Stepwise Approach to Problematic Hypoglycemia in Korea: Educational, Technological, and Transplant Interventions
Article information
Abstract
Impaired awareness of hypoglycemia has been found to be prevalent in 20% to 40% of people with type 1 diabetes. If a similar prevalence exists in Koreans with type 1 diabetes, at a minimum, thousands of people with type 1 diabetes suffer at least one unpredicted episode of severe hypoglycemia per year in Korea. For patients with problematic hypoglycemia, an evidence-based stepwise approach was suggested in 2015. The first step is structured education regarding multiple daily injections of an insulin analog, and the second step is adding a technological intervention, such as continuous subcutaneous insulin infusion or real-time continuous glucose monitoring. The next step is a sensor-augmented pump, preferably with a low glucose suspension feature or very frequent contact, and the final step is islet or pancreas transplantation. In Korea, however, none of these treatments are reimbursed by the National Health Insurance, and thus have not been widely implemented. The low prevalence of type 1 diabetes means that Korean physicians are relatively unfamiliar with the new technologies in this field. Therefore, the roles of new technologies and pancreas or islet transplantation in the treatment of problematic hypoglycemia need to be defined in the current clinical setting of Korea.
INTRODUCTION
Impaired awareness of hypoglycemia has been found to be prevalent in 20% to 40% of people with type 1 diabetes [12]. A prospective study has shown that 19 of 29 patients (66%) with impaired awareness of hypoglycemia had at least one episode of severe hypoglycemia per year, with an overall incidence of 2.8 episodes per patient-year [3]. Although no population-based epidemiological studies of type 1 diabetes in Korea have been conducted, the prevalence of type 1 diabetes has been estimated to be 0.22% to 1.19% of total number of diabetes patients in an analysis of the National Health Insurance Database [4]. Although the relative prevalence of type 1 diabetes in Korea is lower than is found in Western countries, at a minimum, thousands of people with type 1 diabetes must suffer at least one unpredicted episode of severe hypoglycemia per year if the proportion of patients with impaired awareness of hypoglycemia is similar in Korean patients with type 1 diabetes to what has been observed in Western countries.
In 2015, an evidence-based clinical recommendation for the management of problematic hypoglycemia, defined as two or more episodes of severe hypoglycemia or one or more episode of severe hypoglycemia associated with impaired awareness of hypoglycemia, was published [2]. In this statement, the first step is structured education regarding multiple daily injections (MDIs) of an insulin analog or hypoglycemia-specific education, while the second step includes continuous subcutaneous insulin infusion (CSII) or MDI with real-time continuous glucose monitoring (RT-CGM). The next step is the use of a sensor-augmented pump with or without a low glucose suspension feature or very frequent contact (weekly for 3 to 4 months), and the final step is islet or pancreas transplantation.
In Korea, however, none of these treatments, except for insulin analogs themselves and associated consumable products such as blood glucose test strips, are reimbursed by the National Health Insurance. In a recent survey conducted in five tertiary referral hospitals in Seoul, the proportion of patients with type 1 diabetes who used CSII was only ~5%, and an almost negligible proportion used RT-CGM [5]. Moreover, approximately 25% of the patients with type 1 diabetes only used either basal insulin or premixed insulin without using MDI or CSII [5]. This is in contrast to the results of the T1D Exchange Clinic Registry in the United States, in which the proportions of patients using CSII and RT-CGM were 50% and 6%, respectively [6]. The lower proportion of patients with type 1 diabetes adopting recent technological advances in Korea may in part be explained by lack of reimbursement by National Health Insurance and the low prevalence of type 1 diabetes in Korea, which means that Korean physicians are relatively unfamiliar with the new technologies in this field. Therefore, the roles of each therapy (RT-CGM, CSII, sensor-augmented insulin pumps [SAPs], and pancreas or islet transplantation) in the stepwise management of problematic hypoglycemia need to be defined in the current clinical setting of Korea.
EDUCATIONAL AND TECHNOLOGICAL INTERVENTIONS
Structured education for the active adjustment of insulin doses with frequent self-monitoring of blood glucose (SMBG), carbohydrate counting, and additional considerations for exercise, alcohol, and illness has been proven to enable a sustained reduction in the incidence of severe hypoglycemia by 50% to 70% [2789]. These interventions typically require a 30- to 40-hour group learning curriculum [8], or eight weekly sessions of a psychoeducational program [9], which can be challenging to implement in clinical practice. Recently, a brief, partially web-based group intervention, HypoAware, was found to be effective in patients with problematic hypoglycemia; it lead to fewer severe hypoglycemic episodes, significantly improved hypoglycemia awareness, and less hypo-distress in comparison with usual care, and deserves further dissemination [10]. HypoAware consists of three group sessions of 2.5 hours over the course of 4 weeks, combined with two online modules in the weeks between meetings, and for this reason is much more clinically feasible than the original Blood Glucose Awareness Training interventions [11].
Following structured education regarding the MDI of insulin analogs, the second step has been suggested to be either CSII with SMBG, or MDI with RT-CGM [2]. In the second step, it has been suggested that more solid evidence is available regarding CSII with SMBG for patients with problematic hypoglycemia [2].
Recently, a randomized crossover trial proved the efficacy of RT-CGM in patients with an impaired awareness of hypoglycemia. This study documented re-education regarding diabetes management after a 6-week run-in period. In the RT-CGM phase, a significant increase of ~10% in the time spent in normoglycemia (~55% in the SMBG phase vs. ~65% in the real-time phase) and a reduction in both CGM-measured hypoglycemia and severe hypoglycemia were achieved [12]. As in both well controlled and poorly controlled type 1 diabetes, evidence on the efficacy of RT-CGM is expected to accumulate in patients with problematic hypoglycemia.
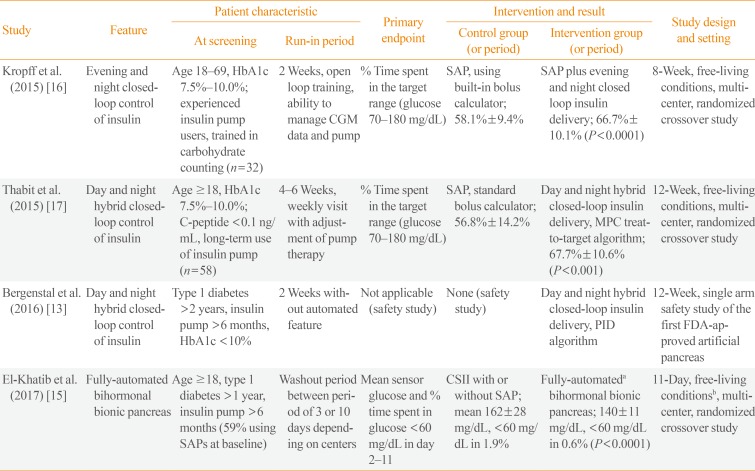
The third step is the combined use of CSII and RT-CGM, in the form of a SAP, preferably with the low glucose suspension feature [2]. Beyond the SAP, which is currently the best tool for the medical care of problematic hypoglycemia, a growing body of evidence supports the superiority of closed-loop insulin delivery (also known as an artificial or bionic pancreas) over SAP in patients with type 1 diabetes. In late 2016, the Food and Drug Administration in the United States approved the first commercially available hybrid artificial pancreas [13]. Although this device still requires the patient's input of carbohydrate counting and calibration of RT-CGM by SMBG, the device partly compensated for the inaccuracy of the patient's carbohydrate count [14]. The superiority of this device over SAP has not been investigated in a randomized controlled trial. Recently, a fully automated bihormonal artificial pancreas, which does not require the input of information about carbohydrate counting, has been investigated in an outpatient setting [15]. However, the superiority of an artificial pancreas over the SAP in problematic hypoglycemia should be determined by further studies specifically including patients with problematic hypoglycemia. The results of major clinical trials of artificial pancreas technologies are summarized in Table 1 [13151617].
TRANSPLANT INTERVENTIONS
The proportion of patients with insulin independence after allogeneic pancreas and islet transplantation is around 70% and 25% to 50%, respectively [18192021]. However, recent international cohort studies have reported that the proportion of recipients with fasting C-peptide levels of >0.3 ng/mL, corresponding to optimal glycemic control without severe hypoglycemia, was approximately 70% at 5 years after allogeneic islet transplantation, which is similar to the results found after pancreas transplantation [1920]. Therefore, both islet and pancreas transplantation are equally efficient for abolishing severe hypoglycemia. Several cases with partial islet graft function have been reported in Korea [2223], with the first case of allogeneic islet transplantation being reported in 1999 [23]. A single-center retrospective study in Korea has also shown pancreas transplantation to have favorable outcomes, comparable to those reported in Western countries [24].
The choice of pancreas or islet transplantation should be individualized. The higher long-term insulin independence associated with pancreas transplantation and the very low procedural complication rate and low mortality rate of islet transplantation should be balanced according to the characteristics of the recipient. The typical recipient criteria for pancreas transplantation are being non-obese, being <50 years of age, and not having coronary artery disease. Islet transplantation can be used in recipients who fail to meet the above criteria for pancreas transplantation, and also can also be used complementarily for donors with certain characteristics, such as those who are obese and older donors unsuitable for the donation of the whole pancreas.
CONCLUSIONS
Problematic hypoglycemia should be treated by a stepwise approach, including structured education, as well as technological and transplant interventions. In Korea, none of the educational and technological interventions for problematic hypoglycemia are reimbursed by the National Health Insurance, and low awareness about the management of type 1 diabetes among both physicians and society prevents their widespread use in the clinic. However, this national reimbursement policy, providing no choice other than MDI with SMBG, is against the abundant international evidence regarding the efficacy of various educational and technological interventions. On the other hand, the annual number of organ donations is gradually increasing, and now more than 500 donated pancreases are available each year. Since the use of donated pancreases for pancreas transplantation is far less than the availability of donated pancreases, and the optimal donor characteristics for pancreas and islet transplantation are complementary to each other, the role of islet transplantation will continue even in the future.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.