Recent Advances in Diagnostic Strategies for Diabetic Peripheral Neuropathy
Article information
Abstract
Diabetes is an increasing epidemic in Korea, and associated diabetic peripheral neuropathy (DPN) is its most common and disabling complication. DPN has an insidious onset and heterogeneous clinical manifestations, making it difficult to detect high-risk patients of DPN. Early diagnosis is recommended and is the key factor for a better prognosis and preventing diabetic foot ulcers, amputation, or disability. However, diagnostic tests for DPN are not clearly established because of the various pathophysiology developing from the nerve injury to clinical manifestations, differences in mechanisms according to the type of diabetes, comorbidities, and the unclear natural history of DPN. Therefore, DPN remains a challenge for physicians to screen, diagnose, follow up, and evaluate for treatment response. In this review, diagnosing DPN using various methods to assess clinical symptoms and/or signs, sensorineural impairment, and nerve conduction studies will be discussed. Clinicians should rely on established modalities and utilize current available testing as complementary to specific clinical situations.
INTRODUCTION
Diabetes is an important health problem for the Korean population, as it affected 11.0% of adults ≥30 years of age in 2013 [1]. Patients with diabetes have higher rates of premature death, functional disability, and coexisting illnesses compared with those of healthy subjects. Among the serious and deleterious complications of diabetes, diabetic foot ulcers and amputations are the most critical complications, even though most cases are preventable [23]. Diabetic peripheral neuropathy (DPN) can be generally separated into distal symmetric peripheral neuropathies and asymmetric (focal and multifocal) neuropathies (e.g., multiple mononeuropathy, lumbosacral, thoracic, and cervical radiculoplexus neuropathies) [4]. Progressive loss of nerve fibers and resulting instability produce a wide range of clinical manifestations, which differ in their symptoms according to the type of diabetes, population, co-morbidities, and clinical course [5]. Despite the development of diagnostic methods and therapeutic modalities, DPN is not diagnosed or managed properly in some patients. In addition, no reliable estimates are available for the frequency of DPN in different populations, because clear diagnostic guidelines are not existed [2]. Unfortunately, gold standard methods from clinical trials are not useful in the clinical setting, because they are time consuming and require trained specialists and special devices [6]. Moreover, the sensitivity and specificity of different DPN diagnostic methods, as well as their threshold values, have not been determined uniformly.
In this review, the clinical implications of an early diagnosis of DPN and the importance of assessing the signs and symptoms of DPN in patients with diabetes are discussed, as neurological examinations are often omitted in this group of patients. Strategies for diagnosing DPN recommended by expert groups including the Korean Diabetes Association (KDA) that are helpful to clinicians in clinical practice to diagnose DPN are discussed.
DIABETES AND DPN
DPN is "the presence of symptoms and/or signs of peripheral nerve dysfunction in patients with diabetes after excluding other causes" [7]. Thus, DPN should be diagnosed by clinical evaluation, because the absence of symptoms does not always imply the absence of signs. Other causes associated with symptoms or signs mimicking DPN should always be excluded, such as neurotoxins and heavy metal poisoning, alcohol abuse, vitamin B12 deficiency, hypothyroidism, renal disease, chronic inflammatory demyelinating polyradiculoneuropathy, inherited neuropathies, and vasculitis [7].
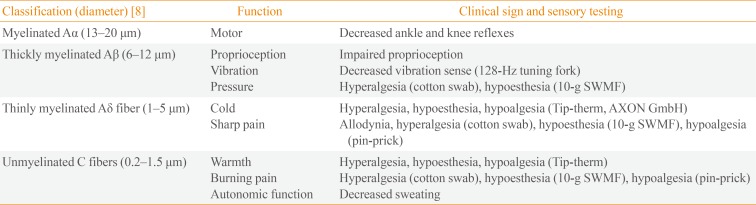
DPN occurs in both patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) because of chronic hyperglycemia. Its onset is usually insidious, so many patients remain asymptomatic for a long period of time, and patient complaints or a routine screening examination often raise suspicion. Peripheral nerve fibers are classified into large myelinated Aβ-fibers (proprioception, vibration, and pressure) and small thinly myelinated Aδ-fibers and unmyelinated C-fibers (warm and cold input and noxious input with high threshold). DPN involves either or both the small and large nerve fibers in limbs in a length-dependent pattern (Table 1) [89]. Small nerve fibers injuries occur earlier [10] than do large ones [11]. Although the pathophysiology of DPN remains unclear, peripheral mechanisms have been suggested, such as neural ion channels (Na+ and Ca2+), abnormal glycemic flux-related damage to the spinal cord, and central mechanisms, such as impaired central pain processing secondary to functional and structural brain remodeling [12].
DPN is the most common and earliest complication of diabetes that was found to affect 33.5% of patients with T2DM at multiple hospitals across Korea (n=3,999), compared with retinopathy (21.0%) and nephropathy (15.7%) [2]. Painful DPN was reported among 14.4% of that population and was associated with decreased quality of life (QOL) and sleep disturbance [3].
Increased vibration and thermal thresholds occur early during the course of the disease in both patients with T1DM and T2DM [11]. However, DPN symptoms may develop much earlier in the course of T1DM than in T2DM [13], suggesting a difference in the natural history or different mechanisms of nerve injury in DPN between diabetes types: predominant involvement of small nerve fibers in patients with T2DM versus large myelinated fibers in patients with T1DM, respectively, even in those with a similar severity of neuropathy [14]. These differences were supported by large interventional studies that explored the effects of intensive glucose control on microvascular outcomes; patients with T1DM and T2DM responded differently to enhanced glucose control [15]. More intensive treatment of hyperglycemia in patients with T1DM leads to a substantially lower incidence of neuropathy [16], whereas it has a minimal effect on preventing neuropathy in patients with T2DM [17]. The large effect of glucose control in patients with T1DM suggests that hyperglycemia is the primary driver of nerve injury, whereas the lack of this effect in patients with T2DM suggests that factors other than hyperglycemia are important, including obesity, hypertension, low high density lipoprotein concentrations, and hypertriglyceridemia, which possibly contribute to nerve injury and often cluster together with diabetes. However, the precise temporal sequence should be clarified in further prospective studies.
IMPORTANCE OF EARLY DETECTION
Half of the patients with DPN are asymptomatic. Typical symptoms of DPN are symmetric numbness, paresthesia, or pain in the distal lower limbs involving more than a single nerve distribution, which progresses in a centripetal direction [6]. Symmetrical sensory loss ("stocking or glove sensory loss") in the feet, above the ankles, and in the hands is evident on clinical examination. The ankle and Achilles reflexes are usually reduced or absent, which can result in foot abnormalities. These symptoms collectively result in disturbed proprioception and abnormal muscle sensory function. However, many patients with DPN have trouble describing their symptoms accurately, which confounds the results of clinical trials and comparisons of drug efficacy. Therefore, validated and quantified measures applicable to Korean patients must be developed to assess the nature and extent of DPN at the early stage.
Painful DPN has a negative impact on physical and mental QOL compared with non-painful DPN. Patients with painful DPN have significantly decreased QOL scores because of pain and impaired balance and mobility. Pain has a large effect on QOL, including quality of sleep, mood, energy, and mobility. Therefore, an early diagnosis of DPN is critical for a good prognosis, and timely comprehensive care can help prevent falls and reduce the negative impacts on patients' QOL [3].
No clinical test is available to identify or predict the development or worsening of symptoms in patients at risk of or with DPN. In addition, no consensus exists for the precise algorithm of medications to treat DPN, and the only known effective disease modifying treatment for DPN is enhanced glucose control [15]. Therefore, identifying patients during the early course will provide a window to identify targeted therapy to modify the course of DPN.
An observational study in Korea indicated that only 12.6% patients with DPN are aware of their disease as a complication of diabetes, and a greater proportion of patients with DPN are not receiving treatment even though they are more likely to develop foot ulcers [2]. Painful DPN is under-recognized and undertreated, indicating that an early diagnosis provides an opportunity for improved patient care.
SCREENING AND DIAGNOSTIC MODALITIES FOR DPN
Assessments of pressure sensation, vibration, thermal, and pain thresholds are used as screening tools for patients "at risk" for foot ulcerations [6]. Although there is a lack of uniform guidelines on diagnosis and interpretation of the results from a neurological examination, it is generally accepted that DPN should be diagnosed based on more than one diagnostic test rather than on one symptom, sign, or test alone [7].
Assessment of risk factors for DPN
DPN develops with chronic hyperglycemia and subsequent potential mechanisms induced by chronic hyperglycemia, including oxidative injury, activation of the polyol glucose metabolic pathway, deposition of advanced glycosylation end-products within nerves, and vascular insufficiency. A number of studies have reported that DPN is related to diabetes duration, hyperglycemia, current cigarette smoking, dyslipidemia, and hypertension [1819]. In agreement with these observations, the results from a nationwide survey conducted by the Diabetic Neuropathy Study Group of the KDA in 2010 showed that older age, female sex, long duration of diabetes, presence of retinopathy, hypertension, or dyslipidemia, hyperglycemic status (i.e., being treated with insulin), and history of cerebrovascular disease or foot ulcers are independently associated with DPN [2].
Assessment of patients' symptoms and/or signs using scoring systems
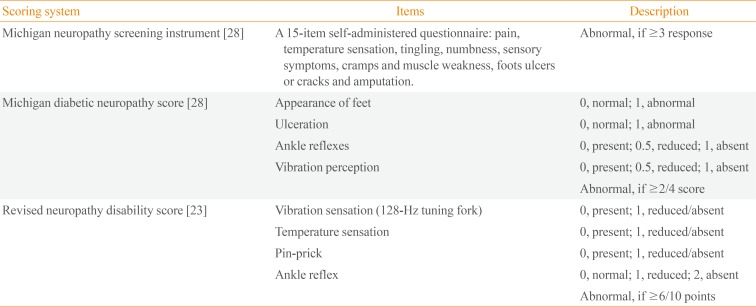
Composite scoring systems that use symptoms, signs, or both have been developed to quantify general neuropathic deficits, such as the neurological symptom score of Dyck [20], the neuropathy symptom profile [21], and the neuropathy disability score (NDS) [21]. The DPN-specific symptom scoring system, the diabetic neuropathy symptom score [22], the modified NDS, which uses a sensory score (vibration perception threshold using a 128-Hz tuning fork and temperature perception, pin-prick) and reflex score (Achilles reflex) [23], neuropathy impairment score (NIS) [24], NIS of the lower limbs [25], a diabetic neuropathy examination [26], and the Toronto clinical neuropathy score are now available [27]. The Michigan neuropathy screening instrument (MNSI) for outpatients questionnaire has high specificity of 92%, and the Korean version has been validated [228]. The Michigan diabetic neuropathy score (MDNS) along with the MNSI score includes assessments of foot deformities and clinical sensory nerve tests to score DPN [28]. The Neuropathy Study Group of the KDA recommends the MNSI, MDNS, and modified NDS to evaluate patients with diabetes (Table 2). These scoring systems enhance diagnostic accuracy by combining the results of individual examinations. The severity of neuropathic pain and response to treatment in patients with painful DPN can be assessed using a visual analog scale or valid scales and questionnaires, such as the Korean version of the Brief Pain Inventory [3].
Assessment of sensorineural impairment
Clinical sensory nerve tests conducted at bedside use various devices to generate specific physical vibratory (128-Hz tuning fork) [29], pressure (10-g Semmes-Weinstein monofilament) [30], noxious (pin-prick) [31], or thermal stimuli (Tip-therm, AXON GmbH, Dusseldorf, Germany) [32], which deliver electrical signals along the sensory pathway (Table 1, Fig. 1) [333435].
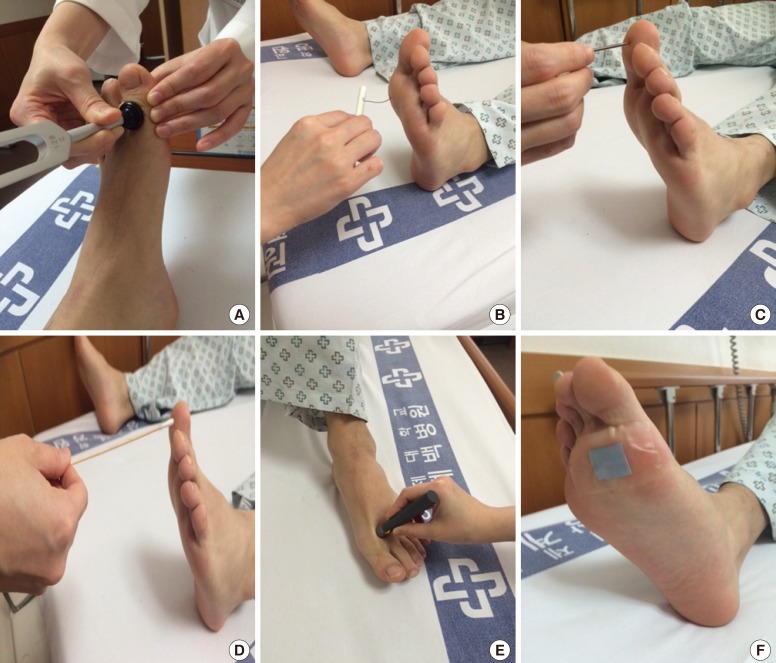
Bedside neurological and sensory nerve testing. (A) Vibration. Patients are notified when they cannot feel the vibrations from a 128-Hz tuning fork (first interphalangeal joint of the great toe) when the toes are extended, and the investigator feels the vibration and measures the time when the feeling disappeared. A time difference ≥10 seconds between the investigator and the patient is considered abnormal [33]. (B) Pressure: 10-g monofilaments are pressed on 10 points on the sole and dorsum of the feet until the monofilament begins to bend (100 mN). If the patient has sensation in fewer than seven points, the results is considered abnormal [33]. Four sites per foot, such as the hallux and metatarsal heads 1, 3, and 5, should be screened [4]. (C) Noxious stimuli and (D) light touch. The patient is touched on the foot using a sterile pin, toothpick, and cotton wisp and asked to identify a "sharp or dull" or "light touch" with their eyes closed [33]. (E) Warm/cold. Tip-therm (temperature discriminator; AXON GmbH) is a pen-like device with a plastic cylinder on one end and a metal cylinder on the other end, which is applied to the dorsum of each foot at irregular intervals so patients can identify the sensation as cold or not with their eyes closed [32]. (F) Sudomotor function. Indicator tests (Neuropad, miro Verbandstoffe) are applied to both soles at the level of the first and second metatarsal heads. The time to color change from blue to pink is more than 10 seconds; the result is considered abnormal [34].
Quantitative sensory testing (QST) is a quantitative method usually graded using a continuous numerical scale to detect the threshold of thermal perception (cold or warm), vibration perception, current perception, pressure pain, and sudomotor function (Table 1) [2829323637]. Vibration thresholds are particularly sensitive to detect mild or subclinical neuropathy and correlate well with other QST measures [4]. The current perception threshold (CPT) to 2,000-Hz stimulation is correlated best with vibratory thresholds, and the CPT to 5-Hz stimulation is correlated with thermal perception [37]. Sweating abnormalities may be an early manifestation of DPN. Sudoscan is a novel method to detect electrochemical skin conductance, which is proportional to the number of functional sweat glands [36].
While a sensory stimulus is an objective physical event, the response is highly subjective and depends on the examiner's experience, patient's cooperation, and confounding factors (age, sex, obesity, and smoking or alcohol consumption). This differs from an electrophysiological study of nerve conduction velocity (NCV) in which the stimulus generates evoked stimuli independent of the subjective response [38]. The QST is probably effective for documenting sensory abnormalities and changes in sensory thresholds during a longitudinal evaluation of patients with DPN. The Rochester Diabetic Neuropathy Study reported that "the QST should not be used as the sole criterion for diagnosing DPN but should be accompanied by at least one other defined abnormality before the diagnosis of DPN can be made"; therefore, the QST should be complementary to a thorough clinical assessment [39]. Although the QST has been used in clinical practice and clinical trials, it is not extensively used in clinical practice in Korea. Further studies are needed to develop the standardized test procedure, QST algorithms, and reference values from healthy test subjects.
Role of electrophysiological studies
The nerve conduction study (NCS) is a reliable and objective diagnostic method to evaluate the DPN treatment response [40]. The pathological findings of DPN are axonal loss, axonal regeneration, and demyelination in some patients [4142]. The NCV is used to detect slowing of nerve conduction in nerve axons resulting from segmental demyelination and to measure the speed of both motor and sensory conduction, amplitude, distal latency, distance, F wave latency, and other factors [41]. The American Academy of Neurology (AAN) in conjunction with the American Association of Electrodiagnostic Medicine and the American Academy of Physical Medicine and Rehabilitation has a recommended protocol for NCS [40], which includes unilateral studies of sural, ulnar, and medial sensory nerves and peroneal, tibial, medial, and ulnar motor nerves with F waves with a minimum case detection criterion to confirm distal symmetric polyneuropathy for clinical research; "an abnormality (≥ 99th or ≤1st percentile) of any nerve conduction attribute in two separate nerves, one of which must be the sural nerve." The Nerve Conduction Criteria Study indicated subclinical distal symmetric polyneuropathy to be clinically acceptable if "≥1 among abnormal attributes in ≥2 separate nerves" for clinical practice [43]. Although a NCS is regarded as the gold standard in clinical research, it is not useful in clinical practice because it is time consuming, requires special devices and trained examiners, and has no general consensus regarding its criteria, even after multiple investigations [40]. In addition, a NCS is sensitive enough to detect abnormalities in large nerve fibers but is not sensitive enough to detect to small nerve neuropathy, which is the earliest detectable sign of DPN [44].
Challenging modalities for diagnosing DPN
A nerve biopsy, typically of the sural nerve, is rarely used in clinical practice, due to its invasiveness [6]. However, a skin biopsy assessment of intraepidermal nerve fiber density (unmyelinated C fibers and small myelinated fibers) has been proposed as a valid method to detect early small nerve neuropathy, even when signs of DPN are minimal or absent and when myelinated nerve fiber morphology is still normal [45]. A nerve biopsy may detect pathological changes in small nerve fibers and correlate well with the structural pathology of axons. However, this procedure is invasive to patients and normal reference value in Korean subjects must be established.
Corneal confocal microscopy is used to assess the pathology of the corneal subbasal plexus of nerve fibers originating from the ophthalmic division of the trigeminal nerve, particularly corneal nerve fiber length, which is highly reproducible [46]. This noninvasive technique is sensitive to detect corneal nerve fiber damage during the earlier stages of DPN, and the extent of corneal nerve damage and repair correlates with peripheral nerve function. The nerve fiber regeneration response to therapeutic intervention enables documentation of the natural history of DPN in patients at follow-up [47]. This method, however, needs expensive device and expert examiner for examination.
EVOLVING STRATEGIES FOR DIAGNOSING DPN
The consensus regarding the diagnosis of DPN has changed since 2004. The AAN recommends a NCV with both neuropathic symptoms and signs as confirmation of DPN [40]. Then, the European Federation of Neurological Societies proposed a skin biopsy and intraepidermal nerve fiber density as sensitive measures to detect small nerve neuropathy [48]. Lastly, the Toronto consensus panel defined "possible" neuropathy as having symptoms, signs, or abnormal reflexes, "probable" as having any two or more of symptoms, signs, or abnormal reflexes, "confirmed" as having either symptoms or signs and NCS results or skin biopsy, and "subclinical" as having neither symptoms nor signs but rather abnormal nerve conduction or a validated measure of small nerve neuropathy. In addition, they recommended severity assessments using a staged approach based on the nerve conduction abnormality (Fig. 2) [5643].

Definition and severity assessment of diabetic peripheral neuropathy (DPN) proposed by the Toronto Diabetic Neuropathy Expert Group. Numbers in each column refer to the definitions of the minimal criteria for DPN, and the number in parentheses is the stage of severity: 1 ("possible"), 2 ("probable"), or 3 ("confirmed") for clinical practice and 3 or 4 ("subclinical") for research studies [6]. Severity is staged based on the symptoms, signs, and nerve conduction (NC) abnormalities: stage 0, no NC abnormality; 1a, subclinical but without symptoms or signs; 1b, subclinical with signs but no symptoms; 2a, subclinical with symptoms regardless of signs; and 2b (not shown here), subclinical with unequivocal weakness of ankle dorsiflexion [5].
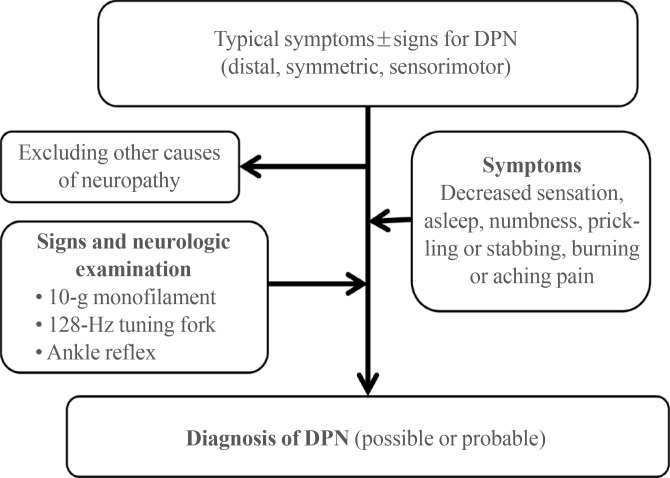
The KDA guidelines recommend screening for DPN regularly at patient's visits, after a diagnosis of diabetes. In agreement with the American Diabetes Association's recommendation [49], all patients should be screened for DPN at the time of the T2DM diagnosis and 5 years after a T1DM diagnosis and at least annually thereafter. The KDA also recommends that physicians perform a foot examination at each visit to inspect the feet for deformities, cracks, ulcerations, and wounds in addition to sensation. DPN should be screened by surveys for neu-ropathic symptoms and signs, including the MNSI, clinical sensory nerve tests, the QST, and ankle and Achilles reflexes. Specifically, the KDA recommends screening via the 10-g monofilament, vibration perception clinical bedside tests using a 128-Hz tuning fork, and assessment of ankle reflexes (Fig. 3) [7]. An assessment of distal pulses is also recommended, and the ankle brachial index should be measured if peripheral arterial disease needs to be evaluated.
CONCLUSIONS
Early diagnosis of DPN is critical for successful management of diabetes and preventing DPN-related patient and social disease burdens. Hyperglycemia and other risk factors should be controlled in patients during the early stages of DPN. In addition, symptoms and/or signs must be assessed, and composite scoring systems, the QST, as well as a NCS are complementary to diagnose DPN in patients with diabetes. Further research is needed to investigate the role of the QST and NCS for early detection and predicting DPN in Korean patients with T2DM. Although the diagnosis is clearly improving with current diagnostic tools, longitudinal investigations are needed to better understand the significance of abnormalities detected by each modality, including emerging new methods and their cost-effectiveness.
ACKNOWLEDGMENTS
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020224).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.