Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(1); 2023 > Article
-
Original ArticleThyroid Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
 Keypoint
Keypoint
This study compared the outcomes of thyroid cancer in patients detected by screening and clinical suspicion in Korea. Patients diagnosed based on clinical suspicion had larger tumors, more advanced stages, and higher mortality rates. Adjusted Cox regression showed higher risks of all-cause and thyroid cancer-specific mortality in patients diagnosed based on clinical suspicion. Thyroid-specific symptoms directly increased cancer-specific mortality and indirectly affected mortality through tumor size and advanced clinicopathologic status. The study suggests that early detection through screening may provide a survival advantage over symptomatic thyroid cancer diagnosis. -
Shinje Moon1
 , Eun Kyung Lee2
, Eun Kyung Lee2 , Hoonsung Choi3, Sue K. Park4,5, Young Joo Park6,7
, Hoonsung Choi3, Sue K. Park4,5, Young Joo Park6,7
-
Endocrinology and Metabolism 2023;38(1):81-92.
DOI: https://doi.org/10.3803/EnM.2023.1668
Published online: February 27, 2023
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
2Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
3Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
4Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
5Integrated Major in Innovative Medical Science, Seoul National University College of Medicine, Seoul, Korea
6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
7Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- Corresponding authors: Young Joo Park Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4183, Fax: +82-2-764-2199, E-mail: yjparkmd@snu.ac.kr
- Eun Kyung Lee Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea Tel: +82-31-920-1743, Fax: +82-31-920-2798, E-mail: eklee@ncc.re.kr
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The true benefit of thyroid cancer screening is incompletely understood. This study investigated the impact of ultrasound screening on thyroid cancer outcomes through a comparison with symptomatic thyroid cancer using data from a nationwide cohort study in Korea.
-
Methods
- Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortality. Considering the possible bias arising from age, sex, year of thyroid cancer registration, and confounding factors for mortality (including smoking/drinking status, diabetes, and hypertension), all analyses were conducted with stabilized inverse probability of treatment weighting (IPTW) according to the route of detection.
-
Results
- Of 5,796 patients with thyroid cancer, 4,145 were included and 1,651 were excluded due to insufficient data. In comparison with the screening group, the clinical suspicion group was associated with large tumors (17.2±14.6 mm vs. 10.4±7.9 mm), advanced T stage (3–4) (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.09 to 1.41), extrathyroidal extension (OR, 1.16; 95% CI, 1.02 to 1.32), and advanced stage (III–IV) (OR, 1.16; 95% CI, 1.00 to 1.35). In IPTW-adjusted Cox regression analysis, the clinical suspicion group had significantly higher risks of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29). Mediation analysis showed that the presence of thyroid-specific symptoms was directly associated with a higher risk of cancer-specific mortality. Thyroid-specific symptoms also indirectly affected thyroid cancer-specific mortality, mediated by tumor size and advanced clinicopathologic status.
-
Conclusion
- Our findings provide important evidence for the survival benefit of early detection of thyroid cancer compared to symptomatic thyroid cancer.
- The incidence of thyroid cancer has increased worldwide over the past 40 years [1]. Uniquely, the estimated age-standardized incidence of thyroid cancer is higher in high-income countries than in low-to middle-income countries [2]. This geographically unequal distribution of thyroid cancer incidence results from multiple causes, including diagnostic practices, health care systems, environmental exposures, and individual factors [3-5].
- A profound increase in the incidence of thyroid cancer in Korea has taken place. The age-standardized incidence rate of thyroid cancer in Korea peaked in 2012 (74.83 per 100,000), declined until 2015 (42.52 per 100,000), and only fluctuated slightly until 2018 (48.62 per 100,000) according to joinpoint regression analysis [6]. Interestingly, this recent increase in the incidence of thyroid cancer was not correlated with the number of thyroid fine-needle aspiration procedures [7]. Li et al. [8] suggested that between 2008 and 2012, 90% of cases of thyroid cancer in Korean women were due to overdiagnosis. Although thyroid cancer screening is not included in the national screening programs of Korea, high access to medical services may have contributed to the unique characteristics of thyroid cancer screening in Korea, with a high proportion of thyroid cancers diagnosed by screening tests.
- Several studies have suggested that thyroid cancers detected by screening were smaller and patients were younger at diagnosis than symptomatic thyroid nodules [9,10]. However, the impact of screening on mortality remained controversial [11]. In a systematic review of 18 observational studies, when comparing incidental and non-incidental thyroid nodules, the risks of thyroid cancer were similar in both groups (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.63 to 1.70), and incidentally detected thyroid cancer had better progression-free and overall survival [12]. The major limitation of these large-scale retrospective studies regarding the impact of screening was the difficulty in adjusting the innate imbalance between both groups in factors such as age, sex, and the size and histologic subtype of tumors [13].
- This study aimed to compare the mortality of thyroid cancer according to the detection route using a publicly accessible nationwide cohort database. The National Epidemiological Survey of Thyroid cancer (NEST) was constructed to investigate secular trends in the clinicopathological features of Korean thyroid cancer patients by a two-stage sampling method at three time points (1999, 2005, and 2008) [14]. In addition, to eliminate the potential bias arising from differences in age, sex, and the year of thyroid cancer registration, inverse probability of treatment weighting (IPTW), as the inverse of the propensity score, was used in all analyses.
INTRODUCTION
- Study design and participants
- The NEST study was a retrospective nationwide study containing data from patients with thyroid cancer in 1999, 2005, and 2008 extracted from the Korea National Cancer Incidence Database by December 2010. Twenty-four hospitals were finally selected, including at least one hospital in each of the 12 provinciallevel divisions in Korea. Thyroid cancer patients were randomly selected from local hospitals according to the proportion of registered thyroid cancer patients in each administrative district.
- A total of 6,846 patients with thyroid cancer were selected as the sample population. The number of thyroid cancer patients registered in the Korean National Cancer Incidence Database (KNCI DB) was 3,342 in 1999, 12,659 in 2005, and 26,890 in 2008, and different proportions of thyroid cancer patients were sampled for different years of study: 33% in 1999 (n=1,103 patients), 22% in 2005 (n=2,785 patients), and 11% in 2008 (n=2,958). Of 6,846 sampled patients with thyroid cancer, 5,796 patients (84.7%) were included in the NEST study after excluding 960 patients due to the refusal of two hospitals to participate and 90 patients due to missing or inadequate data. For this study, 4,145 patients were included in the study after excluding 1,651 patients due to insufficient data on the route of thyroid cancer detection and confounding factors associated with mortality, including smoking status, alcohol drinking status, diabetes mellitus, and hypertension.
- Baseline information and classification of the routes of thyroid cancer detection
- Clinical information, including age, sex, comorbidities, the route of diagnosis for thyroid cancer, histology, tumor, node, metastasis (TNM) stage (defined by the American Joint Committee on Cancer [AJCC] sixth edition) from the postoperative pathology, and treatment was collected through medical record review. Mortality data, including the date and cause of mortality, were extracted from the cause-of-death database of Statistics Korea by December 31, 2020 and linked to the NEST dataset. Causes of mortality were presented as International Classification of Diseases, 10th revision codes, and we defined thyroid cancer-specific mortality as occurring when the cause of mortality was coded as thyroid cancer (C73).
- The NEST study collected information about the detection route through electronic medical record review (screening vs. clinical suspicion). We divided subjects according to the route of thyroid cancer detection into the screening group (diagnosed during cancer screening or incidentally diagnosed during treatment for diseases other than thyroid disease) and the clinical suspicion group (diagnosed from an examination of symptoms related to thyroid disease, such as throat pain or a palpable mass).
- Statistical analysis
- Continuous variables are presented as means with standard deviation, with P values calculated according to the route of thyroid cancer detection using the t test. Categorical variables are presented as numbers (%) with P values calculated using the chi-square test. Considering the possible bias arising from age, sex, and year of thyroid cancer registration, we calculated the stabilized IPTW as the inverse of the propensity score with age, sex, year of thyroid cancer registration, smoking status, alcohol drinking status, diabetes, and hypertension according to the route of detection using R package “ipw” (R Foundation for Statistical Computing, Vienna, Austria). We revalidated the balance of covariates after weighting using standardized differences. A standardized difference of less than 0.1 was considered to indicate a well-balanced covariate.
- We compared the cumulative mortality rates between the screening group and clinical suspicion group using Kaplan-Meier plots with the log-rank test for IPTW-adjusted samples. Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortalities with and without IPTW.
- To investigate the direct and indirect effects of the route of thyroid cancer detection on thyroid cancer-specific mortality through the clinicopathologic status of thyroid cancer, we conducted a regression-based causal mediation analysis using the package “Regmedint” developed by Li et al. [15]. We calculated the total natural indirect effect, total natural direct effect, and total effect according to the presence of thyroid-related symptoms, adjusting for age, sex, year of thyroid cancer registration, smoking status, alcohol drinking status, diabetes, and hypertension.
- The statistical analysis was performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA) and R version 3.1.0 (R Foundation for Statistical Computing; www.r-project.org). P values <0.05 were considered statistically significant.
- Ethical considerations
- The research protocol for the NEST study was approved by the Institutional Review Board (IRB) of the National Cancer Center (no. NCC2017-0070). All study procedures followed the ethical standards outlined by the IRB of the National Cancer Center for human participants and were in line with the Declaration of Helsinki. Informed consent was not required because all data were fully anonymized before access. The NEST data are publicly opened and freely available.
METHODS
- Baseline characteristics according to the route of thyroid cancer detection
- Of 5,796 patients with thyroid cancer, 4,145 patients were included in the study after excluding 1,651 patients due to insufficient data on the route of thyroid cancer detection. The mean age of the patients was 46.8±12.4 years, and 84.6% of them were women. A total of 345 patients (8.3%) died during a median follow-up period of 170 months (interquartile range, 148 to 187). Of those, 84 deaths (2.0%) were due to thyroid cancer.
- Table 1 summarizes the baseline characteristics according to the route of thyroid cancer detection, which were included in IPTW matching. Although no significant differences in age were found according to the route of thyroid cancer detection, a significant difference in the proportions of sex and year of registration was found. After IPTW, patients’ baseline characteristics were well-balanced according to the route of thyroid cancer detection (all standardized differences <0.1). The clinicopathologic characteristics are summarized in Table 2. The tumor size was significantly larger in the clinical suspicion group than in the screening group. In addition, all-cause mortality and thyroid cancer-specific mortality were higher in the clinical suspicion group than in the screening group. After IPTW, the clinical suspicion group also showed a higher incidence of all-cause and thyroid cancer-specific mortality than the screening group (Table 2).
- Association between the route of thyroid cancer detection and clinicopathologic characteristics of thyroid cancer
- In comparison with the screening group, the clinical suspicion group was associated with large tumor size (Table 2) and advanced T stage (Table 3) in both unweighted and weighted analyses. Although the associations with the risk of lymph node and distant metastasis did not reach statistical significance, the clinical suspicion group showed a moderate association with extrathyroidal extension and advanced stage in the weighted analysis.
- Association between the route of thyroid cancer detection and thyroid cancer-specific mortality
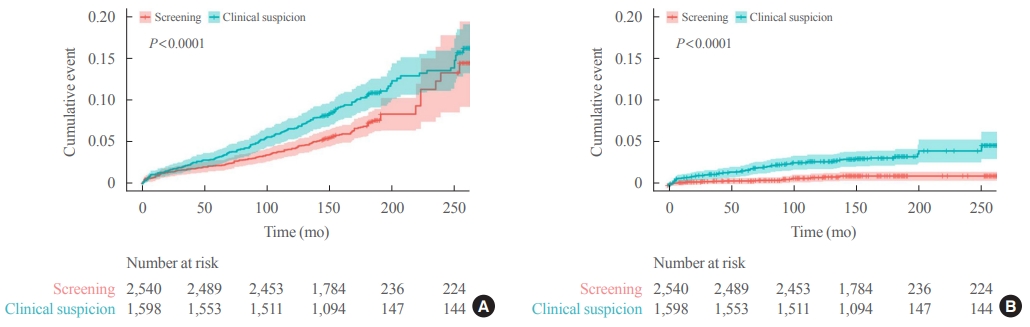
- Kaplan-Meier survival curves with IPTW-adjusted data revealed significantly higher rates of all-cause and thyroid cancer-specific mortality in the clinical suspicion group than in the screening group (log-rank test, P<0.001) (Fig. 1). In Cox regression analysis adjusted with IPTW, the clinical suspicion group had a significantly higher risk of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29) (Table 4).
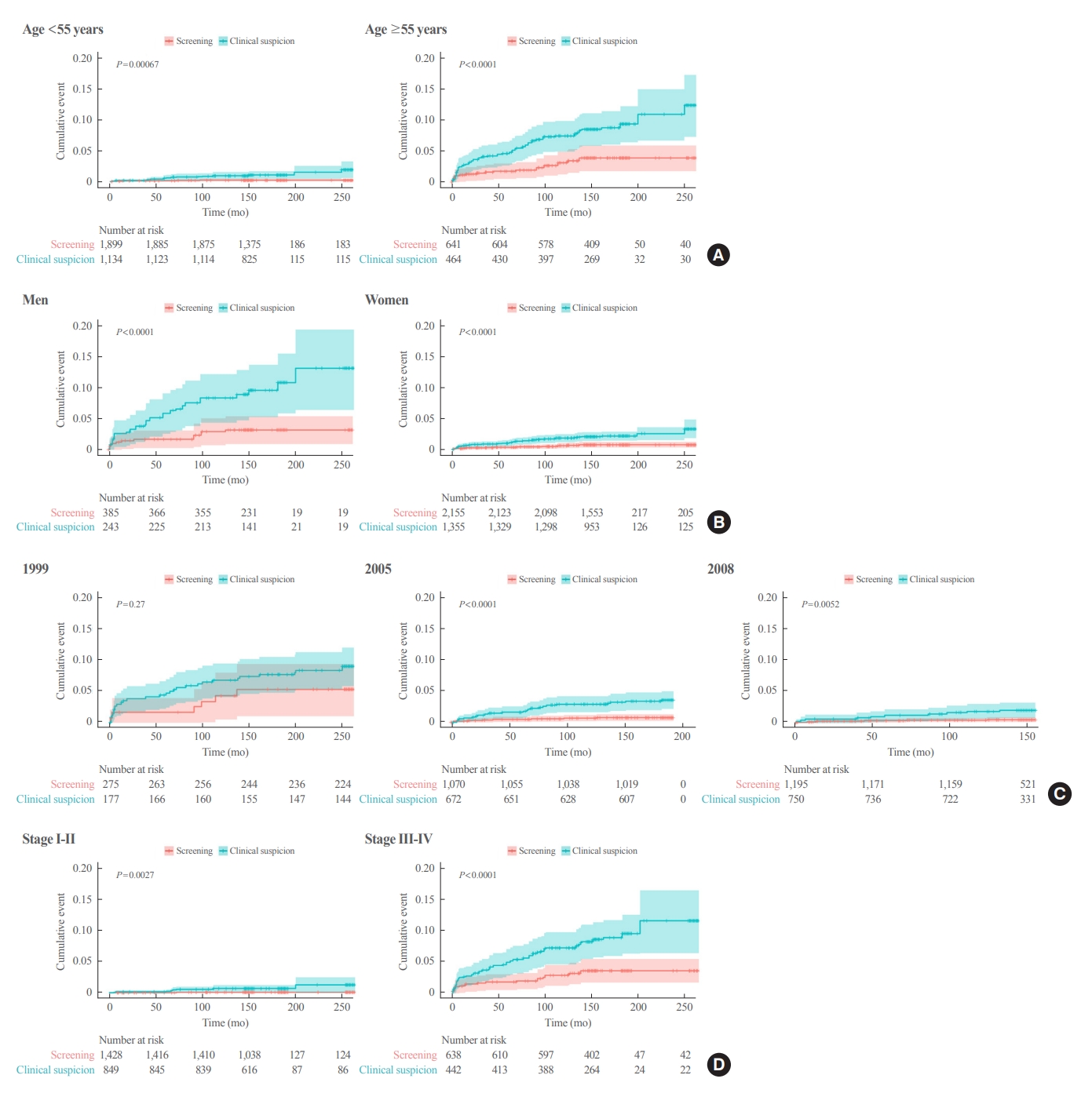
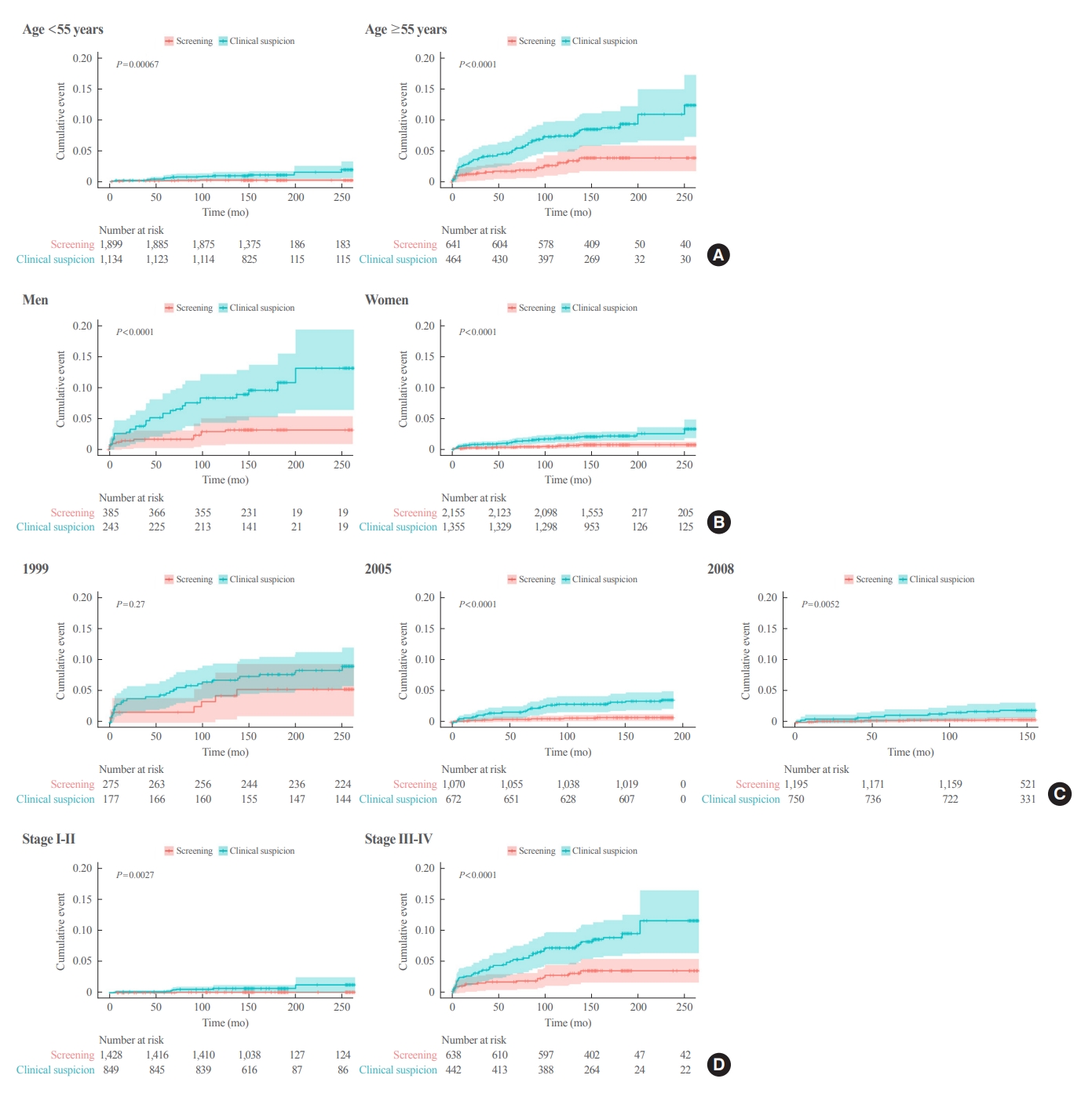
- In the subgroup analysis according to age, Kaplan-Meier survival curves revealed a significantly higher rate of thyroid cancer-specific mortality in the clinical suspicion group than in the screening group in both groups (aged <55 and ≥55 years) (Fig. 2). The adjusted HR in the clinical suspicion group was 4.71 (95% CI, 1.67 to 13.28) in patients aged <55 years and 2.45 (95% CI, 1.32 to 4.56) in patients aged ≥55 years (Table 4).
- In Kaplan-Meier survival curves according to sex, the clinical suspicion group showed a significantly higher rate of thyroid cancer-specific mortality than screening group in both men and women (Fig. 2). The adjusted HR of the clinical suspicion group was 3.25 (95% CI, 1.40 to 7.53) in men and 2.94 (95% CI, 1.44 to 6.02) in women (Table 4). Although few cases of thyroid cancer-specific mortality were reported in the subgroup with early-stage thyroid cancer (AJCC sixth edition stage I and II), Kaplan-Meier survival curves revealed a significantly higher rate of thyroid cancer-specific mortality in the clinical suspicion group than in the screening group (log-rank test, P=0.003) (Fig. 2). The risk for thyroid cancer-specific mortality was higher in the clinical suspicion group (adjusted HR, 12.15; 95% CI, 1.50 to 98.55) than in the screening group (Table 4).
- In the subgroup with an advanced stage of thyroid cancer (stage III and IV), a higher rate of thyroid cancer-specific mortality was found in the clinical suspicion group than in the screening group (log-rank test, P<0.001) (Fig. 2). The adjusted HR for thyroid cancer-specific mortality was 2.59 (95% CI, 1.38 to 4.86) in the clinical suspicion group with an advanced stage of thyroid cancer (Table 4). A subgroup analysis according to lymph node metastasis and distant metastasis showed that the clinical suspicion group had a significantly higher risk of thyroid cancer-specific mortality regardless of the presence of lymph node metastasis and distant metastasis (Table 4).
- Effect of clinically detected thyroid cancer on mortality and its mediating factors
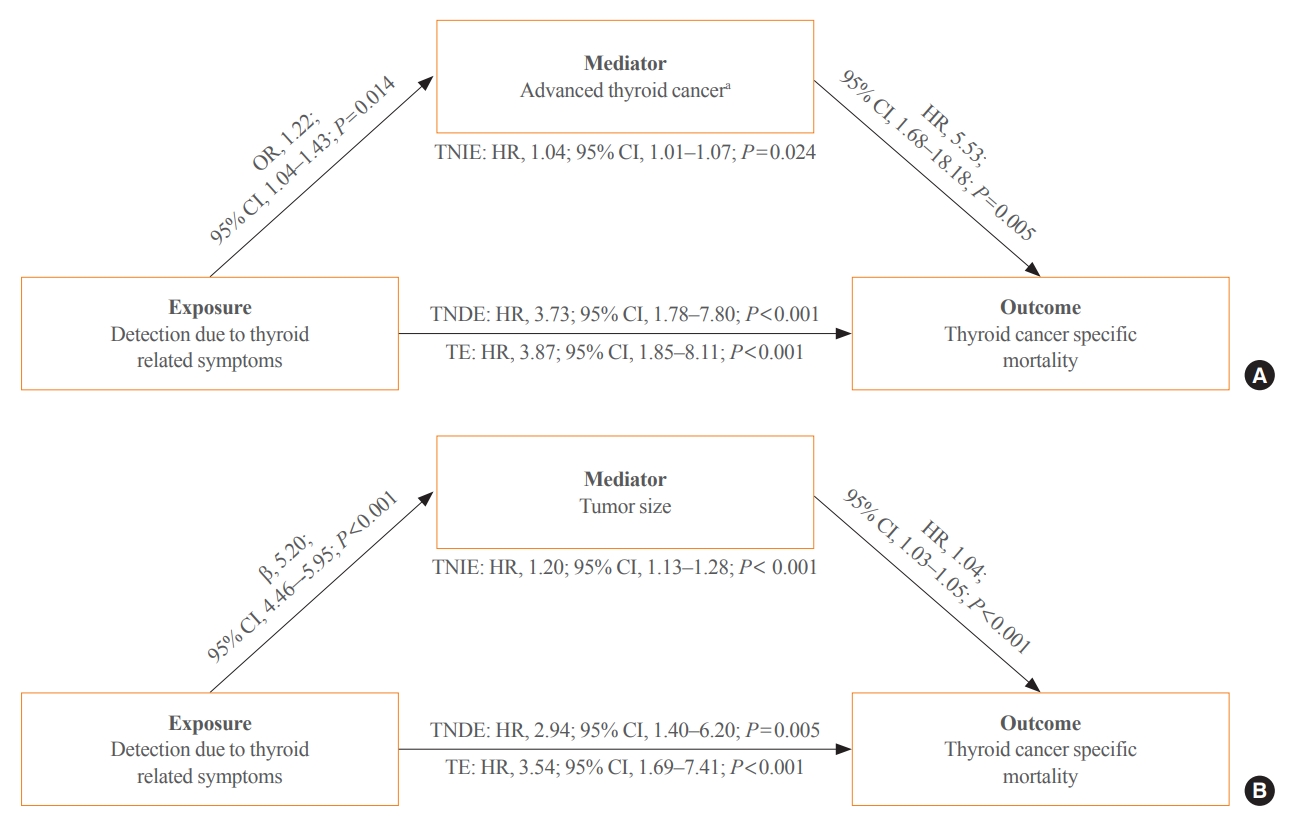
- Using mediation analysis, we present a conceptual model in which clinically detected thyroid cancer is directly associated with thyroid cancer-specific mortality and indirectly through advanced thyroid cancer (T3–4, lymph node metastasis or distant metastasis) (Fig. 3A). Thyroid cancer detected due to symptoms showed a significant direct effect on thyroid cancer-specific mortality, as the total natural direct effect according to detection based on symptoms demonstrated a 3.73 times higher risk of thyroid cancer-specific mortality (Fig. 3A). In addition, clinically detected thyroid cancer was also associated with a higher risk of advanced thyroid cancer (OR, 1.22; 95% CI, 1.04 to 1.43), which was associated with thyroid cancer-specific mortality (HR, 5.53; 95% CI, 1.68 to 18.18) (Fig. 3A). A significant indirect effect indicates that advanced thyroid cancer detected based on clinical symptoms partially increased the risk of thyroid cancer death. We also conducted a mediation analysis with tumor size. Clinically detected thyroid cancer is directly associated with thyroid cancer-specific mortality (Fig. 3B). Clinically detected thyroid cancer showed a significant association with larger tumor size (β=5.20; 95% CI, 4.46 to 5.95), which increased the risk of thyroid cancer-specific mortality (HR, 1.04; 95% CI, 1.03 to 1.50) (Fig. 3B). Clinically detected thyroid cancer indirectly increased the risk of thyroid cancer-specific death, mediated by large tumor size (HR, 1.20; 95% CI, 1.13 to 1.28) (Fig. 3B).
RESULTS
- This study found that all-cause and thyroid cancer-specific mortality rates were higher in patients with clinically detected thyroid cancer than in those diagnosed by screening. Larger tumor size and advanced T stage (stages 3 and 4) were significantly associated with the clinical suspicion group in comparison to the screening group. The clinical suspicion group also had higher risks of extrathyroidal extension and advanced stage in a weighted analysis.
- Although thyroid cancer incidence has increased, it is still inconclusive whether thyroid cancer-related mortality has worsened in a way that reflects the rising incidence, has decreased due to early intervention following early diagnosis, or has remained stable due to overdiagnosis [16]. Pizzato et al. [13] analyzed age-standardized incidence and mortality rates in the international Global Cancer Observatory: Cancer Today (GLOBOCAN) database in 2020 and reported that although the geographical variability in thyroid cancer incidence rates was large, the mortality rates were similar regardless of the region. When analyzing the International World Health Organization Mortality Database using an age-period-cohort model, age-specific mortality curves consistently showed similar long-term declines across countries and time periods [17]. In contrast, Lim et al. [18] showed increases in the incidence and mortality of thyroid cancer for advanced thyroid cancer, suggesting a true increase in thyroid cancer incidence in the United States, using the Surveillance, Epidemiology, and End Results (SEER) database during 1974 to 2013. Megwalu and Moon [19] analyzed the SEER database for 2000 to 2018 and suggested that incidence-based mortality has continued to increase despite a decrease in thyroid cancer incidence since 2014. This inconsistency stems from the fact that those large databases do not provide detailed results for the histological subtype or stage, making subgroup analysis or stratification not applicable; therefore, it is difficult to weigh the benefits and harms of thyroid cancer screening based on those analyses.
- In order to compare mortality rates according to thyroid cancer screening experience along with detailed clinicopathological findings, we analyzed the NEST database, which was established to evaluate temporal trends in thyroid cancer characteristics in Korea. Previously, Jung et al. [20] analyzed the NEST database and found that the survival rate of stage 3–4 thyroid cancer was 63% higher in the screening group than in the clinical diagnosis group, but without a significant difference in stage 1–2 thyroid cancer. However, in that study, a majority of deaths (n=329, 85.5%) were excluded from the 385 deaths after propensity score matching. To minimize the number of deaths missed in the analysis, we reanalyzed the database by adjusting the two groups with IPTW instead of propensity score matching. As a result, of the 4,439 patients included in this analysis, 385 (8.7%) died of any cause during a median follow-up of 170 months. After adjustment using IPTW, the clinical suspicion group had a 69% higher risk of death than the screening group. These results are consistent with previous studies comparing mortality between incidental and non-incidental thyroid cancer [1,21-30].
- In another study, Jun et al. [11] investigated the association between thyroid cancer screening and mortality by defining patients who died of thyroid cancer in the NEST cohort as the death-case group and 1:10-matched newly diagnosed thyroid cancer patients in the National Cancer Screening Program (NCSP) cohort as the survival-control group, and mortality rates were compared. Thyroid cancer patients who received screening had a higher risk of death, but without statistical significance (OR, 1.44; 95% CI, 0.68 to 3.05). However, that study had two limitations. First, the route of cancer detection, which is a major factor in determining group allocation, was inconsistently defined in the NEST cohort (symptoms, screening tests, or incidental findings) and NCSP cohort (whether or not an individual underwent thyroid ultrasound screening). In addition, the proportion of the screening group was only 9.4% in the survival-control group (NCSP), which was significantly lower than the proportion (49.1%) in the death-case group (NEST). In contrast, Kim et al. [31] asked about the diagnostic motives of thyroid cancer patients prospectively and reported that the proportion of cancers detected by screening has rapidly increased since 2000, comprising more than half of patients in 2000 to 2005 and up to 90% in 2011 to 2013. In the entire NEST database, we found that 56.2% of patients were detected clinically out of the 385 deaths, which was consistent with Kim et al. [31].
- Comparing the clinicopathological characteristics, we found that the clinical suspicion group had larger tumors and were more likely to have advanced T stage than the screening group. We also found that the benefit of screening for cumulative mortality by cancer was greater in patients older than 55 years, men, and stage III–IV patients in subgroup analyses. Using mediation analysis to assess the direct effect of screening, thyroid cancers detected due to thyroid-related symptoms were associated with a high risk of thyroid cancer-specific mortality, which was significantly mediated by an advanced stage of thyroid cancer and tumor size. These histological features have shown associations with recurrence, but not with mortality. However, opposing evidence has suggested that repeated recurrence is associated with reduced survival [32]. A recent analysis showed that although individual clinicopathologic factors were not strongly associated with cancer-specific survival or overall survival, there was a significant synergistic effect when multiple factors were involved in terms of the attributable ratio and synergy index [33].
- Regarding the period of thyroid cancer diagnosis, cumulative mortality was highest in participants diagnosed in 1999, followed by those diagnosed in 2005 and 2008, respectively. The difference in mortality between the clinical detection group and the screening group persisted at all three time points, suggesting that thyroid cancer screening has the potential to reduce aggressiveness through early diagnosis and intervention. In a meta-analysis of 29 autopsy studies involving 8,750 patients, Robenshtok et al. [34] reported that unfavorable histological features, such as minimal extrathyroidal extension, lymph node metastasis, multifocality, and vascular invasion were commonly observed in occult differentiated thyroid carcinomas. Thus, patients with undesirable histological features, which are unpredictable prior to screening, may be candidates for survival through screening.
- Our study has several limitations. Firstly, the database used for analysis was a retrospective national cohort that was sampled at three different time points. To mitigate this issue, we adjusted for year of registration, age, and sex to remove lag time bias and innate bias between the two groups due to the study design. Secondly, healthy controls were not included in the comparison of baseline characteristics. However, since the aim of this study was to compare the outcomes of incidentally detected thyroid cancer versus symptomatic thyroid cancer, controls were not essential for the analysis. Finally, there were cases where the method of detection was difficult to distinguish between screening and clinical suspicion. For instance, incidental thyroid cancers discovered during the evaluation of other comorbidities could not be distinguished from cases with the sole purpose of screening. Additionally, nonspecific symptoms related to the upper aerodigestive tract were not specific signs of thyroid cancer, but were a common reason for patients to visit the clinic and receive screening for thyroid cancer. In this study, out of 4,145 thyroid cancer patients, 214 had other types of cancer, with 169 being in the screening group, and three of them died from thyroid cancer. Conversely, among the 45 symptomatic patients with other types of cancer, three also died from thyroid cancer. A higher mortality risk was observed in this group, but the number of deaths from thyroid cancer was too small to conduct a detailed analysis.
- The strengths of this study are that it is the first study to directly compare the mortality rates according to whether thyroid cancer was detected based on clinical suspicion or screening based on the mortality database of Statistics Korea, and that it is linked to a national database established through a comprehensive medical record review. In addition, this cohort was well-designed, with two-stage random sampling stratified by age, sex, and region. Second, to eliminate potential biases that could arise from differences between the two groups in terms of age, sex, and year of thyroid cancer diagnosis, IPTW was used, reducing the possibility of case loss after propensity score matching. Sampling at the three time points also helped reflect the impact of evolving diagnostic and treatment strategies for thyroid cancer.
- In conclusion, our findings provide important evidence that the early detection of thyroid cancer has a survival advantage over the diagnosis of symptomatic thyroid cancer. In particular, discussions on the need for cancer screening are moving toward discussions on the best management strategy, such as active surveillance, reduction of the surgical range, and minimization of indications for radioactive iodine therapy. Therefore, treatment following early diagnosis is not always harmful and can develop into a well-balanced treatment strategy.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: E.K.L., Y.J.P. Acquisition, analysis, or interpretation of data: S.M., E.K.L., H.C., S.K.P., Y.J.P. Drafting the work or revising: S.M., E.K.L. Final approval of the manuscript: S.M., E.K.L., Y.J.P.
Article information
-
Acknowledgements
- We thank the Medical Research Collaborating Center (MRCC) at Seoul National University for their assistance in the statistical analyses.
- This study was supported by Korean Thyroid Association and research funding from the National Cancer Center (grant number 2210521-2). All authors had full access to all the study data; the corresponding authors had final responsibility for the decision to submit for publication.
| Variable |
Unweighted |
IPTW-adjusted (95% CI) | |
|---|---|---|---|
| Crude OR (95% CI) | Adjusted ORa (95% CI) | ||
| T 3–4 | 1.22 (1.07–1.38) | 1.26 (1.1–1.44) | 1.24 (1.09–1.41) |
| Extrathyroidal extension | 1.11 (0.97–1.26) | 1.17 (1.02–1.34) | 1.16 (1.02–1.32) |
| Lymph node metastasis | 1.20 (1.04–1.38) | 1.13 (0.98–1.31) | 1.13 (0.98–1.29) |
| Distant metastasis | 1.62 (0.79–3.32) | 0.87 (0.40–1.91) | 0.91 (0.46–1.81) |
| TNM stage III–IV | 1.16 (1.00–1.34) | 1.17 (0.96–1.43) | 1.16 (1.00–1.35) |
| Group |
All-cause mortality |
Thyroid cancer-specific mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/Total | Crude HR (95% CI) | Adjusted HRa (95% CI) | IPTW-adjusted HR (95% CI) | Events/Total | Crude HR (95% CI) | Adjusted HRa (95% CI) | IPTW-adjusted HR (95% CI) | ||
| Total | 345/4,145 | 1.64 (1.32–2.04) | 1.32 (1.05–1.65) | 1.43 (1.14–1.80) | 84/4,145 | 4.57 (2.79–7.51) | 2.79 (1.66–4.71) | 3.07 (1.77–5.29) | |
| Age group, yr | |||||||||
| <55 | 90/3,047 | 1.67 (1.10–2.55) | 1.67 (1.07–2.61) | 1.55 (0.99–2.43) | 21/3,048 | 4.93 (1.72–13.57) | 4.16 (1.43–12.11) | 4.71 (1.67–13.28) | |
| ≥55 | 254/1,097 | 1.44 (1.12–1.84) | 1.18 (0.91–1.53) | 1.27 (0.97–1.65) | 63/1,097 | 3.85 (2.18–6.81) | 2.35 (1.29–4.27) | 2.45 (1.32–4.56) | |
| Sex | |||||||||
| Men | 100/638 | 2.31 (1.54–3.45) | 1.65 (1.06–2.56) | 1.91 (1.24–2.92) | 32/638 | 4.98 (2.29–10.79) | 3.1 (1.34–7.15) | 3.25 (1.40–7.53) | |
| Women | 244/3,506 | 1.52 (1.18–1.96) | 1.23 (0.95–1.6) | 1.28 (0.98–1.68) | 52/3,506 | 4.86 (2.54–9.29) | 2.98 (1.52–5.86) | 2.94 (1.44–6.02) | |
| Year | |||||||||
| 1999 | 92/456 | 1.04 (0.64–1.68) | 1.13 (0.69–1.84) | 1.07 (0.66–1.73) | 36/456 | 1.63 (0.68–3.90) | 1.74 (0.71–4.23) | 1.72 (0.72–4.14) | |
| 2005 | 151/1,742 | 1.71 (1.25–2.36) | 1.52 (1.09–2.11) | 1.75 (1.27–2.41) | 31/1,742 | 4.32 (1.93–9.66) | 3.38 (1.46–7.83) | 4.75 (2.12–10.64) | |
| 2008 | 101/1,946 | 1.34 (0.89–2.02) | 1.16 (0.77–1.77) | 1.36 (0.9–2.05) | 17/1,946 | 3.62 (1.38–9.51) | 2.9 (1.05–8) | 3.97 (1.51–10.46) | |
| T stage | |||||||||
| T1–T2 | 106/2,068 | 0.85 (0.57–1.27) | 0.77 (0.5–1.17) | 0.78 (0.51–1.2) | 9/2,068 | 1.22 (0.32–4.71) | 0.92 (0.22–3.84) | 0.85 (0.20–3.53) | |
| T3–T4 | 189/1,908 | 2.44 (1.80–3.3) | 1.98 (1.45–2.71) | 1.99 (1.44–2.76) | 55/1,908 | 6.48 (3.25–12.89) | 4.13 (2.01–8.49) | 3.84 (1.82–8.12) | |
| Extrathyroidal extension | |||||||||
| Absent | 124/2,138 | 1.04 (0.73–1.5) | 0.93 (0.63–1.36) | 0.94 (0.64–1.38) | 15/2,138 | 2.71 (0.90–8.15) | 2.07 (0.65–6.64) | 2.04 (0.62–6.76) | |
| Present | 167/1,852 | 2.36 (1.71–3.24) | 1.89 (1.35–2.63) | 2.04 (1.45–2.86) | 48/1,852 | 5.88 (2.93–11.82) | 3.61 (1.74–7.52) | 3.55 (1.66–7.58) | |
| Lymph node metastasis | |||||||||
| Absent | 124/1,884 | 1.48 (1.04–2.11) | 1.26 (0.87–1.82) | 1.41 (0.97–2.04) | 16/1,884 | 7.95 (2.27–27.90) | 4.88 (1.31–18.12) | 6.62 (1.84–23.76) | |
| Present | 123/1,458 | 1.74 (1.21–2.51) | 1.33 (0.9–1.97) | 1.38 (0.93–2.04) | 44/1,458 | 4.31 (2.17–8.57) | 2.64 (1.27–5.48) | 2.52 (1.21–5.26) | |
| Distant metastasis | |||||||||
| Absent | 289/3,950 | 1.62 (1.28–2.05) | 1.35 (1.05–1.73) | 1.47 (1.14–1.88) | 55/3,950 | 4.85 (2.59–9.07) | 2.93 (1.5–5.72) | 3.12 (1.57–6.21) | |
| Present | 21/30 | 1.90 (0.79–4.57) | 2.42 (0.71–8.25) | 2.52 (0.98–6.51) | 18/30 | 2.76 (1.02–7.43) | 3.05 (0.82–11.26) | 4.56 (1.57–13.26) | |
| TNM stage | |||||||||
| I–II | 74/2,298 | 1.05 (0.66–1.68) | 1.15 (0.71–1.87) | 1.05 (0.64–1.71) | 9/2,298 | 12.3 (1.52–99.35) | 10.57 (1.22–91.32) | 12.15 (1.50–98.55) | |
| III–IV | 169/1,064 | 2.10 (1.54–2.86) | 1.55 (1.12–2.14) | 1.67 (1.20–2.33) | 58/1,064 | 3.93 (2.21–7.00) | 2.29 (1.25–4.17) | 2.59 (1.38–4.86) | |
- 1. Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid 2015;25:999–1007.ArticlePubMedPMC
- 2. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 2020;16:17–29.ArticlePubMedPDF
- 3. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global cancer observatory: cancer today; Lyon: International Agency for Research on Cancer; 2018.
- 4. Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid 2016;26:306–18.ArticlePubMedPMC
- 5. Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol 2015;181:883–8.ArticlePubMedPMC
- 6. Choi YM, Lee J, Kwak MK, Jeon MJ, Kim TY, Hong EG, et al. Recent changes in the incidence of thyroid cancer in Korea between 2005 and 2018: analysis of Korean National Data. Endocrinol Metab (Seoul) 2022;37:791–9.ArticlePubMedPMCPDF
- 7. Jung CK, Bae JS, Park YJ. Re-increasing trends in thyroid cancer incidence after a short period of decrease in Korea: reigniting the debate on ultrasound screening. Endocrinol Metab (Seoul) 2022;37:816–8.ArticlePubMedPMCPDF
- 8. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020;8:468–70.ArticlePubMed
- 9. Lincango-Naranjo E, Solis-Pazmino P, El Kawkgi O, Salazar-Vega J, Garcia C, Ledesma T, et al. Triggers of thyroid cancer diagnosis: a systematic review and meta-analysis. Endocrine 2021;72:644–59.ArticlePubMedPDF
- 10. Chen Z, Mosha SS, Zhang T, Xu M, Li Y, Hu Z, et al. Incidence of microcarcinoma and non-microcarcinoma in ultrasound-found thyroid nodules. BMC Endocr Disord 2021;21:38.ArticlePubMedPMCPDF
- 11. Jun JK, Hwang SY, Hong S, Suh M, Choi KS, Jung KW. Association of screening by thyroid ultrasonography with mortality in thyroid cancer: a case-control study using data from two national surveys. Thyroid 2020;30:396–400.ArticlePubMed
- 12. Chooi JE, Ravindiran A, Balasubramanian SP. The influence of incidental detection of thyroid nodule on thyroid cancer risk and prognosis: a systematic review. Clin Endocrinol (Oxf) 2022;96:246–54.ArticlePubMedPDF
- 13. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol 2022;10:264–72.ArticlePubMed
- 14. Oh CM, Kong HJ, Kim E, Kim H, Jung KW, Park S, et al. National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Epidemiol Health 2018;40:e2018052.ArticlePubMedPMC
- 15. Li Y, Yoshida K, Kaufman JS, Mathur MB. A brief primer on conducting regression-based causal mediation analysis. OSF Preprints 2022 Mar 28 [Preprint]. https://doi.org/10.31219/osf.io/jath7.Article
- 16. Krajewska J, Kukulska A, Oczko-Wojciechowska M, Kotecka-Blicharz A, Drosik-Rutowicz K, Haras-Gil M, et al. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol (Lausanne) 2020;11:571421.ArticlePubMedPMC
- 17. Li M, Brito JP, Vaccarella S. Long-term declines of thyroid cancer mortality: an international age-period-cohort analysis. Thyroid 2020;30:838–46.ArticlePubMed
- 18. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338–48.ArticlePubMedPMC
- 19. Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid 2022;32:560–70.ArticlePubMed
- 20. Jung YS, Oh CM, Kim Y, Jung KW, Ryu J, Won YJ. Long-term survival of patients with thyroid cancer according to the methods of tumor detection: a nationwide cohort study in Korea. PLoS One 2018;13:e0194743.ArticlePubMedPMC
- 21. Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients. World J Surg 2014;38:1312–7.ArticlePubMedPDF
- 22. Choi YJ, Park YL, Koh JH. Prevalence of thyroid cancer at a medical screening center: pathological features of screen-detected thyroid carcinomas. Yonsei Med J 2008;49:748–56.ArticlePubMedPMC
- 23. Chung WY, Chang HS, Kim EK, Park CS. Ultrasonographic mass screening for thyroid carcinoma: a study in women scheduled to undergo a breast examination. Surg Today 2001;31:763–7.ArticlePubMedPDF
- 24. Evranos B, Polat SB, Cuhaci FN, Baser H, Topaloglu O, Kilicarslan A, et al. A cancer of undetermined significance: Incidental thyroid carcinoma. Diagn Cytopathol 2019;47:412–6.ArticlePubMedPDF
- 25. Kim H, Park SY, Jung J, Kim JH, Hahn SY, Shin JH, et al. Improved survival after early detection of asymptomatic distant metastasis in patients with thyroid cancer. Sci Rep 2019;9:18745.ArticlePubMedPMCPDF
- 26. Kim SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Differences in the recurrence and survival of patients with symptomatic and asymptomatic papillary thyroid carcinoma: an observational study of 11,265 person-years of follow-up. Thyroid 2016;26:1472–9.ArticlePubMed
- 27. Zagzag J, Malone MK, Lopresti MA, Ogilvie JB, Patel KN, Heller KS. Method of detection of well-differentiated thyroid cancers in obese and non-obese patients. PLoS One 2016;11:e0152768.ArticlePubMedPMC
- 28. Marina M, Ceda GP, Aldigeri R, Ceresini G. Causes of referral to the first endocrine visit of patients with thyroid carcinoma in a mildly iodine-deficient area. Endocrine 2017;57:247–55.ArticlePubMedPDF
- 29. Pisanu A, Reccia I, Nardello O, Uccheddu A. Risk factors for nodal metastasis and recurrence among patients with papillary thyroid microcarcinoma: differences in clinical relevance between nonincidental and incidental tumors. World J Surg 2009;33:460–8.ArticlePubMedPDF
- 30. Shakil J, Ansari MZ, Brady J, Xu J, Robbins RJ. Lower rates of residual/recurrent disease in patients with incidentally discovered thyroid carcinoma. Endocr Pract 2017;23:163–9.ArticlePubMed
- 31. Kim H, Hwangbo Y, Kong SH, Song YS, Kim MJ, Cho SW, et al. Secular trends for diagnostic motives and environmental risk factors in thyroid cancer using questionnaire survey. Int J Thyroidol 2017;10:82–8.ArticlePDF
- 32. Palme CE, Waseem Z, Raza SN, Eski S, Walfish P, Freeman JL. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2004;130:819–24.ArticlePubMed
- 33. Hu D, Zhou W, Huang Y, Chen S, Zeng W, Wei W, et al. Synergistic effect of clinicopathological factors on mortality risk in patients with differentiated thyroid cancer: an analysis using the SEER database. Surg Oncol 2020;34:96–102.ArticlePubMed
- 34. Robenshtok E, Neeman B, Reches L, Ritter A, Bachar G, Kaminer K, et al. Adverse histological features of differentiated thyroid cancer are commonly found in autopsy studies: implications for treatment guidelines. Thyroid 2022;32:37–45.ArticlePubMed
References
Figure & Data
References
Citations

- Clinical Characteristics, Diagnostic Approach and Outcome of Thyroid Incidental Findings vs. Clinically Overt Thyroid Nodules: An Observational Single-Centre Study
Tom Jansen, Nike Stikkelbroeck, Annenienke van de Ven, Ilse van Engen-van Grunsven, Marcel Janssen, Han Bonenkamp, Martin Gotthardt, Romana T. Netea-Maier
Cancers.2023; 15(8): 2350. CrossRef - Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinology and Metabolism.2023; 38(1): 93. CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinology and Metabolism.2023; 38(1): 72. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite