Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(2); 2023 > Article
-
Original ArticleMiscellaneous Association between N-Terminal Prohormone Brain Natriuretic Peptide and Decreased Skeletal Muscle Mass in a Healthy Adult Population: A Cross-Sectional Study

 AudioslideKeypoint
AudioslideKeypoint
This cross-sectional study examined the association between NT-proBNP levels and skeletal muscle mass in asymptomatic healthy adults. Participants were categorized into control, mildly low skeletal muscle mass, and severely low skeletal muscle mass groups based on the skeletal muscle mass index. The prevalence of elevated NT-proBNP was higher in the low skeletal muscle mass groups. Adjusted odds ratios showed a significant association between severely low skeletal muscle mass and elevated NT-proBNP levels. The study suggests a link between skeletal muscle mass and NT-proBNP levels in a young and healthy adult population. -
Tae Kyung Yoo1
 , Marie Yung-Chen Wu1, Moon Soo Kim1, Mi-Yeon Lee2, Yong-Taek Lee3, Kyung Jae Yoon3, Chul-Hyun Park3
, Marie Yung-Chen Wu1, Moon Soo Kim1, Mi-Yeon Lee2, Yong-Taek Lee3, Kyung Jae Yoon3, Chul-Hyun Park3
-
Endocrinology and Metabolism 2023;38(2):269-276.
DOI: https://doi.org/10.3803/EnM.2022.1588
Published online: March 13, 2023
1Department of Medicine, MetroWest Medical Center, Framingham, MA, USA
2Division of Biostatistics, Department of R&D Management, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Physical and Rehabilitation Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding author: Chul-Hyun Park. Department of Physical and Rehabilitation Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea Tel: +82-2-2001-8487, Fax: +82-2-2001-2176, E-mail: chpark0930@gmail.com
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,812 Views
- 76 Download
ABSTRACT
-
Background
- Although an inverse association between the N-terminal prohormone brain natriuretic peptide (NT-proBNP) and obesity exists, only few major studies have assessed the association between NT-proBNP levels and skeletal muscle mass in asymptomatic healthy adults. Therefore, this cross-sectional study was conducted.
-
Methods
- We assessed participants who underwent health examinations at Kangbuk Samsung Hospital in South Korea from January 2012 to December 2019. Appendicular skeletal muscle mass was measured using a bioelectrical impedance analyzer, and the skeletal muscle mass index (SMI) was calculated. Participants were divided into the control, mildly low skeletal muscle mass (LMM) (−2 standard deviation [SD] < SMI ≤−1 [SD]), and severely LMM groups (SD ≤−2) based on their SMI. The association between elevated NT-proBNP level (≥125 pg/mL) and skeletal muscle mass was assessed using multivariable logistic regression analysis with adjustment for confounding factors.
-
Results
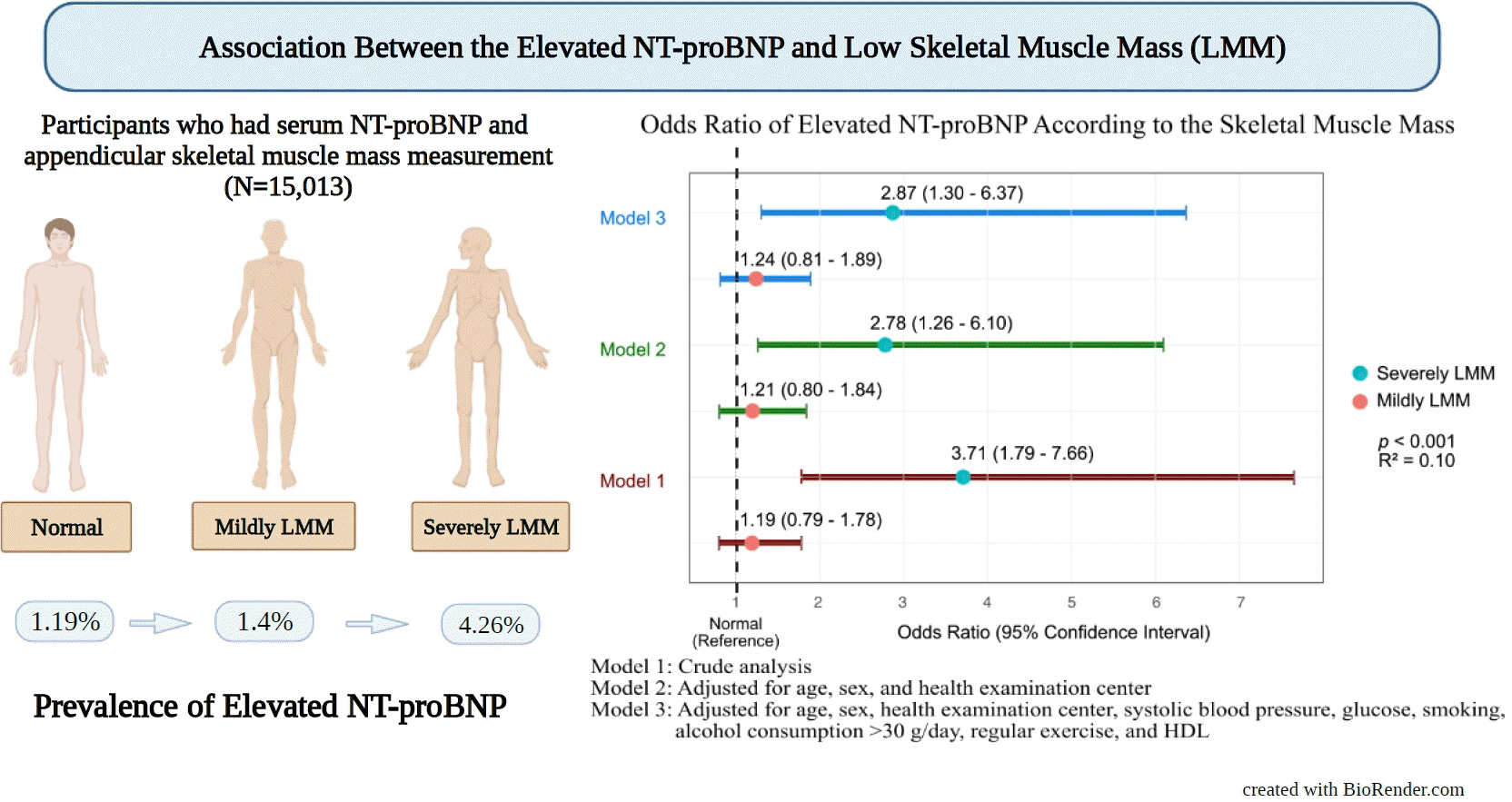
- This study enrolled 15,013 participants (mean age, 37.52±9.52; men, 54.24%; control, n=12,827; mildly LMM, n=1,998; severely LMM, n=188). Prevalence of elevated NT-proBNP was higher in mildly and severely LMM groups than in the control group (control, 1.19%; mildly LMM, 1.4%; severely LMM, 4.26%; P=0.001). The adjusted odds ratio (OR) of elevated NT-proBNP was significantly higher in severely LMM (OR, 2.87; 95% confidence interval [CI], 1.3 to 6.37) than in control (OR, 1.00; reference) or mildly LMM groups (OR, 1.24; 95% CI, 0.81 to 1.89).
-
Conclusion
- Our results showed that NT-proBNP elevation were more prevalent in participants with LMM. In addition, our study showed an association between skeletal muscle mass and NT-proBNP level in a relatively young and healthy adult population.
- Loss of muscle mass has been demonstrated to be a significant public health issue, with an estimated 10% prevalence of individuals (both sexes) with decreased skeletal muscle mass worldwide [1]. Decrease in the skeletal muscle mass is generally associated with the aging process, and this age-related decline in skeletal muscle mass may begin as early as >30 years of age [2]. In addition, owing to decreased physical activity in the middle-aged adult population, decreased skeletal muscle mass has become an increasingly important issue in the relatively young adult population [3].
- N-terminal prohormone brain natriuretic peptide (NT-proBNP) is widely used in clinical settings, is associated with increased severity and acuity of heart failure (HF) [4], and is elevated in populations with impaired renal function [5,6].
- NT-proBNP is also decreased among obese individuals, particularly men [7]. Nevertheless, despite the close relationship between skeletal muscle mass and obesity, both of which contribute to the nexus of body composition, the body of literature examining the association between NT-proBNP and skeletal muscle mass is limited. Most of the previous studies that assessed the association between low skeletal muscle mass (LMM) and NT-proBNP focused on a specific population, namely, HF or hemodialysis population, and only a few studies evaluated the association in young and healthy adult populations [8,9].
- To fill this gap in clinical science, we conducted a cross-sectional study to characterize the association between decreased skeletal muscle mass and NT-proBNP levels in an asymptomatic healthy adult population.
INTRODUCTION
- Kangbuk Samsung Health Study and study period
- Details of the cohort explanation and measurement methods have been provided in the previous studies by our team [10,11]. In brief, this was a population-based cross-sectional study based on the Kangbuk Samsung Health Study, which is a cohort study that includes individuals who participated in an annual or biennial health checkup program in South Korea.
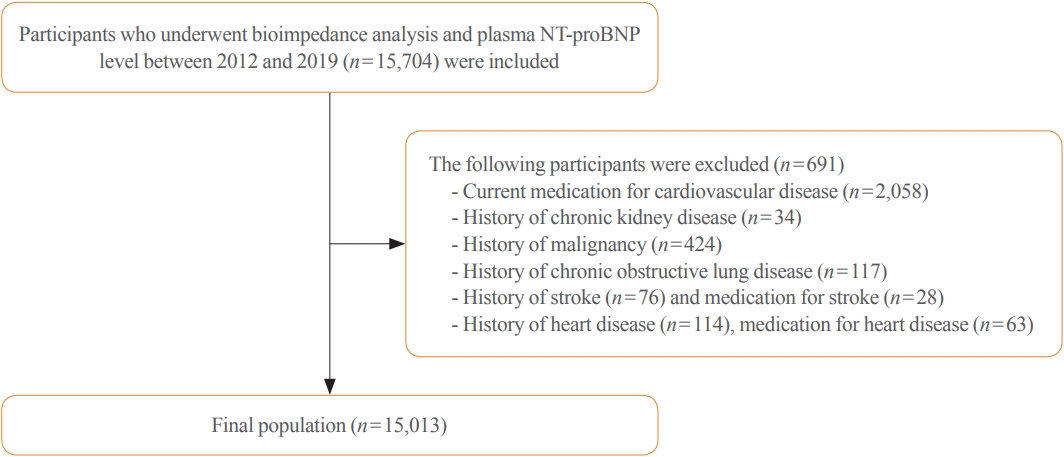
- The data were collected from January 3, 2012 to December 31, 2019. This study initially enrolled 15,704 individuals with skeletal muscle mass index (SMI) and plasma NT-proBNP level measurements. The following participants were excluded: history of heart disease (angina, myocardial infarction, atrial fibrillation, heart valve diseases, HF, hypertrophic myocardial disease, debrillators, or an artificial heart machine, n=114), chronic kidney disease (CKD) (n=34), malignancy (n=424), chronic obstructive lung disease (n=117), stroke (n=76), and under medication for heart diseases (n=63) and stroke (n=28). Hence, after exclusion, of the 15,704 participants, 15,013 were included in the study (Fig. 1).
- Anthropometric data, demographic information, and medical history of the participants were collected. Blood samples were collected and analyzed for NT-ProBNP levels and laboratory data. The appendicular skeletal muscle mass (kg) was measured using a bioelectrical impedance analyzer (BIA). The SMI was calculated by dividing appendicular skeletal muscle mass by the square of the height (kg/m2) [10]. Participants were considered to be engaged in regular exercise if they participated in >150 minutes of moderate-intensity exercise in a week [12].
- The study protocol was approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (IRB no. KBSMC 2022-08-075). The requirement for informed consent was waived by the IRB of Kangbuk Samsung Hospital because we used unidentifiable datasets that were collected as part of the routine health checkup. Our study was done in accordance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Declaration of Helsinki in 1975.
- Definition of LMM and elevated NT-proBNP
- Participants were classified by the LMM category using the following criteria: control (SMI >–1 standard deviation [SD]), mildly LMM (–2 SD < SMI ≤–1 SD), and severely LMM (SMI ≤–2 SD) [13]. During the study period, the cut-off values for mildly LMM in men and women were 7.40 and 5.47 kg/m2 and that for severely LMM were 6.71 and 4.87 kg/m2, respectively (Table 1). NT-proBNP level of >125 pg/mL was defined as an elevated status [14].
- Data availability
- The datasets used in the current study cannot be made publicly available to protect patient information. However, the corresponding author can provide a dataset upon reasonable request.
- Statistical analyses
- Participants were categorized into three groups according to their muscle mass and were used as categorical variables in the analysis. NT-proBNP status was considered a binary variable (normal or elevated) based on the abovementioned cut-off value. Chi-square test and one-way analysis of variance were used to compare the means of variables between groups. Post hoc Bonferroni analyses were performed to compare groups. Multivariable logistic regression analysis was conducted to assess the cross-sectional association between the LMM groups and NT-proBNP levels. We used three models with adjusted confounding factors: model 1, crude analysis; model 2, adjusted for age, sex, and health examination center; and model 3, adjusted for age, sex, health examination center, systolic blood pressure, serum glucose level, smoking, heavy alcohol consumption (alcohol >30 g), regular exercise, and high-density lipoprotein. Odds ratios (OR) with 95% confidence intervals (CI) were calculated.
- In addition, we assessed the association between NT-proBNP and LMM, with NT-proBNP as a continuous variable. NT-proBNP values were log-transformed owing to its skewed distribution. Analysis of covariance was used to compare adjusted means of log-transformed NT-proBNP values of each group after adjusting for confounding factors in model 3. IBM SPSS version 26.0 (IBM Co., Armonk, NY, USA) was used for all statistical analyses, and a two-tailed P value of <0.05 was considered statistically significant.
METHODS
- Baseline demographic characteristics
- Table 2 shows the baseline characteristics of the participants in the control and LMM groups. The control, mildly LMM, and severely LMM groups comprised 85.44% (n=12,827), 13.31% (n=1,998), and 1.25% (n=188) of the study population, respectively. The mean age of the participants in the control, mildly LMM, and severely LMM groups was 37.52±9.52 (>18 years old), 36.89±10.85, and 40.74±14.55, respectively. The control, mildly LMM, and severely LMM groups comprised 54.24% (n=6,957), 55.76% (n=1,114), and 64.89% (n=112) (P=0.007) men, respectively. The SMI of the participants in the control, mildly LMM, and severely LMM groups was 7.31±1.13, 6.32±0.95, and 5.87±0.87 kg/m2 (P<0.001), respectively. The appendicular skeletal muscle mass of the participants in the control, mildly LMM, and severely LMM groups was 21.09± 5.04, 17.52±4.06, and 15.75±3.57 kg (P<0.001), respectively. The percentage of the participants with regular exercise (>3 times/week) in the control, mildly LMM, and severely LMM groups was 14.1 (n=1,809), 8.06 (n=161), and 3.19 (n=6) (P<0.001), respectively.
- Prevalence of elevated NT-proBNP in control, mildly LMM, and severely LMM groups
- The prevalence of elevated NT-proBNP was 1.19% in control, 1.4% in mildly LMM, and 4.26% in severely LMM groups. The prevalence of normal NT-proBNP levels among the control, mildly LMM, and severely LMM groups was 98.81%, 98.6%, and 95.74%, respectively (P<0.001) (Table 3).
- Comparison of natural log transformed NT-proBNP level values between each group
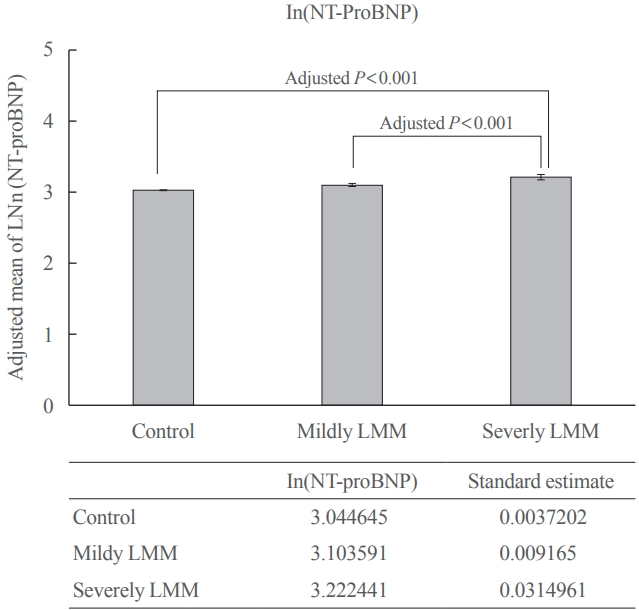
- Fig. 2 shows the association between log-transformed NT-proBNP levels (as a continuous variable) and the LMM groups. Log transformed adjusted mean of proBNP level was 3.04 (standard estimate, 0.004) in the control, 3.10 (standard estimate, 0.009) in mildly LMM, and 3.22 (standard estimate, 0.031) in severely LMM groups. There were significant differences in the comparisons between all the group (all post hoc Bonferroni, P<0.001).
- Association between LMM and elevated NT-proBNP
- Table 4 shows the association between LMM groups and NT-proBNP levels. In crude model, mild LMM group was not associated with elevated NT-proBNP levels; however, severe LMM group was significantly associated with elevated NT-proBNP levels (model 1, control, reference; mildly LMM [OR, 1.19; 95% CI, 0.79 to 1.78]; severely LMM [OR, 3.71; 95% CI, 1.79 to 7.66]). After the adjustment for age, sex, and health examination center, mildly LMM group was not associated with elevated proBNP levels, but severely LMM group was significantly associated with elevated NT-proBNP levels (model 2, mildly LMM [OR, 1.21; 95% CI, 0.80 to 1.84]; severely LMM [OR, 2.78; 95% CI, 1.26 to 6.10]). The trend was the same after full adjustment. Mildly LMM group was not associated with elevated NT-proBNP levels, but severely LMM group was significantly associated with elevated NT-proBNP levels (model 3, mildly LMM [OR, 1.24; 95% CI, 0.81 to 1.89]; severe LMM [OR, 2.87; 95% CI, 1.30 to 6.37]).
RESULTS
- To the best of our knowledge, this is one of the few major studies to assess the relationship between LMM and elevated NT-proBNP levels in an asymptomatic population using BIA to assess skeletal muscle mass. Our study showed that elevated NT-proBNP levels were more prevalent in the LMM groups than in the control group. In addition, severely LMM group was significantly associated with elevated NT-proBNP levels. This association was significant, even after adjusting for multiple confounding factors. This finding indicates that skeletal muscle mass can affect NT-proBNP levels.
- NT-proBNP is a useful biomarker for the diagnosis and assessment prognosis of patients with HF [15]. NT-proBNP is inversely associated with body composition such as visceral fat, body mass index, and waist circumference [16]. Patients with chronic HF and cachexia tend to have higher NT-proBNP levels than those without cachexia [8]. Selvaraj et al. [17] showed that axial muscle mass had a strong inverse relationship with NT-proBNP levels in patients with HF. However, their study had several limitations because they used axial muscle mass instead of appendicular muscle mass, even though appendicular muscle mass has more study data [17,18]. In addition, the study participants were mainly men and patients with HF [17]. By excluding patients with heart diseases at baseline and including both men and women in our study, we expanded the previous findings to the healthy adult population group. Furthermore, we used axial skeletal muscle mass, the sum of the muscle mass of the arms and legs, which is more widely used to assess muscle mass [18].
- Ikeda et al. [8] used dual-energy X-ray absorptiometry (DEXA) to assess the association between NT-proBNP levels and loss of muscle mass in patients undergoing hemodialysis and showed that NT-proBNP levels were significantly higher in such patients experiencing muscle loss. The authors explained that this finding may be due to the lipolytic effect of natriuretic peptides [8,19]. Increased natriuretic peptides in the circulation can cause continuous release of free fatty acids, which can eventually cause increased energy dissipation by the skeletal muscle [20]. The authors stated that NT-proBNP level may be a useful marker of muscle mass loss in patients undergoing dialysis [8]. However, their study needs cautious interpretation as the participants were limited to patients undergoing hemodialysis. Additionally, the total number of participants was relatively small (n=238) [8]. Our study is novel in that it included a larger number of healthy cohorts without a history of CKD.
- Yamashita et al. [9] showed that in participants with decreased thigh muscle cross-sectional area, the BNP levels were higher compared to that of the normal participants. Authors postulated that increased arterial stiffness, which is associated with loss of muscle mass, could have affected the hemodynamic change [9,21]. This altered change may mechanically stimulate BNP secretion [9,22]. In addition, the authors hypothesized that BNP levels increase to protect against further muscle damage, as BNP was found to reduce skeletal muscle mitochondrial dysfunction and oxidative stress after ischemia-reperfusion [9]. Although the study was meaningful in that it proposed a potential mechanism of increased BNP in participants with decreased skeletal muscle mass, the study is limited as it used thigh muscle cross-sectional area to assess the muscle mass of participants, which is not a commonly used method, compared to BIA or DEXA, to assess skeletal muscle mass [9,18].
- The exact mechanisms of how muscle mass may relate to NT-proBNP are unclear [17]. Previous studies suggested several possible mechanisms. It was postulated that a substance produced in the lean mass suppresses the synthesis or release of natriuretic peptides from cardiomyocytes [23]. Another hypothesis was that sex steroid hormones, such as androgen, associated with increased lean mass, or estrogen, associated with lower lean mass, may affect natriuretic peptide levels [24]. Lastly, lean mass might secrete a substance that leads to NT-proBNP degradation or clearance [23].
- Overall, our study is in line with previous studies showing that LMM is related to elevated NT-proBNP levels. In addition, compared with previous studies, our study has several strengths. First, we used BIA to assess the SMI, which correlates well with skeletal muscle mass and is widely available in clinical settings [25]. Second, compared to previous studies, we included a relatively larger number of participants, increasing the validity of our results. Third, we adjusted the participants’ physical activity levels as a confounding factor, which affects skeletal muscle and NT-proBNP levels [16].
- Despite these strengths, our study has several limitations. First, it was a cross-sectional study. A causal inference between LMM and NT-proBNP levels could not be established. Second, there was a relatively small number of participants with severely LMM in our study population, manifesting as a small number of individuals with elevated levels of NT-proBNP. This is likely owing to the relatively young and healthy cohorts. In addition, the generalizability of our findings beyond this population requires cautious interpretation and judgement. Third, our study was conducted in a single ethnicity (Korean). Fourth, the LMM groups were less physically active than the other group. To overcome this limitation, we adjusted the physical activity level as a confounding factor to overcome this heterogeneity. Lastly, those with excessively elevated proBNP levels might have had subclinical abnormalities, which might have affected the relationship. However, this is less likely because the prevalence or incidence of HF and end-stage kidney disease in our cohort’s age group was very low in Korea [26,27]. Future prospective studies incorporating broader ethnicities and age groups are required to validate the study results.
- In conclusion, this study demonstrated that the prevalence of elevated NT-proBNP levels was higher in the LMM populations. In addition, there was a significant association between severely LMM and elevated NT-proBNP levels in a relatively healthy adult population. Clinicians may need to consider skeletal muscle mass when interpreting elevated NT-proBNP levels in patients without HF or CKD.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: T.K.Y., C.H.P. Acquisition, analysis, or interpretation of data: T.K.Y., M.Y.L., C.H.P. Drafting the work or revising: T.K.Y., M.Y.C.W., M.S.K. Final approval of the manuscript: T.K.Y., M.Y.C.W., M.S.K., M.Y.L., Y.T.L., K.J.Y., C.H.P.
Article information
-
Acknowledgements
- This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1F1A107487511).
| Characteristic |
Overall |
P value | Post hoc analysis (Bonferroni) | |||
|---|---|---|---|---|---|---|
| Control (a) | Mildly LMM (b) | Severely LMM (c) | ||||
| Number | 12,827 (85.44) | 1,998 (13.31) | 188 (1.25) | |||
| Age, yr | 37.52±9.52 | 36.89±10.85 | 40.74±14.55 | <0.001 | a≠c, b≠c | |
| Male sex | 6,957 (54.24) | 1,114 (55.76) | 122 (64.89) | 0.007 | a≠c, b≠c | |
| Screening center (Seoul) | 3,625 (28.26) | 497 (24.87) | 51 (27.13) | 0.007 | a≠b | |
| Height, cm | 168.6±8.75 | 165.37±8.22 | 162.71±8.29 | <0.001 | a≠b, a≠c, b≠c | |
| Weight, kg | 68.65±13.78 | 56.43±9.18 | 51.1±8.05 | <0.001 | a≠b, a≠c, b≠c | |
| BMI, kg/m2 | 23.99±3.52 | 20.53±2.2 | 19.22±2.09 | <0.001 | a≠b, a≠c, b≠c | |
| Appendicular skeletal muscle mass, kg | 21.09±5.04 | 17.52±4.06 | 15.75±3.57 | <0.001 | a≠b, a≠c, b≠c | |
| SMIa, kg/m2 | 7.31±1.13 | 6.32±0.95 | 5.87±0.87 | <0.001 | a≠b, a≠c, b≠c | |
| Smoker status | <0.001 | a≠b, b≠c | ||||
| Never smoker | 8,146 (63.51) | 1,356 (67.87) | 104 (55.32) | |||
| Former smoker | 3,217 (25.08) | 452 (22.62) | 57 (30.32) | |||
| Current smoker | 1,310 (10.21) | 172 (8.61) | 22 (11.7) | |||
| Heavy alcohol | 2,271 (17.7) | 275 (13.76) | 30 (15.96) | <0.001 | a≠b | |
| Regular exercise | 1,809 (14.1) | 161 (8.06) | 6 (3.19) | <0.001 | a≠b, a≠c | |
| Comorbidities | ||||||
| Diabetes | 286 (2.23) | 44 (2.2) | 6 (3.19) | 0.470 | ||
| Hypertension | 1,018 (7.94) | 107 (5.36) | 18 (9.57) | <0.001 | a≠b, a≠c | |
| Dyslipidemia | 1,893 (14.76) | 235 (11.76) | 29 (15.43) | 0.004 | a≠b | |
| Laboratory finding | ||||||
| Fasting glucose | 95.15±13.13 | 93.24±13.09 | 96.76±28.26 | <0.001 | b≠c | |
| HbA1c | 5.47±0.49 | 5.39±0.46 | 5.51±0.84 | <0.001 | a≠b, b≠c | |
| Insulina | 6.64 (4.57–9.55) | 5.52 (3.92–7.68) | 4.8 (3.51–6.99) | <0.001 | a≠b, a≠c, b≠c | |
| Total cholesterol | 185.62±32.72 | 185.52±31.88 | 189.72±34.48 | 0.226 | ||
| LDL | 123.8±32.16 | 121.94±31.59 | 124.85±32.65 | 0.047 | a≠b, a≠c | |
| HDL | 60.98±16.53 | 65.66±16.65 | 66.48±18.43 | <0.001 | a≠b, a≠c | |
| Tga | 89 (64–135) | 80.5 (60–116) | 86.5 (65–128.5) | <0.001 | a≠b | |
| SBP | 110.14±12.31 | 106.21±11.77 | 107.13±11.87 | <0.001 | a≠b, a≠c | |
| DBP | 69.43±9.2 | 68.07±8.87 | 69.29±8.45 | <0.001 | a≠b | |
Values are expressed as number (%), mean±standard deviation, or median (interquartile range).
LMM, low skeletal muscle mass; BMI, body mass index; SMI, skeletal muscle mass index; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; Tg, triglyceride; SBP, systolic blood pressure; DBP, diastolic blood pressure.
a SMI (%), appendicular skeletal muscle mass (kg)/height(m)2×100.
| LMM |
NT-proBNP |
P value | |
|---|---|---|---|
| <125 pg/mL | ≥125 pg/mL | ||
| Control | 12,675 (98.81) | 152 (1.19) | 0.001 |
| Mildly LMM | 1,970 (98.6) | 28 (1.4) | |
| Severly LMM | 180 (95.74) | 8 (4.26) | |
Values are expressed as odds ratio (95% confidence interval). Model 1: crude analysis; Model 2: adjusted for age, sex, and health examination center; Model 3: adjusted for age, sex, health examination center, systolic blood pressure, glucose, smoking, alcohol consumption >30 g/day, regular exercise, and high-density lipoprotein cholesterol.
NT-proBNP, N-terminal prohormone brain natriuretic peptide; LMM, low skeletal muscle mass.
- 1. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord 2017;16:21.ArticlePubMedPMCPDF
- 2. Li Z, Tong X, Ma Y, Bao T, Yue J. Relationship between low skeletal muscle mass and arteriosclerosis in western China: a cross-sectional study. Front Cardiovasc Med 2021;8:735262.ArticlePubMedPMC
- 3. Nikitara K, Odani S, Demenagas N, Rachiotis G, Symvoulakis E, Vardavas C. Prevalence and correlates of physical inactivity in adults across 28 European countries. Eur J Public Health 2021;31:840–5.ArticlePubMedPMCPDF
- 4. Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2004;110:1780–6.ArticlePubMed
- 5. Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, et al. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc 2001;76:1111–9.ArticlePubMed
- 6. Mueller C, Laule-Kilian K, Scholer A, Nusbaumer C, Zeller T, Staub D, et al. B-type natriuretic peptide for acute dyspnea in patients with kidney disease: insights from a randomized comparison. Kidney Int 2005;67:278–84.ArticlePubMed
- 7. McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, et al. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med 2004;164:2247–52.ArticlePubMed
- 8. Ikeda M, Honda H, Takahashi K, Shishido K, Shibata T. Nterminal pro-B-type natriuretic peptide as a biomarker for loss of muscle mass in prevalent hemodialysis patients. PLoS One 2016;11:e0166804.ArticlePubMedPMC
- 9. Yamashita T, Kohara K, Tabara Y, Ochi M, Nagai T, Okada Y, et al. Muscle mass, visceral fat, and plasma levels of Btype natriuretic peptide in healthy individuals (from the JSHIPP Study). Am J Cardiol 2014;114:635–40.ArticlePubMed
- 10. Yoo TK, Rhim HC, Lee YT, Yoon KJ, Park CH. Relationship between hyperhomocysteinemia and coexisting obesity with low skeletal muscle mass in asymptomatic adult population. Sci Rep 2022;12:12439.ArticlePubMedPMCPDF
- 11. Park CH, Lizarraga AD, Lee YT, Yoon KJ, Yoo TK. Increased carcinoembryonic antigen (CEA) level is highly associated with low skeletal muscle mass in asymptomatic adults: a population-based study. J Clin Med 2022;11:5009.ArticlePubMedPMC
- 12. Yoo TK, Park SH, Park SJ, Lee JY. Impact of sex on the association between flexibility and arterial stiffness in older adults. Medicina (Kaunas) 2022;58:789.ArticlePubMedPMC
- 13. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–96.ArticlePubMed
- 14. Corteville DC, Bibbins-Domingo K, Wu AH, Ali S, Schiller NB, Whooley MA. N-terminal pro-B-type natriuretic peptide as a diagnostic test for ventricular dysfunction in patients with coronary disease: data from the heart and soul study. Arch Intern Med 2007;167:483–9.ArticlePubMedPMC
- 15. Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int J Mol Sci 2019;20:1820.ArticlePubMedPMC
- 16. Hamasaki H. The effects of exercise on natriuretic peptides in individuals without heart failure. Sports (Basel) 2016;4:32.ArticlePubMedPMC
- 17. Selvaraj S, Kim J, Ansari BA, Zhao L, Cvijic ME, Fronheiser M, et al. Body composition, natriuretic peptides, and adverse outcomes in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging 2021;14:203–15.ArticlePubMedPMC
- 18. Koo BK. Assessment of muscle quantity, quality and function. J Obes Metab Syndr 2022;31:9–16.ArticlePubMedPMC
- 19. Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J 2000;14:1345–51.ArticlePubMedPDF
- 20. Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol 2013;304:H358–68.ArticlePubMed
- 21. Kohara K, Ochi M, Tabara Y, Nagai T, Igase M, Miki T. Arterial stiffness in sarcopenic visceral obesity in the elderly: J-SHIPP study. Int J Cardiol 2012;158:146–8.ArticlePubMed
- 22. Maeder MT, Mariani JA, Kaye DM. Hemodynamic determinants of myocardial B-type natriuretic peptide release: relative contributions of systolic and diastolic wall stress. Hypertension 2010;56:682–9.ArticlePubMed
- 23. Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163–8.ArticlePubMed
- 24. Maffei S, Del Ry S, Prontera C, Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci (Lond) 2001;101:447–53.ArticlePubMed
- 25. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89:465–71.ArticlePubMed
- 26. Park JJ, Lee CJ, Park SJ, Choi JO, Choi S, Park SM, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail 2021;3:224–36.ArticlePubMedPMCPDF
- 27. Lee MJ, Ha KH, Kim DJ, Park I. Trends in the incidence, prevalence, and mortality of end-stage kidney disease in South Korea. Diabetes Metab J 2020;44:933–7.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 KES
KES

 PubReader
PubReader ePub Link
ePub Link Cite
Cite