama.daiki.753@m.kyush-u.ac.jp
- *These authors contributed equally to this work.
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- An excess of thyroid hormones in Graves’ disease (GD) has profound effects on systemic energy metabolism that are currently partially understood. In this study, we aimed to provide a comprehensive understanding of the metabolite changes that occur when patients with GD transition from hyperthyroidism to euthyroidism with methimazole treatment.
-
Methods
- Eighteen patients (mean age, 38.6±14.7 years; 66.7% female) with newly diagnosed or relapsed GD attending the endocrinology outpatient clinics in a single institution were recruited between January 2019 and July 2020. All subjects were treated with methimazole to achieve euthyroidism. We explored metabolomics by performing liquid chromatography-mass spectrometry analysis of plasma samples of these patients and then performed multivariate statistical analysis of the metabolomics data.
-
Results
- Two hundred metabolites were measured before and after 12 weeks of methimazole treatment in patients with GD. The levels of 61 metabolites, including palmitic acid (C16:0) and oleic acid (C18:1), were elevated in methimazole-naïve patients with GD, and these levels were decreased by methimazole treatment. The levels of another 15 metabolites, including glycine and creatinine, were increased after recovery of euthyroidism upon methimazole treatment in patients with GD. Pathway analysis of metabolomics data showed that hyperthyroidism was closely related to aminoacyl-transfer ribonucleic acid biosynthesis and branched-chain amino acid biosynthesis pathways.
-
Conclusion
- In this study, significant variations of plasma metabolomic patterns that occur during the transition from hyperthyroidism to euthyroidism were detected in patients with GD via untargeted metabolomics analysis.
- Graves’ disease (GD) is an autoimmune thyroid disorder that results in overproduction of thyroid hormones. GD-mediated thyrotoxicosis increases resting energy expenditure, body temperature, and heart rate [1]. Patients with GD usually exhibit accelerated whole-body catabolism due to increased oxygen consumption and thermogenesis, resulting in weight loss [2,3]. Moreover, an excess of thyroid hormones induces lipolysis and gluconeogenesis as well as a reduction of cholesterol levels [4-6]. More specifically, expression and activity of carnitine palmitoyl transferase 1, a key regulatory enzyme for fatty acid oxidation, are high in livers with hyperthyroidism [7]. Triiodothyronine (T3) is also involved in mitochondrial biogenesis and tricarboxylic acid cycle activity [8]. However, it is unclear which metabolites are altered upon methimazole treatment in patients with GD.
- Metabolomics has the potential to identify diagnostic biomarkers in order to elucidate the mechanisms underlying diseases. Untargeted metabolomics provides a global profile of information through the simultaneous identification of as many metabolites as possible in specimens, which facilities better understanding of biochemical pathways associated with disease relapse and remission. Multiple studies have been conducted of metabolomic changes in patients with GD. Previous studies indicated that acylcarnitine and polyamine profiles differ between GD patients and the healthy population [9-12]. Hyperthyroidism had a significant impact on aminoacyl-transfer ribonucleic acid (tRNA) biosynthesis, purine and pyrimidine metabolism, and metabolism of several amino acids [12]. Moreover, remarkable changes of arginine and proline metabolic pathways are observed in adult patients with GD. Serum levels of phenylalanine and tyrosine are remarkably increased in pediatric, but not adult, patients with GD [13]. However, longitudinal analysis of changes in plasma metabolites before and after methimazole treatment has not been conducted.
- In the present study, we investigated whether specific plasma metabolites were associated with recovery of euthyroidism in patients with GD using untargeted metabolomics analyzed by liquid chromatography (LC)-mass spectrometry (MS). We also aimed to identify remarkable excess thyroid hormone-mediated changes of key metabolites according to age. Furthermore, we studied whether restoration of euthyroidism by methimazole treatment altered systemic metabolic pathways in patients with GD.
INTRODUCTION
- Study design and participants
- Korean patients with newly diagnosed or relapsed GD who visited the Department of Internal Medicine, Chungnam National University Hospital in Daejeon between January 2019 and July 2020 were recruited. All patients were treated with methimazole for 12 weeks to achieve euthyroidism. Plasma samples were prepared from all 18 patients at the initial visit and 12 weeks later at a follow-up visit. We selected treatment period for 12 weeks. Because we previously published a paper about thyrotoxic myopathy in patient with GD [14]. In that paper, we found that skeletal muscle function and thyroid hormone levels were recovered in most GD patients treated with methimazole for 12 weeks.
- Ethics statement
- This study was approved by the Institutional Review Board of Chungnam National University Hospital (CNUH-2019-02-012). Each participant provided consent and documented by the Department of International Medicine of Chungnam National University Hospital in Korea.
- Sample preparation for plasma metabolomics
- Details have been described previously [14]. Briefly, for whole metabolite extraction, 10 µL of plasma was added to 240 µL of water and 250 µL of ice-cold methanol before being vortexed and centrifuged (14,000 ×g, 4°C, 15 minutes). The supernatant was collected in a 1.5 mL Eppendorf microtube, processed for the extraction of various types of compounds listed below, and used for LC-MS measurement. For water-soluble metabolites, including amino acids and nucleotides, 25 µL of the supernatant was diluted 3-fold with 0.1% formic acid. For acylcarnitines, 30 µL of the supernatant was added to 270 µL of ice-cold methanol, vortexed, sonicated, and centrifuged (14,000 ×g, 4°C, 15 minutes), and the supernatant was collected. For free fatty acids (FFAs), 25 µL of the supernatant was diluted 2-fold with ice-cold methanol. For bile acids, 50 µL of the supernatant was evaporated and dissolved with 25 µL of 20% methanol. For phospholipids, 5 µL of the supernatant was diluted 200-fold with 0.1% formic acid in 20% acetonitrile.
- LC-MS measurements
- Plasma samples were analyzed using LC-MS on a LCMS-8060 instrument (Shimadzu, Kyoto, Japan), essentially as described previously [14]. To measure wide varieties of water-soluble metabolites, the prepared sample was separated on a Discovery HS-F5-3 column (150×2.1 mm, 3 μm particle size; Sigma-Aldrich, St. Louis, MO, USA) with mobile phases consisting of solvent A (0.1% formic acid) and solvent B (0.1% formic acid in acetonitrile). The column oven temperature was 40°C. The gradient elution program was as follows with a flow rate of 0.25 mL/min: 0–2 minutes, 0% B; 2–5 minutes, 0%–25% B; 5–11 minutes, 25%–35% B; 11–15 minutes, 35%–95% B; 15–25 minutes, 95% B; and 25.1–30 minutes, 0% B. The parameters for the heated electrospray ionization (ESI) source in negative/positive ion mode under multiple reaction monitoring (MRM) were as follows: drying gas flow rate, 10 L/min; nebulizer gas flow rate, 3 L/min; heating gas flow rate, 10 L/min; interface temperature, 300°C; desolvation line (DL) temperature, 250°C; heat block temperature, 400°C; and collision-induced dissociation (CID) gas, 270 kPa. For acylcarnitines, the sample was separated on a Luna HILIC column 200A (150×2.0 mm, 3 μm; Phenomenex, Torrance, CA, USA) with mobile phases consisting of solvent A (10 mM ammonium formate) and solvent B (acetonitrile/10 mM ammonium formate [9/1, v/v]). The column oven temperature was 40°C. The gradient elution program was as follows with a flow rate of 0.3 mL/min: 0–2.5 minutes, 100% B; 2.5–4 minutes, 100%–50% B; 4–7.5 minutes, 50%–5% B; 7.5–10 minutes, 5% B; and 10.1–12.5 minutes, 100% B. Acylcarnitine profiles were detected in positive ESI mode under precursor ion scan for m/z 85.5 by changing collision energy according to the lengths of fatty acids as follows: –20 for shortchain acylcarnitines (C0–C8); –35 for middle-chain acylcarnitines (C9–C12); and –45 for long-chain acylcarnitines (C12–C18). For FFAs, the sample was separated on a ACQUITY BEH Amide column (150×2.1 mm, 1.7 μm; Waters, Milford, MA, USA) with mobile phases consisting of solvent A (10 mM ammonium formate in 90% acetonitrile) and solvent B (0.1% formic acid in acetonitrile). The column oven temperature was 40°C. The gradient elution program was as follows with a flow rate of 0.4 mL/min: 0–5 minutes, 0% B; 5.1–7.5 minutes, 100% B; and 7.6–11 minutes, 0% B. FFA profiles were detected in negative ESI mode under selected ion monitoring. For bile acids, the sample was separated on a ACQUITY BEH Amide column (150×2.1 mm, 1.7 μm; Waters) with mobile phases consisting of solvent A (0.1% formic acid) and solvent B (acetonitrile). The column oven temperature was 50°C. The gradient elution program was as follows with a flow rate of 0.3 mL/min: 0–2 minutes, 20% B; 2–10 minutes, 80% B; 10–12 minutes, 80% B; and 12.1–15 minutes, 20% B. Bile acid profiles were detected in negative ESI mode under MRM. For phospholipids, the prepared sample was separated on a Kinetex C8 column (150×2.1 mm, 2.6 μm particle size; Phenomenex) with mobile phases consisting of solvent A (20 mM ammonium formate) and solvent B (acetonitrile/isopropanol [1:1, v/v]). The column oven temperature was 45°C. The gradient elution program was as follows with a flow rate of 0.3 mL/min: 0–1 minutes, 20% B; 1–2 minutes, 40% B; 2–25 minutes, 92.5% B; 25.1–35 minutes, 100% B; and 35.1–38 minutes, 20% B. Phospholipid profiles were detected in positive ESI mode under MRM.
- Metabolomics data processing and analysis
- Data processing was performed using the LabSolutions LC-MS software program (Shimadzu).
- Biochemical measurements
- Peripheral blood was collected in heparin-coated tubes. Plasma levels of thyroid-stimulating hormone (TSH), T3, free thyroxine (T4), and TSH-binding inhibitor immunoglobulin (TBII) were measured by standard methods on an automated analyzer (Cobas 6000, Roche Diagnostics GmbH, Mannheim, Germany).
- Principal component analysis and metabolic pathway analysis of metabolomics data
- MetaboAnalyst was used for pathway analysis and performs metabolite set enrichment analysis, which includes a set of human and mammalian metabolites and a set of chemical grade metabolites. This module accepts a list of compound names, a list of compound names with concentrations, or a table of concentrations. The analysis is based on multiple libraries containing a set of approximately 9,000 biologically significant metabolites collected primarily from human studies, including more than 1,500 chemical classes. A portion of the data utilized for analysis could be analyzed because it was matched to the MetaboAnalyst database. The R program’s FactoMineR function was used to perform principal component analysis (PCA).
- Statistical analysis
- The analysis method was selected after proving the normalcy of the data in accordance with the distribution of each variable. Using the details of statistical tests included in the plot of ggstatsplot, an extension of ggplot2, violin plots could be created. The Student’s t test (two-sided) was used to compare variables between groups (before and after treatment) after assessing the assumption of normality. The Wilcoxon signed-rank test was used to compare paired variables between groups. Statistical analyses were performed using R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). P<0.05 was considered significant.
METHODS
- Characteristics of the study participants
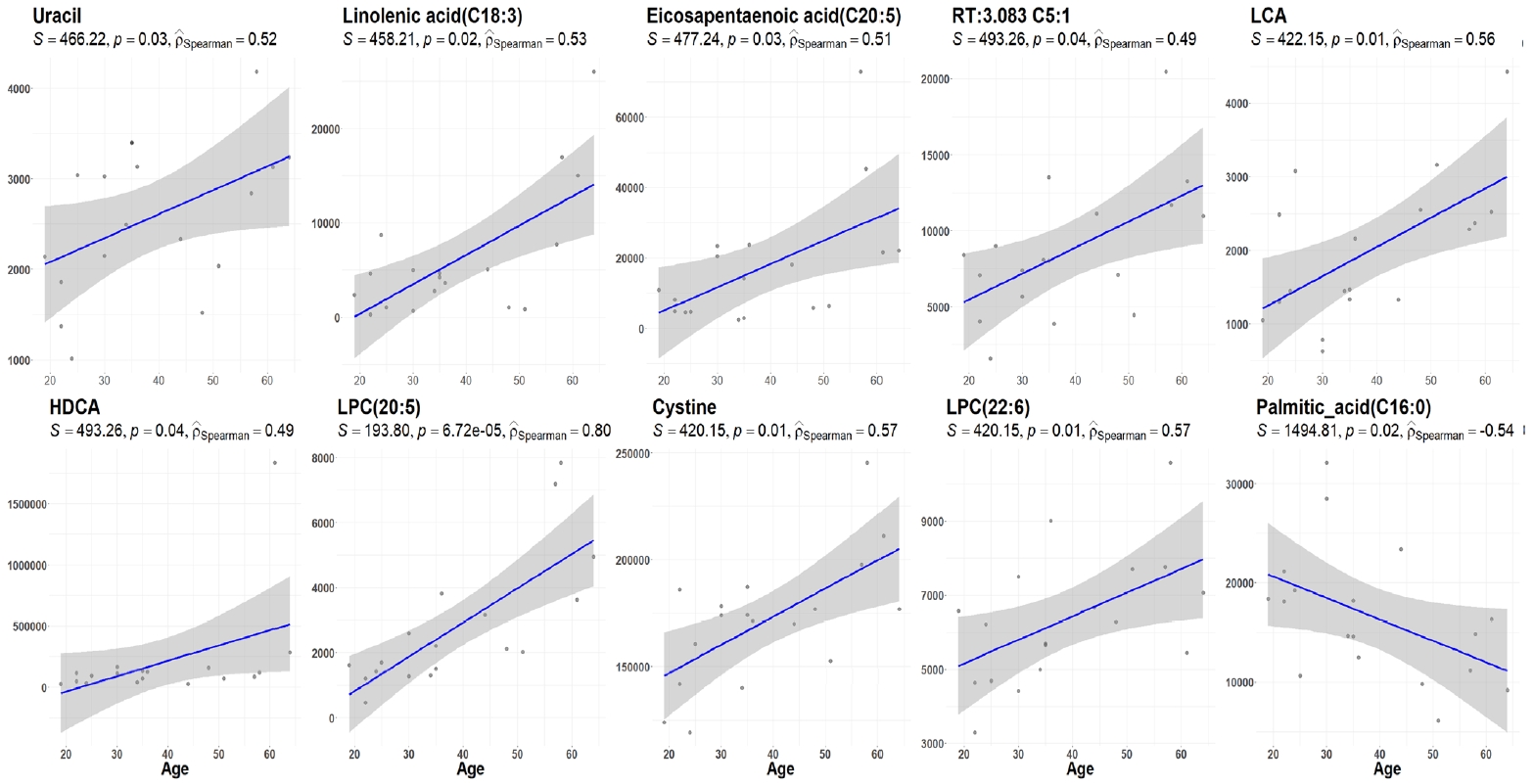
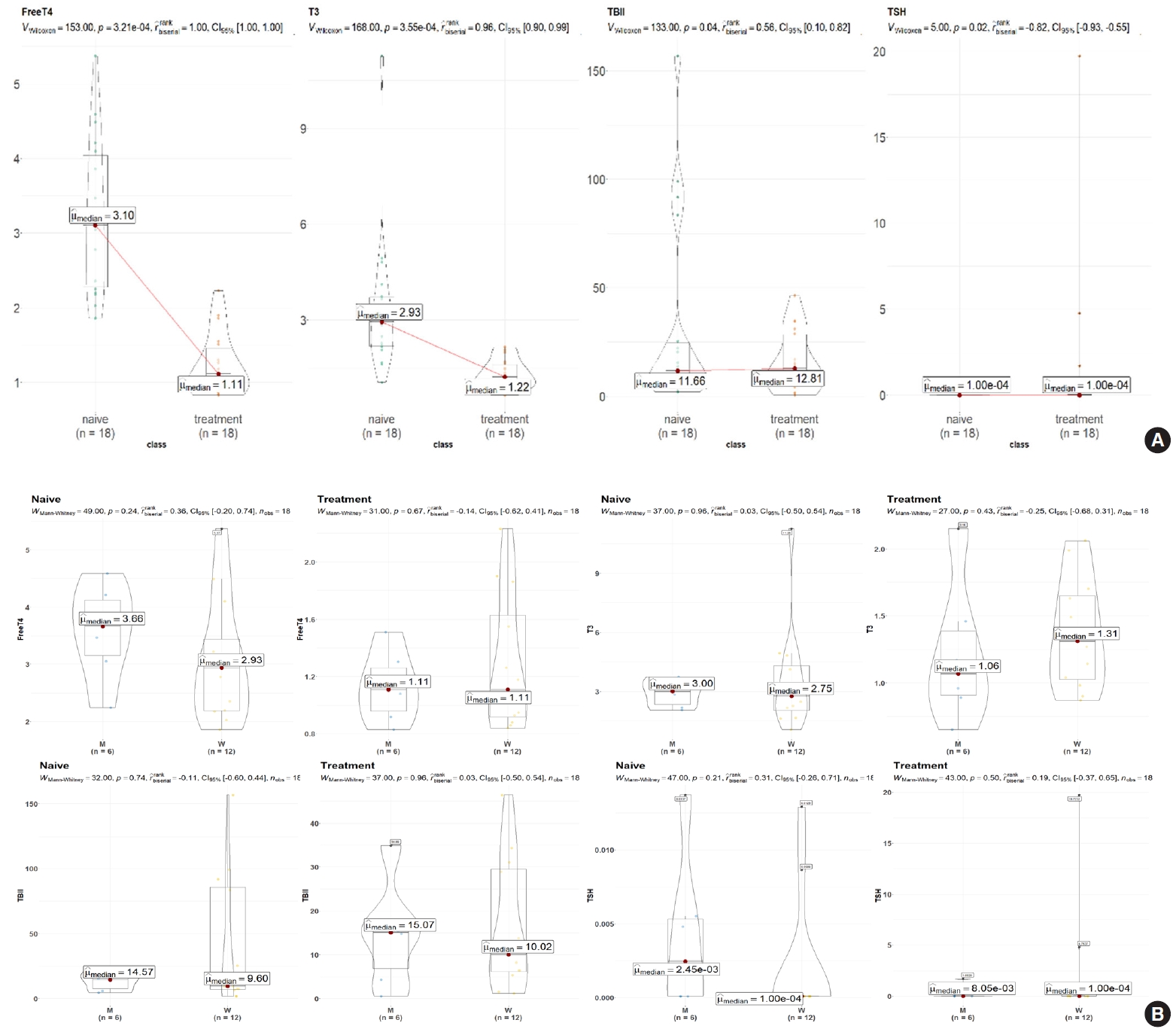
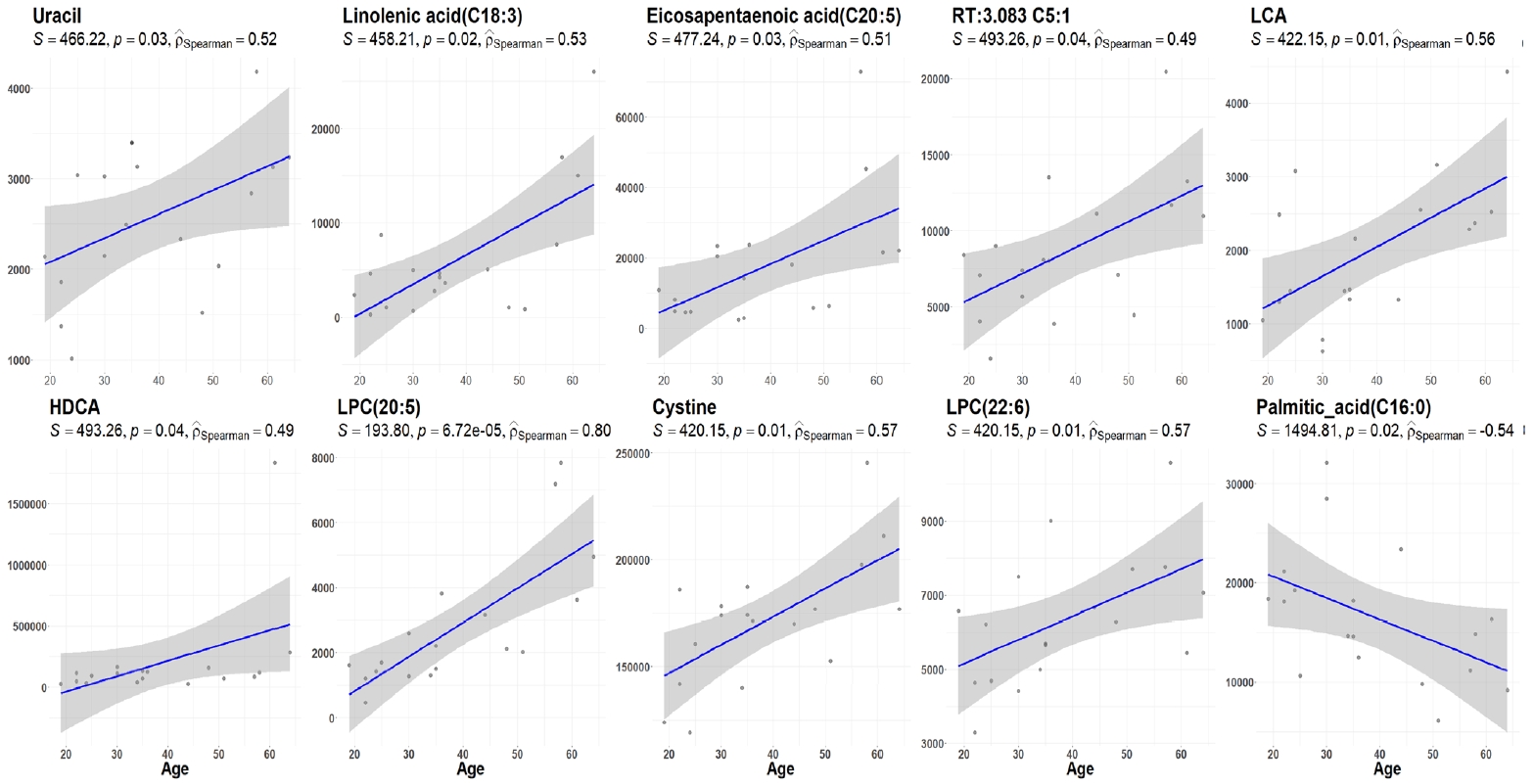
- The demographic and clinical characteristics of 18 patients with GD (male 33%, female 67%) at the initial visit are showed in Table 1. Twelve weeks after methimazole treatment, the thyroid hormone levels of all patients were within the normal range and were significantly different from those before treatment (Fig. 1A). The mean free T4, T3, TSH, and TBII levels of GD patients were similar in males and females (Fig. 1B). The age of the study participants was 22 to 64 years. Among all the measured metabolites, only nine plasma metabolites, including uracil, linolenic acid (C18:3), lysophosphatidylcholine (20:5), and cysteine, had positive correlations (P<0.05) with age in drug-naïve patients with GD. On the other hand, only palmitic acid (C16:0) had a negative correlation with age (Fig. 2).
- Differential metabolomics analysis before and after 12 weeks of methimazole treatment in GD patients
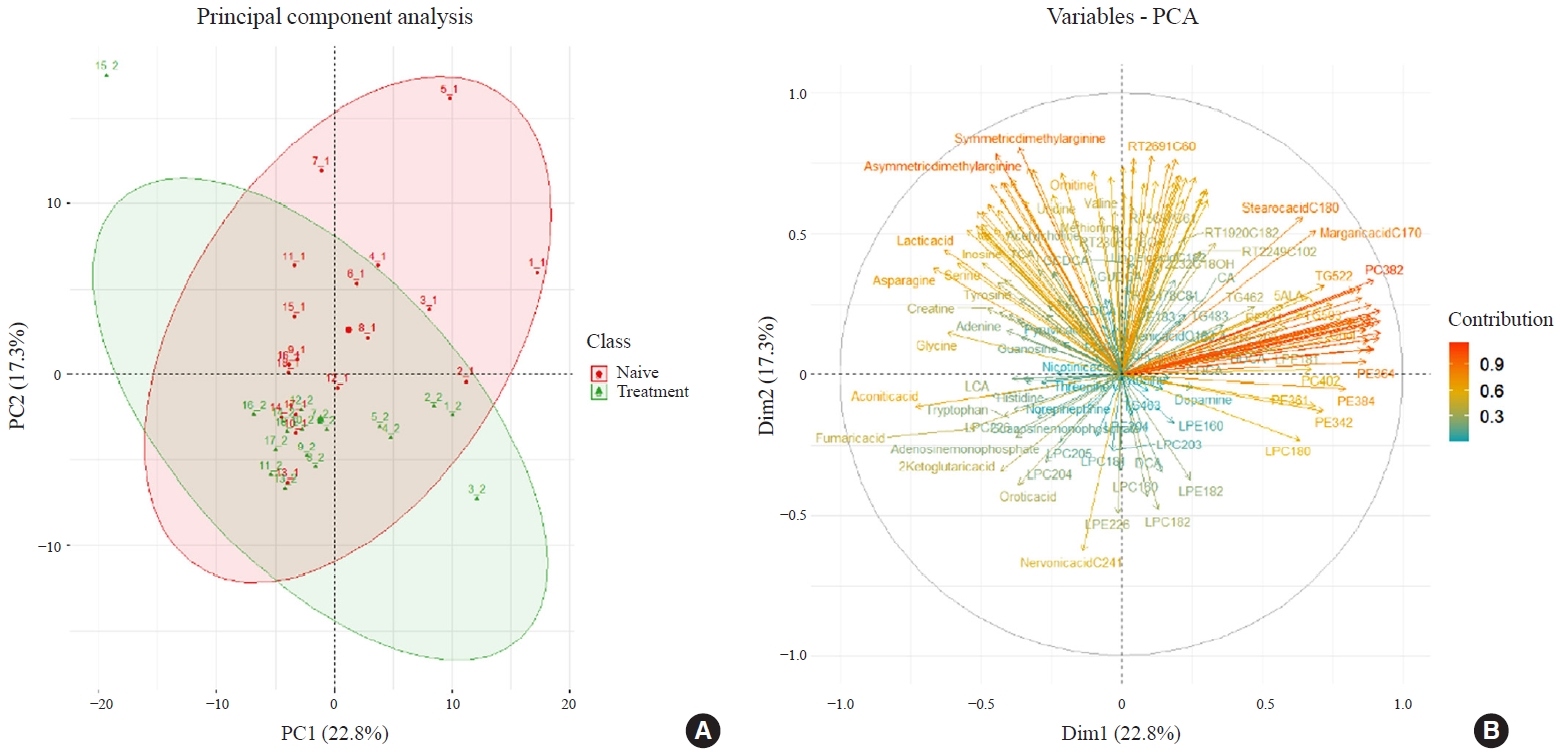
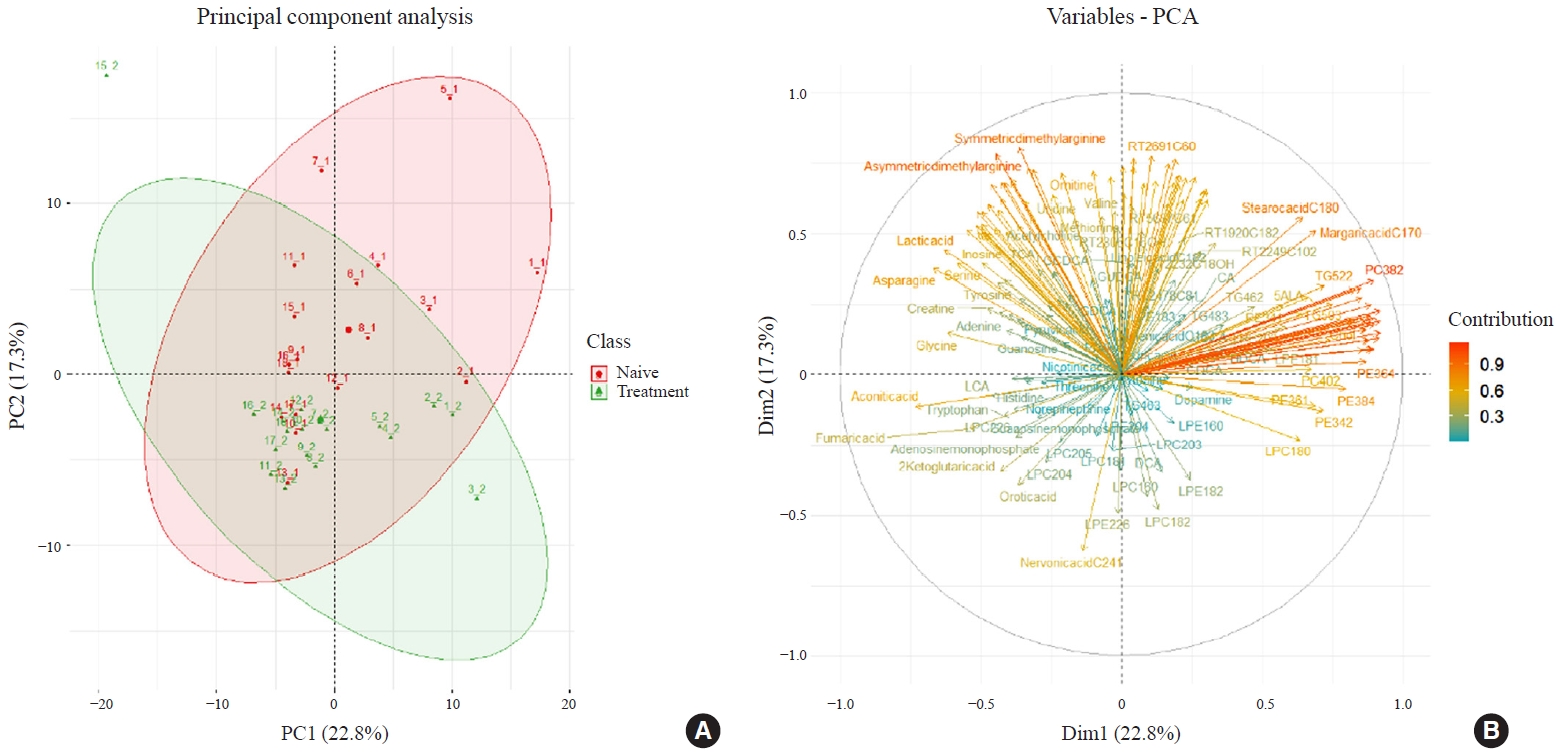
- Two hundred metabolites were measured in plasma samples of all participants with GD before and after methimazole treatment (Supplemental Table S1). PCA score plots showed separate clustering before and after 12 weeks of methimazole treatment in patients with GD (Fig. 3A). Creatine, stearic acid (C18:0), and palmitic acid (C16:0) were enriched in plasma of methimazole-naïve patients with GD. On the other hand, many LPC family members, 2-ketoglutaric acid, and nervonic acid (C24:1) were enriched after methimazole treatment (Fig. 3B).
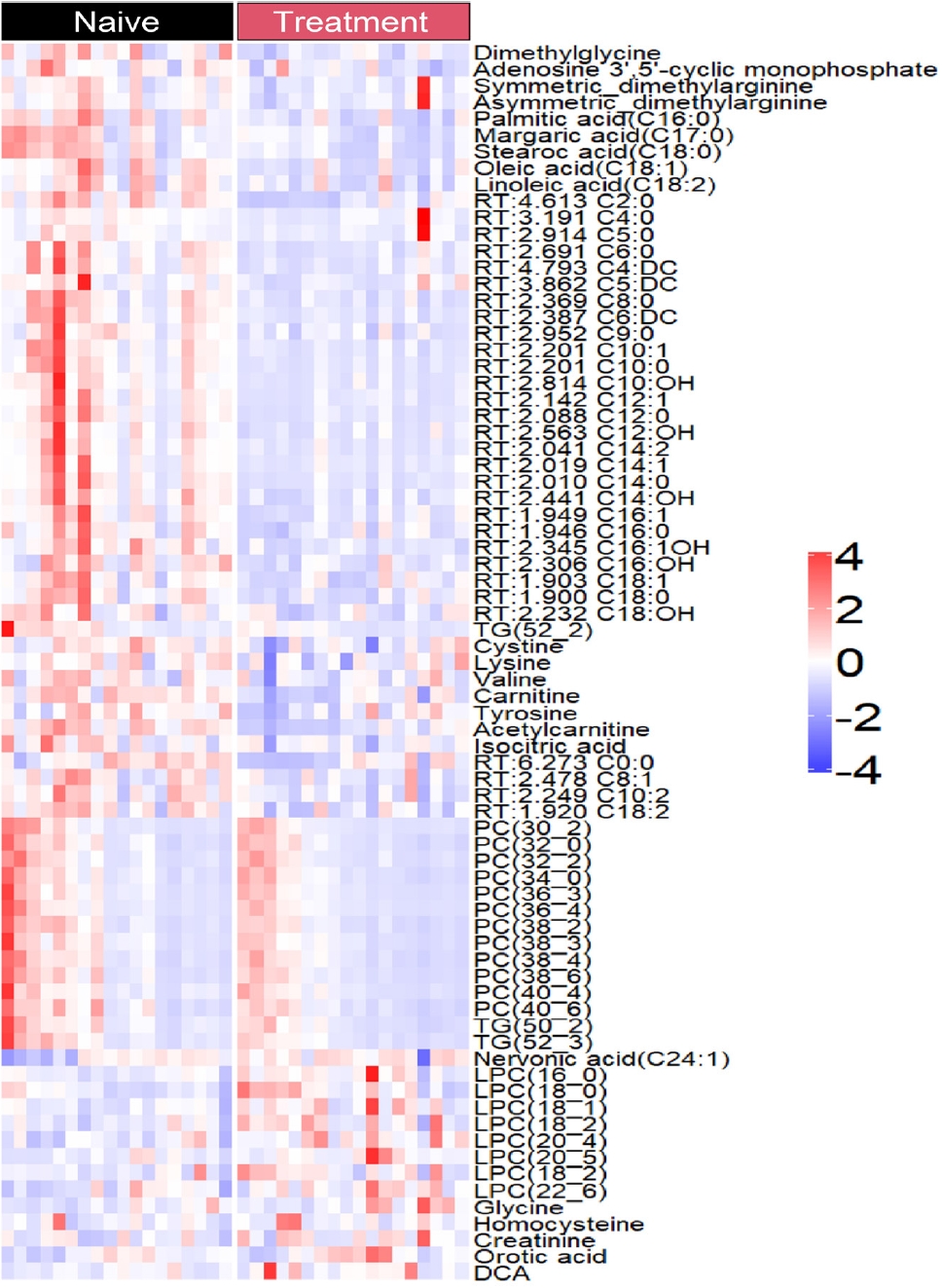
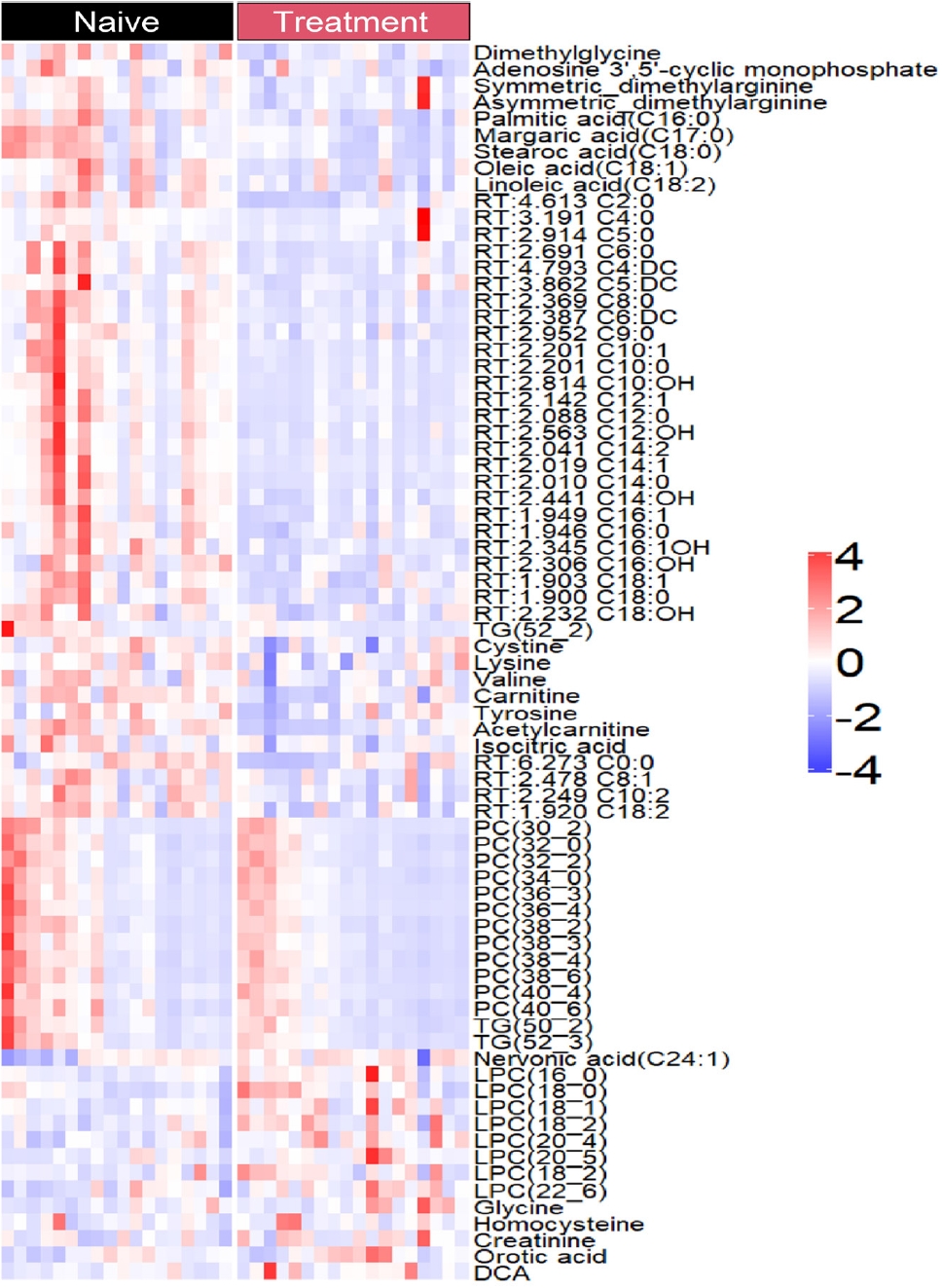
- The levels of only 76 plasma metabolites significantly differed before and after methimazole treatment. The levels of 61 metabolites were increased in drug-naïve patients with GD and the levels of 15 metabolites were increased after methimazole treatment. The levels of fatty acids including palmitic acid (C16:0), stearic acid (C18:0), carnitine, and oleic acid (C18:1), and amino acids including dimethylglycine, cysteine, and valine were elevated in patients with GD at the initial visit, but they were remarkably decreased upon methimazole treatment (Fig. 4). By contrast, the levels of nervonic acid (C24:1), LPC family members including LPC (16:0), (18:0), and (18:1), glycine, and creatinine were significantly increased upon methimazole treatment (Fig. 4 and Supplemental Fig. S1).
- Pathways that differ between GD patients with thyrotoxicosis and euthyroidism
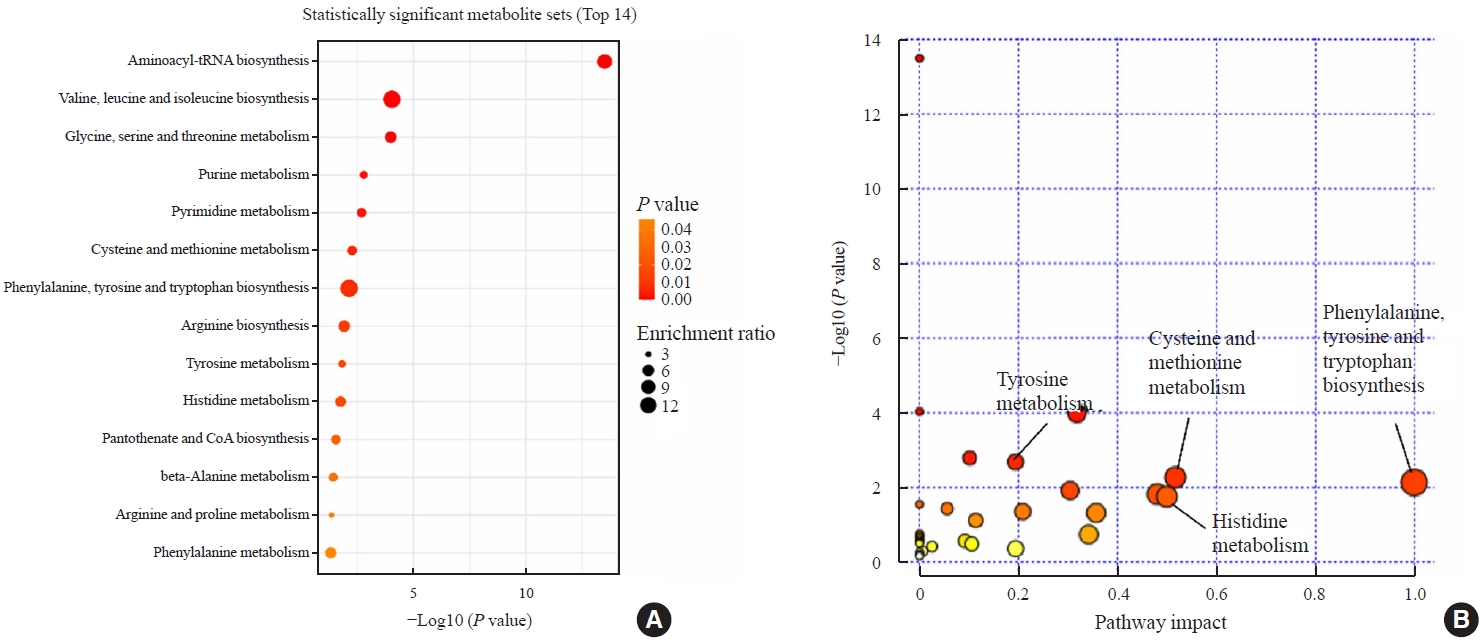
- We observed changes of plasma metabolites before and after methimazole treatment in GD patients. To identify changes in metabolic pathways, we investigated which metabolic pathways differed between the initial visit and 12-week follow-up visit using pathway enrichment analysis. The aminoacyl-tRNA biosynthesis pathway was most significantly altered (Fig. 5). Moreover, various amino acid metabolic pathways, including valine, leucine, and isoleucine biosynthesis and glycine, serine, and threonine metabolism, differed before and after methimazole treatment in patients with GD (Fig. 5). In addition, phenylalanine, tyrosine, and tryptophan biosynthesis- and cysteine and methionine metabolism-related metabolite sets significantly differed between the initial visit and 12-week follow-up visit (Fig. 4B).
RESULTS
- This study provides an overview of alterations of plasma metabolites in GD patients according to age and 12 weeks of methimazole treatment. The levels of 200 plasma metabolites, including amino acids, FFAs, bile acids, and lipids, were measured in patients with GD before and after methimazole treatment. The levels of 76 metabolites significantly differed before and after treatment. Of these, the levels of 61 (80.2%) metabolites were increased in drug-naïve patients with hyperthyroidism and the levels of 15 metabolites (19.8%) were higher in the recovery phase after methimazole treatment. An excess of thyroid hormones induced significant changes of numerous amino acid biosynthesis pathways, especially the aminoacyl-tRNA biosynthesis pathway, which were stabilized upon recovery of euthyroidism. We also found that metabolites varied according to age in GD patients (Fig. 2). A previous report showed that many metabolites including arginine, carnitine, and β-hydroxybutyrate are altered by aging [15], but we found that some metabolites including uracil, LPC family members, and palmitic acid were only changed when thyroid hormones were in excess. A further study is warranted to determine not only the biological meaning of the metabolite changes but also to reveal the roles of each enriched metabolite in GD patients.
- Several studies have investigated the role of thyroid hormones in systemic lipid metabolism. Thyroid hormones regulate lipogenesis and lipolysis [16]. Overproduction of thyroid hormones stimulates systemic lipolysis, elevating the circulating FFA level [17]. The current study shows that overproduction of thyroid hormones increases the levels of fatty acids of different lengths, including acetylcarnitine and acylcarnitine, which are involved in the conversion of fat into energy [18]. Therefore, systemic lipolysis and mitochondrial β-oxidation are stimulated by overproduction of thyroid hormones and decreased by methimazole treatment. Another publication indicated that patients with hyperthyroidism have an increased serum concentration of FFAs and treatment with antithyroid drugs revealed a direct relationship between thyroid activity and the FFA concentration [19]. Our data are consistent with an inverse relationship between thyroid activity and the percentage levels of palmitic acid in FFAs. In a cross-sectional study, Zhou et al. [20] examined the relationship between serum palmitic acid and thyroid function in the United States population using the database of the National Health and Nutrition Examination Survey from 2011 to 2012. They identified a significant negative association between serum palmitic acid and free T4 [20]. In our study, methimazole-naïve patients had an elevated serum level of palmitic acid and this was decreased after methimazole treatment. This discrepancy is thought to originate from a difference in study design. Alterations in serum thyroid hormones are associated with aging [21,22]. In the European population, Hoogendoorn et al. [23] reported that the serum free T4 concentrations increases with age in individuals older than 60 years. These data seem to be consistent with our finding that the serum palmitic acid decreased with age and methimazole treatment in Korean patients. However, another study from Western Australia found no significant change in the free T4 level with aging [21].
- Amino acids are organic compounds involved in protein synthesis and protein-protein interactions, which are essential for the composition of muscle and other tissues [24,25]. Branched-chain amino acids (BCAAs), valine, leucine, and isoleucine, are essential amino acids and serve as substrates for inhibition of proteolysis, protein synthesis, glucose metabolism, and energy production [26,27]. In the present study, various amino acid metabolic pathways differed before and after 12 weeks of methimazole treatment in patients with GD. Levels of valine, leucine, and arginine have significant positive correlations with serum levels of T4 and free T4 [28]. Consistently, valine and leucine biosynthesis was reduced after 12 weeks of treatment in the current study. BCAAs, valine, leucine, and isoleucine have been well studied in the context of muscle protein recovery [29]. The elevation of BCAAs in GD patients is thought to be due to their release from skeletal muscle, which is the initial catabolism site of BCAAs due to high expression of BCAA aminotransferase, the first enzyme in the pathway of BCAA catabolism [27,30]. GD patients exhibit muscle loss and weakness; therefore, the elevation of BCAAs via valine, leucine, and isoleucine biosynthesis is a compensatory effect. Thus, to overcome muscle myopathy in GD patients, there is a compensatory effect to improve muscle function through elevation of BCAA biosynthesis. Glycine and serine were reported to inhibit TSH secretion in rats by enhancing the activity of the periventricular hypothalamus and pituitary gland [31]. The elevated level of glycine after methimazole treatment in our study may reflect the function of glycine in the transition from hyperthyroidism to euthyroidism.
- The main strength of this study is that it acquired serial data from LC-MS-based plasma metabolomics to make findings that may have clinical value for understanding the pathophysiology of GD. However, the present study has several limitations. First and most importantly, as an observational study, it cannot determine a causal association between variables. Second, the study population was exclusively South Korean and therefore the results may not be applicable to other ethnicities with GD. Third, a variety of confounding factors could not be considered in multivariate analyses due to the small sample size. Fourth, the follow-up period of 12 weeks was too short to fully assess euthyroid-induced recovery of metabolic networks. Longer serial follow-up may provide additional information about the long-term systemic metabolic effects of hyperthyroidism. Lastly, the lack of dynamic information precluded interpretation of metabolomics data in the context of metabolic fluxes. It is difficult to determine whether our findings are the result of increased flux from a synthesizing enzyme, decreased flux toward a consuming enzyme, or alteration in transport of a metabolite into or out of cells or tissues.
- In conclusion, this study reports comprehensive metabolite changes that occur during the transition from hyperthyroidism to euthyroidism, and provides new insights into systemic energy homeostasis and metabolic processes, which are beneficial for understanding the clinical features and management of patients with GD.
DISCUSSION
Supplementary Material
Supplementary Fig. S1.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: D.W.C., D.S., H.S.Y. Acquisition, analysis, or interpretation of data: H.Y.L., B.C.S., H.T.N., J.S.M., J.T., N.T.L., D.S., H.S.Y. Drafting the work or revising: H.Y.L., B.C.S., H.T.N., S.H.J., D.W.C., D.S., H.S.Y. Final approval of the manuscript: D.S., H.S.Y.
Article information
-
Acknowledgements
- This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning, Korea (NRF-2021R1A2C4001829 and NRF-2021R1A5-A8029876). H-SY was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HR22C1734) and the CNUH Research Fund, 2022. We would like to thank Ms. Megumi Matsuura for LC-MS measurements. BCS was supported by the BK21 FOUR Program by Chungnam National University Research Grant, 2022.
- 1. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 2008;18:141–4.ArticlePubMed
- 2. Greenlund LJ, Nair KS, Brennan MD. Changes in body composition in women following treatment of overt and subclinical hyperthyroidism. Endocr Pract 2008;14:973–8.ArticlePubMed
- 3. Lee JC, Song BS, Kang YM, Kim YR, Kang YE, Lee JH, et al. Effect of thyroid-stimulating hormone suppression on muscle function after total thyroidectomy in patients with thyroid cancer. Front Endocrinol (Lausanne) 2021;12:769074.ArticlePubMedPMC
- 4. Brent GA. Clinical practice: Graves’ disease. N Engl J Med 2008;358:2594–605.ArticlePubMed
- 5. Song E, Koo MJ, Noh E, Hwang SY, Park MJ, Kim JA, et al. Risk of diabetes in patients with long-standing Graves’ disease: a longitudinal study. Endocrinol Metab (Seoul) 2021;36:1277–86.ArticlePubMedPMCPDF
- 6. Eom YS, Wilson JR, Bernet VJ. Links between thyroid disorders and glucose homeostasis. Diabetes Metab J 2022;46:239–56.ArticlePubMedPMCPDF
- 7. Mynatt RL, Park EA, Thorngate FE, Das HK, Cook GA. Changes in carnitine palmitoyltransferase-I mRNA abundance produced by hyperthyroidism and hypothyroidism parallel changes in activity. Biochem Biophys Res Commun 1994;201:932–7.ArticlePubMed
- 8. Goglia F, Moreno M, Lanni A. Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Lett 1999;452:115–20.ArticlePubMedPDF
- 9. Al-Majdoub M, Lantz M, Spegel P. Treatment of Swedish patients with Graves’ hyperthyroidism is associated with changes in acylcarnitine levels. Thyroid 2017;27:1109–17.ArticlePubMed
- 10. Song J, Shan Z, Mao J, Teng W. Serum polyamine metabolic profile in autoimmune thyroid disease patients. Clin Endocrinol (Oxf) 2019;90:727–36.ArticlePubMedPDF
- 11. Liu J, Fu J, Jia Y, Yang N, Li J, Wang G. Serum metabolomic patterns in patients with autoimmune thyroid disease. Endocr Pract 2020;26:82–96.ArticlePubMed
- 12. Xia Q, Qian W, Chen L, Chen X, Xie R, Zhang D, et al. Comprehensive metabolomics study in children with Graves’ disease. Front Endocrinol (Lausanne) 2021;12:752496.ArticlePubMedPMC
- 13. Timmerman KL, Volpi E. Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care 2008;11:45–9.ArticlePubMedPMC
- 14. Setoyama D, Lee HY, Moon JS, Tian J, Kang YE, Lee JH, et al. Immunometabolic signatures predict recovery from thyrotoxic myopathy in patients with Graves’ disease. J Cachexia Sarcopenia Muscle 2022;13:355–67.ArticlePubMedPMCPDF
- 15. Srivastava S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites 2019;9:301.ArticlePubMedPMC
- 16. Mariash CN. Thyroid hormone and the adipocyte. J Clin Endocrinol Metab 2003;88:5603–4.ArticlePubMed
- 17. Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol 2018;14:259–69.ArticlePubMedPMCPDF
- 18. Mendelson SD. Metabolic syndrome and psychiatric illness: interactions, pathophysiology, assessment and treatment; Boston: Elsevier/Academic Press; 2008.
- 19. Jurand J, Oliver MF. Effect of thyroid activity on fatty acid composition of serum lipids. Atherosclerosis 1970;11:125–40.ArticlePubMed
- 20. Zhou G, Xu Y, Zhai Y, Gong Z, Xu K, Wang G, et al. The association between serum palmitic acid and thyroid function. Front Endocrinol (Lausanne) 2022;13:860634.ArticlePubMedPMC
- 21. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 2012;97:1554–62.ArticlePubMed
- 22. Chen X, Zheng X, Ding Z, Su Y, Wang S, Cui B, et al. Relationship of gender and age on thyroid hormone parameters in a large Chinese population. Arch Endocrinol Metab 2020;64:52–8.ArticlePubMed
- 23. Hoogendoorn EH, Hermus AR, de Vegt F, Ross HA, Verbeek AL, Kiemeney LA, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem 2006;52:104–11.ArticlePubMedPDF
- 24. Nelson DL, Cox MM. Principles of biochemistry; 4th ed. New York: Freeman; 2004.
- 25. Ntountoumi C, Vlastaridis P, Mossialos D, Stathopoulos C, Iliopoulos I, Promponas V, et al. Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved. Nucleic Acids Res 2019;47:9998–10009.ArticlePubMedPMCPDF
- 26. Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, et al. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J 2015;14:67.ArticlePubMedPMCPDF
- 27. Holecek M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 2018;15:33.PubMedPMC
- 28. Krishnamurthy HK, Reddy S, Jayaraman V, Krishna K, Song Q, Rajasekaran KE, et al. Effect of micronutrients on thyroid parameters. J Thyroid Res 2021;2021:1865483.ArticlePubMedPMCPDF
- 29. Louard RJ, Barrett EJ, Gelfand RA. Overnight branchedchain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 1995;44:424–9.ArticlePubMed
- 30. Sun L, Goh HJ, Verma S, Govindharajulu P, Sadananthan SA, Michael N, et al. Brown adipose tissues mediate the metabolism of branched chain amino acids during the transitioning from hyperthyroidism to euthyroidism (TRIBUTE). Sci Rep 2022;12:3693.ArticlePubMedPMCPDF
- 31. Mannisto PT, Mattila J, Tuominen RK, Vesalainen S. Effects of some putative amino acid neurotransmitters on the stimulated TSH secretion in male rats. Horm Res 1983;17:19–26.ArticlePubMed
References
Figure & Data
References
Citations

- Associations of serum keratin 1 with thyroid function and immunity in Graves’ disease
Chao-Wen Cheng, Wen-Fang Fang, Jiunn-Diann Lin, Appuwawadu Mestri Nipun Lakshitha de Silva
PLOS ONE.2023; 18(11): e0289345. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite