Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(1); 2023 > Article
-
Review ArticleCalcium & bone metabolism Cardiovascular Impact of Calcium and Vitamin D Supplements: A Narrative Review
 Keypoint
Keypoint
Calcium and vitamin D are crucial for skeletal health, and supplements are used to prevent fractures in the elderly. Trials have shown inconsistent results for their effectiveness and cardiovascular safety due to heterogeneity in design, prior calcium intake, or the nature of trial outcomes. Current guidelines recommend up to 1,200 mg of calcium intake daily, preferably from the diet. Vitamin D supplementation recommendations vary, but well-conducted trials have shown it to be safe, without cardiovascular benefits or harms. This review provides an overview of important trials and systematic reviews, emphasizing the effectiveness and cardiovascular safety of calcium and vitamin D supplementation. -
Fatima Zarzour1
 , Ahmad Didi1, Mohammed Almohaya2, David Kendler1
, Ahmad Didi1, Mohammed Almohaya2, David Kendler1
-
Endocrinology and Metabolism 2023;38(1):56-68.
DOI: https://doi.org/10.3803/EnM.2022.1644
Published online: February 16, 2023
1Department of Medicine, University of British Columbia, Vancouver, BC, Canada
2King Fahad Medical City, Riyadh, Saudi Arabia
- Corresponding author: David Kendler Department of Medicine, University of British Columbia, 150-943 West Broadway, Vancouver, BC V5Z 4E1, Canada Tel: +1-604-263-3661, Fax: +1-604-263-3744 E-mail: davidkendler@gmail.com
• Received: December 16, 2022 • Revised: January 15, 2023 • Accepted: January 20, 2023
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- SKELETAL AND EXTRASKELETAL EFFECTS OF CALCIUM AND VITAMIN D
- EFFECT OF CALCIUM/VITAMIN D SUPPLEMENTATION ON THE CARDIOVASCULAR SYSTEM
- EVIDENCE FOR THE CARDIOVASCULAR EFFECTS OF CALCIUM WITH OR WITHOUT VITAMIN D SUPPLEMENTATION
- EFFECT OF VITAMIN D ON CARDIOVASCULAR ENDPOINTS
- CALCIUM AND VITAMIN D SUPPLEMENTATION RECOMMENDATIONS FROM GUIDELINES
- CONCLUSIONS
- Article information
- References
ABSTRACT
- Calcium and vitamin D play an important role in mineral homeostasis and the maintenance of skeletal health. Calcium and vitamin D supplements have been widely used for fracture prevention in elderly populations. Many trials have studied the effectiveness and cardiovascular safety of calcium and vitamin D supplementation, with disparate results. In this review, we summarize the most important trials and systematic reviews. There is significant heterogeneity in clinical trial design, differences in the nature of trial outcomes (self-reported vs. verified), prior calcium intake, and trial size. Inconsistent results have been reported concerning the effects of calcium and vitamin D supplementation on cardiovascular outcomes. Most current guidelines recommend calcium intake of up to 1,200 mg daily, preferably from the diet, without concern for cardiovascular risk. Recommendations regarding vitamin D supplementation vary widely. There is compelling evidence from well-conducted randomized trials that modest vitamin D supplementation is safe but does not confer cardiovascular benefit or cardiovascular harm.
- Calcium is required for bone mineralization as well as a wide variety of physiologic processes. The skeleton is both essential for muscle attachment, enabling ambulation, and as a source of calcium to maintain homeostasis at times of low enteral calcium availability. The plasma concentration of calcium is maintained by the interplay among intestinal absorption, renal calcium tubular reabsorption, and endocrine factors, primarily parathyroid hormone (PTH) and vitamin D metabolites. Besides its importance for skeletal health, calcium plays roles in cell division, muscle and neurologic function, blood coagulation, exocytosis, and metabolic regulation [1]. Vitamin D metabolites increase the intestinal absorption of calcium, and vitamin D deficiency is associated with decreased calcium absorption with increases in PTH and secondary hyperparathyroidism. Increased PTH increases calcium absorption by stimulating the conversion of 25-hydroxyvitamin D (25-OHD) to 1,25-dihydroxyvitamin D, which in turn acts as a hormone, stimulating intestinal calcium absorption [2]. In addition, increased PTH directly stimulates osteoclastic bone resorption through the receptor activator of NF-κB (RANK) ligand pathway, drawing in calcium from bone and leading to bone loss [2]. Vitamin D is also required for bone mineralization; severe vitamin D deficiency results in osteomalacia, a condition where osteoid remains unmineralized. With age, multiple physiological changes occur and can contribute to calcium and vitamin D insufficiency. Elderly people often consume inadequate calcium, have reduced calcium absorption, and have decreased skin vitamin D production and decreased vitamin D activation in the kidney [3]. Because of these metabolic changes, the elderly may require calcium and/or vitamin D supplementation. However, excessive supplemental calcium and vitamin D intake sometimes occurs in an attempt to maintain skeletal integrity or reverse osteoporosis. Excessive calcium supplementation may lead to bloating, constipation, and an increased risk of nephrolithiasis [4].
- There has been controversy in the literature over the past 8 years regarding the cardiovascular safety of calcium supplements, and extensive data have been published in attempts to determine whether this risk exists. This narrative review summarizes the most important randomized controlled trials (RCTs) and systematic reviews that address the cardiovascular safety of calcium and vitamin D supplementation in elderly individuals.
INTRODUCTION
- A recent network meta-analysis demonstrated that a combination of calcium and vitamin D was associated with a 19% relative risk (RR) reduction for hip fractures compared to placebo (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.71 to 0.93); there was no effect on vertebral fractures [5]. Monotherapy with calcium or vitamin D alone was not associated with fracture risk reduction [5]. In one of the earliest controlled trials of calcium and vitamin D, Chapuy et al. [6] in 1992 studied over 3,000 elderly residents in an ambulatory setting in southern France. Participants were randomized to supplementation with 1,200 mg of elemental calcium and 800 IU of vitamin D daily or placebo [6]. The combination of calcium and vitamin D was associated with a 43% reduction in hip fractures compared to placebo. At baseline, the participants were vitamin D-insufficient (serum 25-OHD: 16±11 ng/mL in the intervention group and 13±9 ng/mL in the control) [6]. The Decalyos II study demonstrated the effectiveness of calcium and vitamin D supplementation (1,200 mg of calcium and 800 IU of vitamin D) in vitamin D-deficient subjects in reversing secondary hyperparathyroidism as early as 6 months after initiating supplements [7]. There was no statistically significant difference in the probability of hip and non-vertebral fractures between the placebo arm and the calcium and vitamin D arm [7]. Since calcium and vitamin D are threshold nutrients, calcium supplementation is likely to be most beneficial in individuals with insufficient calcium intake. Individuals with adequate dietary calcium intake are unlikely to benefit from additional supplemental calcium. No additional bone health benefits were observed with vitamin D supplementation in vitamin D-replete community-dwelling individuals. However, it is prudent to recommend vitamin D supplementation in institutionalized and frail older individuals and in patients on osteoporosis treatment, since there is likely a synergy between calcium and vitamin D supplementation and the therapeutic effects of osteoporosis medication. Several randomized trials have shown that vitamin D supplementation leads to a signification reduction in the risk of falls in vitamin D-deficient patients [8-10]. A positive effect of vitamin D supplementation on autoimmune diseases (rheumatoid arthritis [RA], systemic lupus erythematosus [SLE], and multiple sclerosis [MS]) has been observed in animal models [11]. However, in human studies, vitamin D supplementation was associated with a lower risk of developing MS, but not SLE or RA [12]. A RCT by Lappe et al. [13] showed that calcium and vitamin D supplementation did not lead to a decreased risk of cancer in elderly vitamin D-replete women.
- On the other hand, excessive calcium supplementation can be associated with a high-risk of nephrolithiasis. The Women’s Health Initiative (WHI) trial showed that a total calcium intake of 2,100 mg per day was associated with a 17% increased risk of kidney stones [14]. Hypercalciuria in this setting depends not only on total calcium intake, but also on the timing of calcium relative to meals, as well as sodium and oxalate intake. It is well established that calcium taken with meals acts as a chelator for oxalate, which interferes with its absorption and urinary excretion [15]. Thus, calcium restriction is not recommended for the prevention of calcium stone formation [15]. The timing of calcium supplementation relative to meals was studied in 32 healthy males. This study showed that calcium carbonate supplementation (either 1 g three times daily with meals or 3 g at bedtime) led to an increase in calciuria compared to baseline, but only calcium taken with meals was associated with a decline of oxalate excretion compared to baseline [16].
SKELETAL AND EXTRASKELETAL EFFECTS OF CALCIUM AND VITAMIN D
- Effect of calcium supplementation on the cardiovascular system
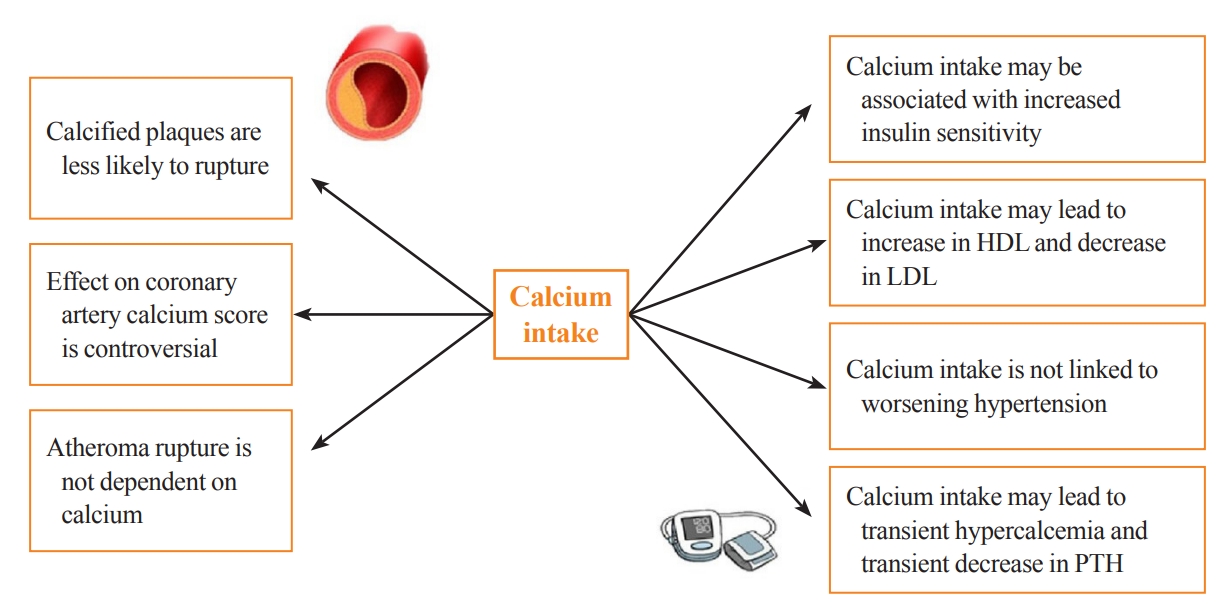
- Calcium supplementation is usually associated with a small increase in serum calcium and a consequent decrease in serum PTH, with both usually remaining in the normal range. This modest rise in serum calcium is considered unlikely to lead to extraskeletal calcification [17]. Clinical trials of PTH and its analogs in osteoporosis subjects have demonstrated mild transient increases in serum calcium, which do not appear to have detrimental cardiovascular effects [18]. That being said, some observational studies still question the link between transient hypercalcemia, endothelial dysfunction, atherosclerosis, and arterial stiffness [19]. These findings are in line with recent Mendelian randomization studies. These studies take a genetic epidemiological approach to estimate causality and have suggested a positive causal influence of transient increases in serum calcium on increased cardiovascular disease risk, especially for myocardial infarction (MI) [20-23]. However, this increase in cardiovascular disease risk was not observed with higher intake of dietary calcium [24]. Calcium supplementation could have favorable effects on lipids and blood pressure. An RCT examining the effect of calcium supplementation (1 g of calcium citrate) compared to placebo on lipid profiles in 223 postmenopausal females showed that calcium citrate supplementation was associated with a 7% increase in high-density lipoprotein (P=0.01) and a 6% decrease in low-density lipoprotein (P=0.09) at 1 year of follow-up [25]. Other data suggest that transient hypercalcemia and reduction in PTH in subjects taking calcium supplements may be associated with decreased blood pressure [17,26]. A systematic review of 42 trials examining the effect of calcium supplementation on blood pressure showed a reduction in systolic blood pressure by 1.44 mm Hg (95% CI, –2.20 to –0.68; P<0.001) and in diastolic blood pressure of 0.84 mm Hg (95% CI, –1.44 to –0.24; P<0.001) [26]. A study specifically investigating insulin sensitivity and calcium supplementation showed an association of calcium supplementation with increased insulin sensitivity [27]. This was also a finding in the multicenter Insulin Resistance Atherosclerosis Study, which examined the cross-sectional association between calcium intake (mean±standard deviation: 970 mg of elemental calcium±647 mg) and insulin resistance in 1,036 adults without diabetes [27]. Furthermore, plaque rupture in advanced cardiovascular disease is not thought to involve calcium [28]. In a post hoc analysis of nine randomized trials investigating the effect of calcium supplementation on calcium indices (CaI) and atheroma volume (AV), calcium supplementation was associated with an increase in the annualized CaI (odds ratio, 1.15; P=0.004) and no change in AV. Although the authors adjusted for multiple confounders, one of the limitations of this study is that the participants were asked about their “medication use” rather than their “supplement” use [29]. Manson et al. [30] investigated the effect of calcium supplementation (1,000 mg) and vitamin D (400 IU) on the coronary artery calcium (CAC) score in a substudy of the WHI trial. The CAC score was measured at baseline and after 7 years of study in 754 women aged between 50 and 59 years old. There was no significant difference in the CAC score between calcium and vitamin D-supplemented versus non-supplemented patients [30]. The Multi-Ethnic Study of Atherosclerosis was a 10-year observational study of calcium intake in elderly individuals. This study concluded that high total calcium intake was associated with a decreased risk of atherosclerosis, specifically if this was achieved from diet. However, after accounting for total calcium intake, calcium supplements were associated with incident CAC [24]. Interestingly, it has been shown that calcified carotid plaques are less likely to cause symptoms than non-calcified ones, suggesting that calcium can make plaques more stable and less likely to rupture [31]. The potential effects of calcium supplementation on parameters associated with cardiovascular risk are summarized in Fig. 1.
- Effect of vitamin D supplementation on cardiovascular health
- The cutoff to define vitamin D deficiency is controversial, with most guidelines considering a level less than 20 to 30 ng/mL as deficient [32-34]. Around one-third of the global population is affected by vitamin D deficiency when a cutoff of 20 ng/mL is used to define hypovitaminosis D [33]. Multiple conditions can lead to vitamin D deficiency, including low vitamin D intake, inadequate exposure to sun, use of sunscreens, obesity, aging, malabsorption syndromes (such as celiac disease, bariatric surgery, small intestine disorders) liver disease, chronic renal failure, hypoparathyroidism, antiseizure medications, and certain genetic diseases (defective 25-hydroxyvitamin D-1α hydroxylase) [35].
- A number of observational studies have reported that subjects with low serum 25-OHD have increased risks of cardiovascular diseases, including MI, congestive heart failure, stroke, peripheral vascular diseases, and mortality [36-41]. A meta-analysis of prospective studies with 6,123 cardiovascular events in 65,994 individuals showed a linear, inverse association between circulating 25-OHD in the range of 8 to 24 ng/mL and the risk of cardiovascular disease with a RR of 1.03 (95% CI, 1.00 to 1.06) per 10 ng/mL decrement in 25-OHD levels [42]. Such observational studies are limited by their inability to prove causality and by the presence of multiple confounding factors [43]. The proposed mechanisms for the cardiovascular effects of low vitamin D are activation of the renin angiotensin aldosterone system, hypercoagulability, increased arterial stiffness, and endothelial dysfunction [43,44].
- Mendelian randomization studies have not found a link between genetically determined differences in 25-OHD levels and cardiovascular endpoints [45-47]. In a recent very large Mendelian randomization study, there was an inverse relationship of vitamin D levels with all-cause mortality in people with 25-OHD levels <10 ng/mL; however, the associations with stroke and coronary heart disease were not statistically significant [48].
- Vitamin D receptors are ubiquitous, with expression in many tissues; this explains the pleiotropic effects of vitamin D. Vitamin D receptor-deficient mice have increased renin expression, increased thrombogenesis, and more hypertension and cardiac hypertrophy than controls [49,50]. Chronic kidney disease patients are at risk of vitamin D deficiency, mainly driven by the decline in the activation of 25-OHD to 1,25-dihydroxyvitamin D. Fibroblast growth factor 23 (FGF23) levels are high in chronic kidney disease patients, and it was shown that this elevated level is associated with atherosclerosis and ventricular remodeling [51].
EFFECT OF CALCIUM/VITAMIN D SUPPLEMENTATION ON THE CARDIOVASCULAR SYSTEM
- Evidence from RCTs and observational studies
- The controversies regarding cardiovascular safety of calcium and vitamin D replacement started with the WHI study. The study had a calcium/vitamin D arm where women with generally adequate calcium and vitamin D (including women taking supplements at baseline) were given additional calcium (1,000 mg) and vitamin D (400 IU) daily supplements or placebo. During 7 years of follow-up, neither MI/coronary heart disease, death, nor stroke showed differences between treatment groups (HR, 1.04; 95% CI, 0.92 to 1.18 and HR, 0.95; 95% CI, 0.82 to 1.10, respectively). Although this trial indicated a safe profile of calcium and vitamin D supplementation, it should be noted that the cardiovascular outcomes were prespecified as secondary outcomes (Table 1) [52]. The cardiovascular safety of calcium and vitamin D supplements was accepted until the work of Bolland et al. [53] in 2008 regarding a secondary analysis of an RCT where 1,471 postmenopausal New Zealand females, with a mean age of 74 years, were randomized to 1,000 mg of elemental calcium (without vitamin D) or placebo. Unlike the WHI trial, women taking calcium supplements at baseline were excluded. Although there was a statistically significant increase in the rate of self-reported MI in the calcium group compared to placebo (RR, 2.24; 95% CI, 1.20 to 4.17), the risk of angina, stroke, or the composite outcome (angina, chest pain, MI, or sudden death) was not significantly different between groups (Table 1). Moreover, an analysis of confirmed cardiovascular events attenuated the MI RR to 2.12 (95% CI, 1.01 to 4.47), and there was no significant difference in the composite endpoint of stroke, MI, and sudden death (RR, 1.47; 95% CI, 0.97 to 2.23) [53]. These study results have been challenged for a number of reasons. This was a secondary analysis of a study that was powered to detect bone density and fracture data, but not cardiovascular endpoints; there was a higher baseline risk for CV outcomes in patients assigned to the active calcium arm; and the results were inconsistent between the different cardiovascular outcomes [53]. These questions led Bolland et al. [54] to re-examine the WHI data according to baseline calcium and vitamin D supplementation. They hypothesized that calcium and vitamin D supplementation at baseline in the WHI study may have masked any potential cardiovascular risk of calcium supplementation [54]. Surprisingly, the data showed that in women not taking calcium supplements at baseline, the HR for cardiovascular events in subjects taking calcium and vitamin D supplements ranged from 1.13 to 1.22 (P=0.05 for clinical MI or stroke, P=0.04 for clinical MI or revascularization), while in women taking calcium supplements at baseline, the cardiovascular risk did not differ between treatment arms [54]. The gastrointestinal side effects of calcium supplementation can be mistaken by patients as symptoms of MI when this outcome is defined by patient self-reports; adjudication of cardiovascular events is therefore important [55]. To overcome this, Lewis et al. [56] conducted an RCT comparing calcium (1,200 mg daily) to placebo in 1,460 elderly women, with adjudicated cardiovascular endpoints. In that study, calcium supplementation was not associated with a higher risk of death or first-time hospitalization from atherosclerotic cardiovascular disease (HR,0.938; 95% CI, 0.690 to 1.275) (Table 1) [56]. In the long-term follow-up of the Randomized Evaluation of Calcium Or vitamin D (RECORD) trial, a subsequent randomized trial of calcium and vitamin D supplementation versus placebo, taking supplements was not associated with an increased risk of cardiac events (Table 1) [57]. More recent studies have also failed to demonstrate any increased cardiovascular risk in those taking calcium supplements. In 2013, Cauley et al. [58] reported the 5-year post-intervention outcomes of the WHI study. There were no significant differences in the risk of MI, stroke, and death between the calcium/vitamin D group and placebo. Interestingly, the same results were also seen when patients were examined according to their being on prior calcium at randomization or not [58]. Further safety data were reported from the UK biobank, a large prospective cohort including 475,255 participants (median age 58 years, 55.8% women). In a publication investigating the association between calcium and vitamin D supplementation and incident cardiovascular events including mortality, there was no association between calcium supplements and incident hospital admission for either ischemic heart disease or death [59]. In a large prospective cohort study of 132,823 participants followed for 17.5 years who consumed at baseline 1.5 to 1.7 dairy servings, higher all-cause mortality was observed in men who were taking ≥1,000 mg calcium supplements (RR, 1.17; 95% CI, 1.03 to 1.33) [60]. There was a dose-dependent inverse relationship between calcium supplementation and all-cause mortality in females for calcium intake of 0.1 to <500, 500–<1,000, and ≥1,000 mg/day, respectively (RR, 0.90; 95% CI, 0.87 to 0.94; RR, 0.84; 95% CI, 0.80 to 0.88; and RR, 0.93; 95% CI, 0.87 to 0.99). Interestingly, there was no association between dietary calcium and mortality in both males and females [60]. In a population-based, prospective cohort study of 34,468 patients with a median dietary calcium intake of 792 mg/day (interquartile range, 428 mg/day), calcium intake was not associated with higher cardiovascular death, MI, and stroke [61]. Several observational studies found that dietary calcium (total less than 1,500 mg) levels were not associated with cardiovascular disease endpoints [62,63]. An analysis of data from 35,983 women in the WHI study did not show a reduction in incident heart failure in the calcium and vitamin D group compared to placebo in the overall population (HR, 0.95; P=0.46) but a lower risk for heart failure in the low-risk group (patients without heart failure precursors at baseline, such as coronary artery disease, hypertension, and diabetes) (HR, 0.63, 95% CI, 0.46 to 0.87) [64]. Several other cohort studies reported conflicting results as to whether calcium supplementation can cause cardiovascular harm [65-73].
- Evidence from meta-analyses
- Given the heterogeneity in the results of studies of calcium supplementation, Bolland et al. [74] in 2010 published a meta-analysis of the effect of calcium supplementation on cardiovascular outcomes. The trial-level analysis showed that calcium supplementation was associated with a higher risk of MI (RR, 1.27; 95% CI, 1.01 to 1.59), but not with stroke, composite endpoints, or death. Similarly, the patient-level analysis showed that the risk of MI increased by 31% in the group taking calcium (HR, 1.31; 95% CI, 1.02 to 1.67), with no significant differences in other cardiovascular outcomes [74]. This meta-analysis had multiple limitations, including the fact that cardiovascular outcomes were secondary outcomes, there was some self-reporting of outcomes, and there were differences in the administered calcium supplements [74]. In order to incorporate the WHI patients without baseline calcium supplementation in the meta-analysis, Bolland et al. [54] conducted another systematic review, including three RCTs. The pooled analysis showed again that calcium and vitamin D supplementation was associated with an increased risk of MI (RR, 1.21; P=0.04), stroke (RR, 1.2; P= 0.05), and the composite of MI or stroke (RR, 1.16; P=0.02) [54]. These data are limited by the heterogeneity of the studies and cardiovascular outcomes reported, the selection bias by including only the WHI patients not on baseline supplementation, and the fact that cardiovascular endpoints were secondary outcomes [54]. In 2013, a systematic review of calcium and vitamin D supplementation by Mao et al. [75] showed that calcium and vitamin D supplementation was not associated with a statistically significant difference in major cardiovascular events. In 2015, Lewis et al. [76] published a systematic review of 18 RCTs comparing calcium and vitamin D supplementation versus placebo. They found no significant differences in coronary heart disease hospitalization or death in five trials between calcium with or without vitamin D and control with a pooled RR of 1.02 (95% CI, 0.96 to 1.09; P=0.51). Pooled data from 17 trials showed no difference in all-cause mortality, with an RR of 0.96 (95% CI, 0.91 to 1.02; P=0.18). There were also no significant differences in the individual outcomes of MI, angina, and chronic coronary heart disease. A sensitivity analysis excluding patients in the WHI trial with baseline intake of calcium and vitamin D also showed no cardiovascular associations [76]. In 2016, a systematic review by Chung et al. [77] included clinical trials, prospective cohort studies, and case-control studies. This review again did not show a significant difference in cardiovascular events or mortality between calcium and vitamin D supplementation and placebo, although cardiovascular disease endpoints were secondary outcomes in all included trials [77]. A more recent systematic review in 2020 pooled 89,251 community-dwelling participants from RCTs examining the effect of calcium and vitamin D supplements compared to placebo. There was no significant difference in all-cause mortality between the experimental groups (calcium alone, vitamin D alone, or combination) and the control group. A pooled analysis showed that calcium supplementation was not associated with risk of MI and major adverse cardiovascular events [78].
- Overall, there is no convincing evidence of cardiovascular harm from either dietary calcium or supplemental calcium (total up to 1,200 mg) and vitamin D.
EVIDENCE FOR THE CARDIOVASCULAR EFFECTS OF CALCIUM WITH OR WITHOUT VITAMIN D SUPPLEMENTATION
- Evidence from RCTs
- Historically, the effect of vitamin D on cardiovascular outcomes was controversial and based mainly on studies where the primary endpoints were related to bone health [43]. In the last few years, three large RCTs have helped to answer the question of the cardiovascular effects of vitamin D supplementation. The Vitamin D Assessment Study (ViDA) was a 3.3-year randomized placebo-controlled trial investigating the effect of a vitamin D loading dose of 200,000 IU, followed by 100,000 IU monthly or placebo in 5,110 participants aged 50 to 84 years. The subjects had a mean serum 25-OHD of 25.3±9.5 ng/mL at baseline, and were recruited mostly from family practices in Auckland, New Zealand, with the primary outcomes of incident cardiovascular disease and death. The study showed no significant difference between vitamin D and placebo for all cardiovascular endpoints, with a combined HR of 1.02 (95% CI, 0.87 to 1.20) (Table 2) [79]. However, vitamin D-deficient individuals on vitamin D supplements demonstrated lower systolic blood pressure than controls (–7.5 mm Hg; 95% CI, –14.4 to –0.6 mm Hg; P=0.03) [80].
- The VITamin D and OmegA-3 TriaL (VITAL) trial was an RCT randomizing subjects to vitamin D3 (2,000 IU daily) over 5.3 years with a primary endpoint of cardiovascular events. The trial enrolled 25,871 subjects including men over 50 years and women over 55 years. There was no significant difference in composite major cardiovascular events (MI, stroke, and cardiovascular death) (HR, 0.97; 95% CI, 0.85 to 1.12) (Table 2) [81]. The D-Health trial was a randomized placebo-controlled trial of 21,315 participants over 60 years in Australia comparing 60,000 IU of vitamin D3 monthly to placebo for 5 years. This study showed no significant difference between vitamin D supplementation and placebo in the primary outcome of all-cause mortality and the secondary outcome of cardiovascular mortality (HR, 1.04; 95% CI, 0.93 to 1.18) and 0.96 (95% CI, 0.72 to 1.28) respectively. Serum 25-OHD was not measured as the hypothesis was to examine the potential benefit of vitamin D supplementation in the unscreened population. It is worth mentioning that all-cause mortality was the primary outcome and cause specific mortality was not prespecified in the protocol (Table 2) [82].
- Evidence from meta-analyses
- In a recent meta-analysis of 21 RCTs (including both the ViDA and VITAL trials, but not the D-Health trial) with over 83,000 patients mean age 65.8 years, vitamin D supplementation was not associated with a reduced risk of major adverse cardiovascular events (RR, 1.00; 95% CI, 0.95 to 1.06; P=0.85), individual cardiovascular endpoints (MI, stroke, or cardiovascular disease mortality), or all-cause mortality compared to placebo [83]. The result of these RCTs and a systematic review consistently showed the safety of modest doses of vitamin D supplementation, but a lack of cardiovascular benefits.
EFFECT OF VITAMIN D ON CARDIOVASCULAR ENDPOINTS
- Various professional organizations have established calcium and vitamin D recommendations in their recent guidelines. The Bone Health and Osteoporosis Foundation (formerly the U.S. National Osteoporosis Foundation) recommends “a diet with adequate total calcium intake (1,000 mg/day for men aged 50 to 70 years; 1,200 mg/day for women ≥51 years and men ≥71 years), incorporating calcium supplements if intake is insufficient” [84]. The North American Menopause Society recommends that healthy postmenopausal females and those with osteoporosis should try to achieve a total daily calcium intake of 1,200 mg [85]. The Endocrine Society 2019 osteoporosis guidelines advise an intake of 1,000 to 1,200 mg of calcium from the diet if possible; supplements may be required in some individuals [14]. The American Association of Clinical Endocrinology 2020 osteoporosis guidelines recommend a total calcium intake of 1,200 mg daily including diet and supplements for women 50 years or older [86]. These recommendations are in line with the Institute of Medicine, which recommends 1,000 to 1,200 mg of calcium per day from diet and/or supplements [87]. In postmenopausal females and males over age 50 years at risk for fragility fractures or with osteoporosis, the National Osteoporosis Guidelines Group suggests a minimum intake of 700 mg of calcium, preferably from diet or otherwise from supplements. They recommend the consumption of vitamin D-rich food in addition to at least 800 IU of vitamin D supplements in patients with or at risk of vitamin D insufficiency. The same guidelines acknowledge that in order to achieve the target recommended intake, calcium and vitamin D supplementation is usually required in ambulatory and care facility patients [88]. It is recommended to screen for vitamin D deficiency and to guide vitamin D supplementation in high-risk patients (patients with chronic kidney disease, liver disease, malabsorption, osteoporosis, obesity, or pregnancy and patients on antiepileptic, antifungal, steroids, or cholestyramine) [32].
- Professional society guidelines are consistent in their recommendations regarding the need for calcium and vitamin D nutrition, especially in the elderly and those with osteoporosis [14,84-88]. Although dietary calcium is preferred, they recognize the potential need for supplemental calcium to achieve calcium intake of 1,000 to 1,200 mg per day. The guidelines vary in their vitamin D recommended intake, ranging from 600 to 2,000 IU per day by supplements.
CALCIUM AND VITAMIN D SUPPLEMENTATION RECOMMENDATIONS FROM GUIDELINES
- Although some randomized clinical trials and systematic reviews investigating the cardiovascular effects of calcium and vitamin D supplementation have suggested a risk of cardiovascular endpoints in patients on calcium supplements, the bulk of evidence does not support this. There is inconsistency across different cardiovascular endpoints, including mortality. Overall, there is a neutral effect of calcium and vitamin D supplementation on cardiovascular and mortality endpoints. Despite the importance of dietary calcium and vitamin D intake or supplementation to modest levels in order to maintain optimal bone health in elderly individuals, excessive calcium or vitamin D supplementation may have risks and has no additional benefit. Additional placebo-controlled clinical trials with primary cardiovascular endpoints involving calcium and vitamin D supplementation are unlikely due to ethical concerns. Therefore, with documented bone benefits and neutral cardiovascular concerns, modest calcium intake from the combination of diet and supplements, as well as supplemental vitamin D, should be recommended to our patients after middle age. The current guidelines largely support calcium intake of 1,000 to 1,200 mg, preferentially from diet, with supplements useful in patients unable to achieve adequate intake from dietary sources. Vitamin D supplementation from 800 to 2,000 IU daily would be prudent to maintain calcium homeostasis in older patients and especially those with osteoporosis or fracture risk. The current evidence does not support the use of calcium or vitamin D for the prevention of cardiovascular disease.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
Fig. 1.Potential effects of calcium supplementation on parameters associated with cardiovascular risk. HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTH, parathyroid hormone.
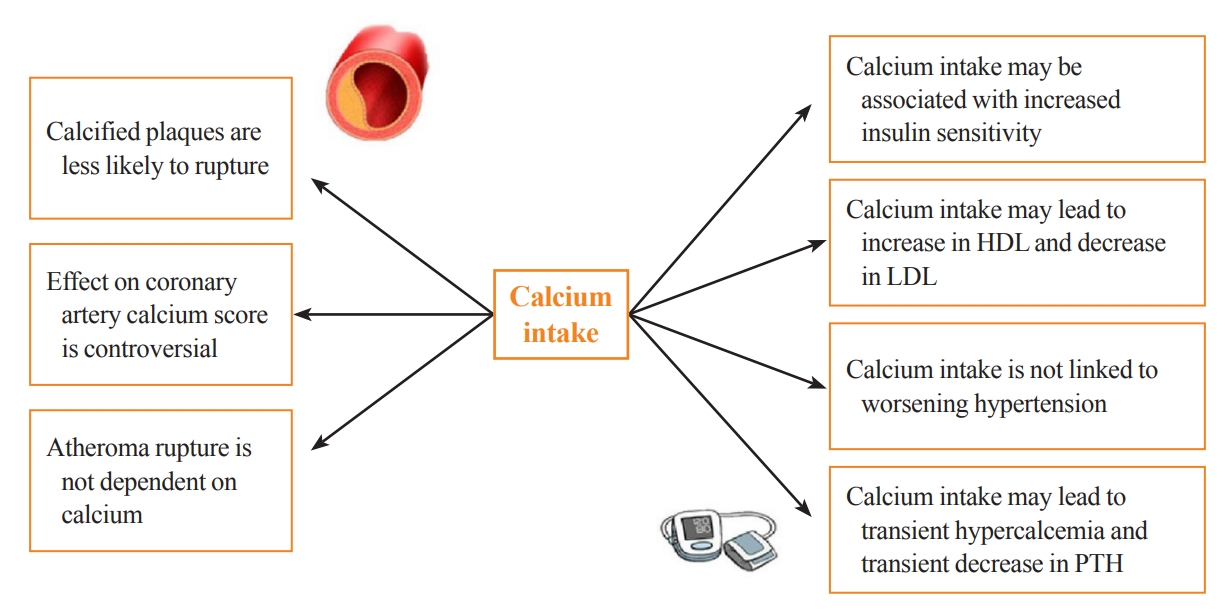

Table 1.Important Trials of Calcium +/– Vitamin D on Cardiovascular Outcomes
| Study | Population | Arms | Background calcium/VD | Duration | Outcome | Comments |
|---|---|---|---|---|---|---|
| Hsia et al. (2007) (WHI) [47] | n=36,282 | Calcium carbonate (500 mg)+vitamin D (200 IU twice daily) | Active arm (calcium: 1,148±654 mg/day; VD: 365±265 IU/day) | 7 years | Prespecified secondary outcomes | Secondary outcomes |
| Postmenopausal females | MI/coronary death: HR, 1.04; 95% CI, 0.92–1.18 | Background calcium/VD continued in the placebo arm | ||||
| Age 50 to 79 years | Placebo arm (calcium: 1,154±658 mg/day; VD: 368±266 IU/day) | |||||
| CV disease at baseline: Ca/VD (4.8%), placebo (4.7%) | Placebo | Stroke: HR, 0.95; 95% CI, 0.82–1.10 | Low VD supplementation | |||
| Bolland et al. (2008) [48] | n=1,471 | 1 g of elemental calcium as citrate | Active arm: 861±390 mg/day | 5 years | Secondary analysis | Secondary analysis |
| Postmenopausal women | MI: RR, 2.24; 95% CI, 1.2–4.17 | Slight difference in baseline CV risk factors | ||||
| Mean age 74 years | Placebo | Placebo: 853±381 mg/day | Angina, chest pain, MI, or sudden death: RR, 0.94; 95% CI, 0.72–1.24 | |||
| Angina: RR, 0.71; 95% CI, 0.50–1.01 | Relatively smaller number compared to WHI | |||||
| Stroke: RR, 1.44; 95% CI, 0.90–2.31 | ||||||
| Avenell et al. (2012) (RECORD trial) [53] | n=5,292 | VD: 800 IU | Calcium: ≤500 mg | 6 years | VD vs. placebo: vascular disease mortality (HR, 0.91; 95% CI, 0.79–1.05) | Prespecified secondary outcomes |
| Majority females | Calcium: 1,000 mg of elemental calcium (as carbonate) | Vitamin D: <200 IU (5 μg) | Poor compliance | |||
| Mean age 77 years | Calcium+VD | Calcium vs. placebo: vascular disease mortality (HR, 1.07; 95% CI, 0.92–1.24) | ||||
| Placebo | ||||||
| Lewis et al. (2011) [56] | n=1,460 | Calcium carbonate (1,200 mg) | Calcium (calcium arm: 961±356 mg; placebo arm: 970±352 mg) | 5 years | Hospitalization or death from ASCVD: HR, 0.938; 95% CI, 0.690–1.275 | Verified events |
| Female | Prespecified outcomes | |||||
| Mean age | Placebo | |||||
| 75.1±2.7 years |
Table 2.Important Trials of Vitamin D Supplementation on Cardiovascular Outcomes
| Study | Population | Intervention vs . control | Baseline 25-OHD level | Duration | Outcome | Comments |
|---|---|---|---|---|---|---|
| Scragg et al. (2017) (ViDA trial) [79] | New Zealand | 200,000 IU of vitamin D3 followed a month later by monthly doses of 100,000 IU | 25.3±9.5 ng/mL | 3.3 years | Incident CVD and death: HR, 1.02; 95% CI, 0.87–1.2 | Primary outcome |
| n=5,110 | High retention rate (87%) | |||||
| Mean age 65.9 years | High adherence (84%) | |||||
| Female and male | Placebo | Short duration of followup, low event rate | ||||
| Manson et al. (2019) (VITAL trial) [81] | United States | 2,000 IU daily of cholecalciferol | 30.8±10 ng/mL | 5.3 years | Composite outcome of major cardiovascular events (MI, stroke, and cardiovascular death): HR, 0.97; 95% CI, 0.85–1.12 | Primary outcome |
| n=25,871 | Large sample size | |||||
| Female (≥55 years) | Placebo | Multiethnic | ||||
| Male (≥50 years) | High rate of adherence | |||||
| High rate of follow-up | ||||||
| Neale et al. (2022) (D-health Trial) [82] | Australian | 60,000 IU monthly of cholecalciferol | Not measured | 5 years | All-cause mortality: HR, 1.04; 95% CI, 0.93–1.18 | All-cause mortality was the primary outcome |
| n=21,315 | Predicted level (ng/mL): | |||||
| ≥60 years | Placebo | Intervention arm: ≥20 (76%) | Cardiovascular mortality: HR, 0.96; 95% CI, 0.72–1.28 | Cause specific mortality was not prespecified in the protocol | ||
| Men and women | Placebo arm: ≥20 (75.2%) |
- 1. Bilezikian JP. The parathyroids; 2nd ed. San Diego: Academic Press; 2001. Chapter 10, Physiology of calcium homeostasis. p. 167–81.
- 2. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501.ArticlePubMed
- 3. Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am 2013;42:319–32.ArticlePubMedPMC
- 4. Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83.PubMed
- 5. Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 2019;104:1623–30.ArticlePubMedPDF
- 6. Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637–42.ArticlePubMed
- 7. Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 2002;13:257–64.ArticlePubMedPDF
- 8. Cangussu LM, Nahas-Neto J, Orsatti CL, Poloni PF, Schmitt EB, Almeida-Filho B, et al. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: a randomized, double-blind, placebo-controlled trial. Menopause 2016;23:267–74.PubMed
- 9. Ozsoy-Unubol T, Candan Z, Atar E, Ok NF, Ata E, Kilac H, et al. The effect of vitamin D and exercise on balance and fall risk in postmenopausal women: a randomised controlled study. Int J Clin Pract 2021;75:e14851.PubMed
- 10. Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med 2008;168:103–8.ArticlePubMed
- 11. Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 2004;80(6 Suppl):1717S–20.ArticlePubMed
- 12. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum 2004;50:72–7.ArticlePubMed
- 13. Lappe J, Watson P, Travers-Gustafson D, Recker R, Garland C, Gorham E, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 2017;317:1234–43.ArticlePubMed
- 14. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2019;104:1595–622.ArticlePubMedPDF
- 15. Bargagli M, Ferraro PM, Vittori M, Lombardi G, Gambaro G, Somani B. Calcium and vitamin D supplementation and their association with kidney stone disease: a narrative review. Nutrients 2021;13:4363.ArticlePubMedPMC
- 16. Domrongkitchaiporn S, Sopassathit W, Stitchantrakul W, Prapaipanich S, Ingsathit A, Rajatanavin R. Schedule of taking calcium supplement and the risk of nephrolithiasis. Kidney Int 2004;65:1835–41.ArticlePubMed
- 17. Heaney RP, Kopecky S, Maki KC, Hathcock J, Mackay D, Wallace TC. A review of calcium supplements and cardiovascular disease risk. Adv Nutr 2012;3:763–71.ArticlePubMedPMC
- 18. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41.ArticlePubMed
- 19. Michos ED, Cainzos-Achirica M, Heravi AS, Appel LJ. Vitamin D, calcium supplements, and implications for cardiovascular health: JACC Focus Seminar. J Am Coll Cardiol 2021;77:437–49.PubMed
- 20. Chen Y, Forgetta V, Richards JB, Zhou S. Health effects of calcium: evidence from Mendelian Randomization Studies. JBMR Plus 2021;5:e10542.ArticlePubMedPMCPDF
- 21. Xu L, Lin SL, Schooling CM. A Mendelian randomization study of the effect of calcium on coronary artery disease, myocardial infarction and their risk factors. Sci Rep 2017;7:42691.ArticlePubMedPMCPDF
- 22. Yuan S, Yu L, Gou W, Wang L, Sun J, Li D, et al. Health effects of high serum calcium levels: updated phenome-wide Mendelian randomisation investigation and review of Mendelian randomisation studies. EBioMedicine 2022;76:103865.ArticlePubMedPMC
- 23. Larsson SC, Burgess S, Michaelsson K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA 2017;318:371–80.ArticlePubMedPMC
- 24. Anderson JJ, Kruszka B, Delaney JA, He K, Burke GL, Alonso A, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the MultiEthnic Study of Atherosclerosis (MESA). J Am Heart Assoc 2016;5:e003815.ArticlePubMedPMC
- 25. Reid IR, Mason B, Horne A, Ames R, Clearwater J, Bava U, et al. Effects of calcium supplementation on serum lipid concentrations in normal older women: a randomized controlled trial. Am J Med 2002;112:343–7.PubMed
- 26. Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated meta-analysis of randomized controlled trials. Am J Hypertens 1999;12(1 Pt 1):84–92.ArticlePubMed
- 27. Ma B, Lawson AB, Liese AD, Bell RA, Mayer-Davis EJ. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: approaches to modeling a dose-dependent association. Am J Epidemiol 2006;164:449–58.ArticlePubMed
- 28. Schuijf JD, van der Wall EE, Bax JJ. Lesions without calcium: lessons from CT angiography. Heart 2009;95:1038–40.ArticlePubMed
- 29. Bazarbashi N, Kapadia SR, Nicholls SJ, Carlo J, Gad MM, Kaur M, et al. Oral calcium supplements associate with serial coronary calcification: insights from intravascular ultrasound. JACC Cardiovasc Imaging 2021;14:259–68.ArticlePubMed
- 30. Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause 2010;17:683–91.ArticlePubMedPMC
- 31. Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am J Roentgenol 2005;184:295–8.ArticlePubMedPMC
- 32. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30.ArticlePubMed
- 33. Giustina A, Bouillon R, Binkley N, Sempos C, Adler RA, Bollerslev J, et al. Controversies in vitamin D: a statement from the Third International Conference. JBMR Plus 2020;4:e10417.ArticlePubMedPMCPDF
- 34. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8.ArticlePubMed
- 35. Minisola S, Colangelo L, Pepe J, Diacinti D, Cipriani C, Rao SD. Osteomalacia and vitamin D status: a clinical update 2020. JBMR Plus 2020;5:e10447.ArticlePubMedPMCPDF
- 36. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174–80.ArticlePubMedPMC
- 37. Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, et al. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail 2015;3:347–56.PubMedPMC
- 38. Schneider AL, Lutsey PL, Selvin E, Mosley TH, Sharrett AR, Carson KA, et al. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol 2015;22:1220–7.ArticlePubMedPMC
- 39. Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 2008;28:1179–85.PubMedPMC
- 40. Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–37.ArticlePubMedPMC
- 41. Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutrition 2012;28:367–71.ArticlePubMedPMC
- 42. Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–29.PubMedPMC
- 43. Heravi AS, Michos ED. Vitamin D and calcium supplements: helpful, harmful, or neutral for cardiovascular risk? Methodist Debakey Cardiovasc J 2019;15:207–13.ArticlePubMedPMC
- 44. Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol 2013;9:337–47.ArticlePubMedPDF
- 45. Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet 2016;9:349–56.ArticlePubMed
- 46. Brondum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol 2015;44:651–61.ArticlePubMed
- 47. Huang T, Afzal S, Yu C, Guo Y, Bian Z, Yang L, et al. Vitamin D and cause-specific vascular disease and mortality: a Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med 2019;17:160.ArticlePubMedPMCPDF
- 48. Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol 2021;9:837–46.PubMedPMC
- 49. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–76.ArticlePubMedPMCPDF
- 50. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40:1109–51.ArticlePubMedPMC
- 51. Russo D, Battaglia Y. Clinical significance of FGF-23 in patients with CKD. Int J Nephrol 2011;2011:364890.ArticlePubMedPMCPDF
- 52. Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–54.ArticlePubMed
- 53. Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008;336:262–6.ArticlePubMedPMC
- 54. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040.ArticlePubMedPMC
- 55. Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J Bone Miner Res 2012;27:719–22.ArticlePubMed
- 56. Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 2011;26:35–41.ArticlePubMed
- 57. Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 2012;97:614–22.ArticlePubMed
- 58. Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt) 2013;22:915–29.ArticlePubMedPMC
- 59. Harvey NC, D’Angelo S, Paccou J, Curtis EM, Edwards M, Raisi-Estabragh Z, et al. Calcium and vitamin D supplementation are not associated with risk of incident ischemic cardiac events or death: findings from the UK Biobank Cohort. J Bone Miner Res 2018;33:803–11.ArticlePubMedPMCPDF
- 60. Yang B, Campbell PT, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, et al. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr 2016;103:886–94.ArticlePubMed
- 61. Khan B, Nowson CA, Daly RM, English DR, Hodge AM, Giles GG, et al. Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: a prospective cohort study of older men and women. J Bone Miner Res 2015;30:1758–66.ArticlePubMedPDF
- 62. Asemi Z, Saneei P, Sabihi SS, Feizi A, Esmaillzadeh A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2015;25:623–34.ArticlePubMed
- 63. Yang C, Shi X, Xia H, Yang X, Liu H, Pan D, et al. The evidence and controversy between dietary calcium intake and calcium supplementation and the risk of cardiovascular disease: a systematic review and meta-analysis of cohort studies and randomized controlled trials. J Am Coll Nutr 2020;39:352–70.ArticlePubMed
- 64. Donneyong MM, Hornung CA, Taylor KC, Baumgartner RN, Myers JA, Eaton CB, et al. Risk of heart failure among postmenopausal women: a secondary analysis of the randomized trial of vitamin D plus calcium of the women’s health initiative. Circ Heart Fail 2015;8:49–56.PubMed
- 65. Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol 2011;174:35–43.ArticlePubMed
- 66. Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA Intern Med 2013;173:639–46.ArticlePubMedPMC
- 67. Michaelsson K, Melhus H, Warensjp Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ 2013;346:f228.ArticlePubMedPMC
- 68. Al-Delaimy WK, Rimm E, Willett WC, Stampfer MJ, Hu FB. A prospective study of calcium intake from diet and supplements and risk of ischemic heart disease among men. Am J Clin Nutr 2003;77:814–8.ArticlePubMed
- 69. Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998;98:1198–204.ArticlePubMed
- 70. Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999;30:1772–9.ArticlePubMed
- 71. Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol 2010;171:801–7.ArticlePubMed
- 72. Larsson SC, Virtanen MJ, Mars M, Mannisto S, Pietinen P, Albanes D, et al. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med 2008;168:459–65.ArticlePubMed
- 73. Sluijs I, Czernichow S, Beulens JW, Boer JM, van der Schouw YT, Verschuren WM, et al. Intakes of potassium, magnesium, and calcium and risk of stroke. Stroke 2014;45:1148–50.ArticlePubMed
- 74. Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010;341:c3691.ArticlePubMedPMC
- 75. Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013;169:106–11.ArticlePubMed
- 76. Lewis JR, Radavelli-Bagatini S, Rejnmark L, Chen JS, Simpson JM, Lappe JM, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015;30:165–75.ArticlePubMedPDF
- 77. Chung M, Tang AM, Fu Z, Wang DD, Newberry SJ. Calcium intake and cardiovascular disease risk: an updated systematic review and meta-analysis. Ann Intern Med 2016;165:856–66.ArticlePubMed
- 78. Zhang Y, Li Y, Liu J, Wei X, Tan N, Zhang J, et al. Association of vitamin D or calcium supplementation with cardiovascular outcomes and mortality: a meta-analysis with trial sequential analysis. J Nutr Health Aging 2021;25:263–70.ArticlePubMedPDF
- 79. Scragg R, Stewart AW, Waayer D, Lawes CM, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol 2017;2:608–16.ArticlePubMedPMC
- 80. Sluyter JD, Camargo CA Jr, Stewart AW, Waayer D, Lawes CM, Toop L, et al. Effect of monthly, high-dose, long-term vitamin d supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc 2017;6:e006802.ArticlePubMedPMC
- 81. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44.ArticlePubMedPMC
- 82. Neale RE, Baxter C, Romero BD, McLeod DS, English DR, Armstrong BK, et al. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol 2022;10:120–8.ArticlePubMed
- 83. Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. Vitamin D supplementation and cardiovascular disease risks in more than 83 000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol 2019;4:765–76.ArticlePubMedPMC
- 84. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2022;33:2049–102.ArticlePubMedPMCPDF
- 85. Management of Osteoporosis in Postmenopausal Women: The 2021 Position Statement of The North American Menopause Society’’ Editorial Panel. Management of osteoporosis in postmenopausal women: the 2021 position statement of the North American Menopause Society. Menopause 2021;28:973–97.ArticlePubMed
- 86. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis: 2020 update. Endocr Pract 2020;26(Suppl 1):1–46.Article
- 87. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Obstet Gynecol Surv 2011;66:356–7.Article
- 88. National Osteoporosis Guideline Group UK. Clinical guideline for the prevention and treatment of osteoporosis [Internet]. NOGG; 2021 [cited 2023 Feb 1]. Available from: https://www.nogg.org.uk/full-guideline.
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Evaluating adherence, tolerability and safety of oral calcium citrate in elderly osteopenic subjects: a real-life non-interventional, prospective, multicenter study
Mariangela Rondanelli, Salvatore Minisola, Marco Barale, Daniele Barbaro, Francesca Mansueto, Santina Battaglia, Gloria Bonaccorsi, Santina Caliri, Alessandro Cavioni, Luciano Colangelo, Sabrina Corbetta, Federica Coretti, Giorgia Dito, Valentina Gavioli,
Aging Clinical and Experimental Research.2024;[Epub] CrossRef - Association between Daily Dietary Calcium Intake and the Risk of Cardiovascular Disease (CVD) in Postmenopausal Korean Women
Jae Kyung Lee, Thi Minh Chau Tran, Euna Choi, Jinkyung Baek, Hae-Rim Kim, Heeyon Kim, Bo Hyon Yun, Seok Kyo Seo
Nutrients.2024; 16(7): 1043. CrossRef - Effect of Denosumab on Bone Density in Postmenopausal Osteoporosis: A Comparison with and without Calcium Supplementation in Patients on Standard Diets in Korea
Chaiho Jeong, Jinyoung Kim, Jeongmin Lee, Yejee Lim, Dong-Jun Lim, Ki-Hyun Baek, Jeonghoon Ha
Journal of Clinical Medicine.2023; 12(21): 6904. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite