Bisphenols and Thyroid Hormone
Article information
Abstract
In recent decades, attention has been directed toward the effects of bisphenol A (BPA) on human health. BPA has estrogenic activity and is regarded as a representative endocrine disruptor. In addition, mounting evidence indicates that BPA can disrupt thyroid hormone and its action. This review examined human epidemiological studies to investigate the association between BPA exposure and thyroid hormone levels, and analyzed in vivo and in vitro experiments to identify the causal relationship and its mechanism of action. BPA is involved in thyroid hormone action not only as a thyroid hormone receptor antagonist, but also through several other mechanisms. Since the use of bisphenols other than BPA has recently increased, we also reviewed the effects of other bisphenols on thyroid hormone action.
INTRODUCTION
Bisphenol A (BPA, 4,4′-isopropylidenediphenol) is used to manufacture polycarbonate plastic and epoxy resins. BPA is widely used in a variety of applications, including baby bottles, food can lining, food packaging, and dental sealants [1]. Ingestion of BPA-containing food is thought to be the primary source of human exposure. BPA is a high-production-volume chemical, and the estimated production of BPA in the United States was approximately 1 million tons in 2004 [2]. As a result, human exposure to BPA is very extensive [3]. BPA is a well-known endocrine-disrupting chemical, and its estrogenic activity was documented in the early stages of its use (1960s). Considering its widespread use and potential harmful effects on human health, especially on reproduction, the use of BPA has been regulated. The United States Environmental Protection Agency has established a reference dose of 50 µg/kg/day and the European Food Safety Authority has set a temporary tolerable daily intake of 4 µg/kg/day [4]. In particular, BPA has been banned from baby bottles in many countries. As concerns about public health and regulations limiting BPA use have increased, the use of other bisphenols as BPA substitutes has become more widespread.
Recently, studies on BPA have increased exponentially, revealing that BPA has other endocrine-disrupting properties in addition to its estrogenic activity. This review focuses on the thyroid-disrupting effects of bisphenols, including BPA.
BPA AND THYROID FUNCTION
Thyroid hormone is essential for development, growth, and metabolism, and plays an especially important role in neurodevelopment. Therefore, alterations of thyroid hormone function can interfere with these vital functions. Thyroid hormones, such as thyroxine (T4), triiodothyronine (T3), and thyroid-stimulating hormone (TSH), can be easily measured in the blood. First, we reviewed the published literature on the association between BPA exposure and thyroid hormone.
Thyroid hormone in humans
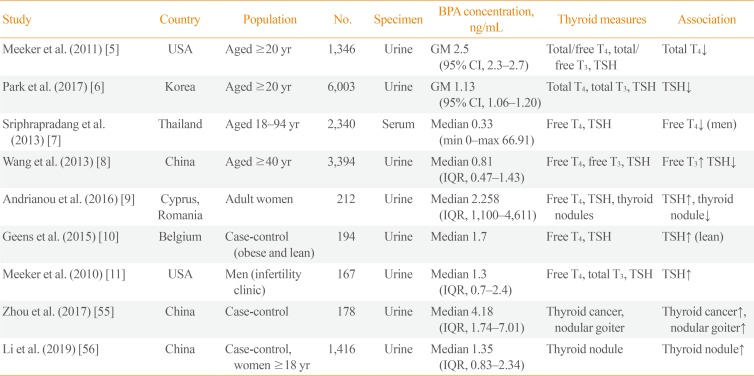
BPA exposure in humans can be evaluated by measuring urinary BPA concentrations. Previous research has demonstrated that BPA is detected in most members of the population [3], and BPA exposure has been found to be associated with thyroid hormone levels [5678]. Several large-scale epidemiological cross-sectional studies have been conducted (Table 1). Urinary BPA concentrations were negatively associated with total T4 in the United States National Health and Nutrition Examination Survey (NHANES) 2007 to 2008 [5]. Urinary BPA concentrations were negatively correlated with TSH levels in Korean National Environmental Health Survey 2012 to 2014 [6]. Serum BPA concentrations had a negative correlation with free T4 in men in the Thai National Health Examination Survey (NHES) 2009 [7]. Urinary BPA concentrations were related to increased free T3 and decreased TSH levels in Chinese adults [8]. Small-scale studies have also shown an association between BPA and TSH [91011]. The leading cause of thyroid dysfunction is autoimmune disease, and the Thai NHES reported that serum BPA concentrations were positively associated with thyroid peroxidase (TPO) antibody positivity [12]. These findings suggest that BPA can induce thyroid autoimmunity, resulting in thyroid dysfunction. However, in the study conducted in China, there was no association between urinary BPA concentrations and thyroid autoantibodies [8].
In pregnant women, BPA exposure can affect thyroid hormone levels [13141516]. Because thyroid hormone plays a pivotal role in fetal neurodevelopment, maternal BPA exposure has a greater clinical significance than exposure in the general population. BPA exposure during pregnancy can affect thyroid hormone levels in newborns (Table 2). A prospective pregnancy and birth cohort study in the United States, reported that urinary BPA concentrations in pregnant women were inversely correlated with TSH levels in boys [16]. Another prospective study also showed an inverse association between maternal urinary BPA concentrations and TSH in girls [17]. Even after birth, maternal BPA exposure can affect children's thyroid hormone levels through breastfeeding [18]. However, some cross-sectional studies found no association between BPA exposure and thyroid hormone in cord blood samples [192021].
Human studies have some limitations. First, a single measurement of BPA in a spot urine sample may not be representative of overall BPA exposure. Because BPA has a short half-life, it leaves the body rapidly and does not bioaccumulate [22]. To assess BPA exposure properly, repeated BPA measurements are needed, but it is difficult to obtain serial BPA measurements in real-world circumstances. Second, the causal relationship between BPA exposure and thyroid hormone changes remains unclear, and is difficult to elucidate. Most of the studies were cross-sectional, and only two studies were longitudinal. Next, humans are exposed to numerous chemicals at once, and several chemicals share similar exposure sources [23], so the findings of those studies may reflect a mixed effect, rather than the effects of BPA alone, which could lead to false positive conclusions. Therefore, the association between BPA and thyroid function in humans reported in the literature is still inconclusive.
Thyroid hormone in animals
To supplement the limitations of human epidemiological studies, several animal experiments have been conducted. BPA was administered directly to animals, and thyroid hormone levels were measured. BPA exposure (40 mg/kg, 15 days, orally) in adult rats increased T4 levels [24]. Neonatal exposure to BPA (2.5 to 6.2 mg/kg, 10 days, subcutaneously) decreased T4 levels and increased TSH levels in adulthood [25]. Maternal exposure to BPA in rats can affect thyroid hormone in the offspring. Zoeller et al. [26] reported that maternal BPA exposure during pregnancy and lactation (1 to 50 mg/kg, orally) increased T4 levels in the offspring (postnatal day [PND] 15). Xu et al. [27] reported that maternal BPA exposure induced a transient increase in T4 levels (PND 7), followed by a decrease of T4 (PND 21) in male offspring. However, other researchers reported that perinatal exposure to BPA (0.0025 to 40 mg/kg, orally or subcutaneously) did not alter TSH and T4 levels in offspring [28293031]. The inconsistent results of rat experiments may be due to different doses, windows of exposure, and routes of exposure to BPA. BPA-induced thyroid hormone changes have also been observed in mice and zebrafish. BPA exposure during puberty decreased T4 levels in mice [32]. BPA exposure to zebrafish larvae increased T3 levels [33]. All these experiments indicate that BPA could affect thyroid function, but the effects might vary according to the route, dose, duration, or age at exposure.
MECHANISM OF BPA
Thyroid hormone is synthesized in the thyroid gland under the regulation of TSH released from the pituitary gland. Synthesized thyroid hormone binds to proteins and circulates in the blood. At the target organ, thyroid hormone binds to the thyroid hormone receptor (TR) and stimulates thyroid hormone signaling pathways. Subsequently, thyroid hormone is metabolized to its inactive form in the liver. We explored the ways in which these processes are disrupted by BPA (Fig. 1) by reviewing the published mechanistic studies.

Chemicals can interfere with thyroid hormone action at several points. (A) The pituitary gland and hypothalamus regulate thyroid hormone synthesis through thyroid-stimulating hormone (TSH) release. (B) Thyroid hormone is synthesized in the thyroid gland. If TSH stimulates thyrocytes, iodine uptake via the sodium iodide symporter (NIS), thyroglobulin (Tg) production, and oxidation by thyroid peroxidase (TPO) occur. (C) Thyroid hormone is carried on binding proteins such as thyroxine-binding globulin (TBG) and transthyretin (TTR). (D) Thyroid hormone is metabolized in the liver by deiodinase (DIO), UDP-glucuronosyltransferase (UGT), or sulfotransferase (SULT) and eliminated in bile. (E) Thyroid hormone binds to the thyroid hormone receptor (TR) in target cells and activates thyroid hormone signaling pathways. T4, thyroxine; T3, triiodothyronine.
Thyroid hormone synthesis
It is possible that BPA acts directly on the thyroid gland, as suggested by the finding that in humans, urinary BPA concentrations were inversely associated with thyroid volume in children [34]. In animal studies, BPA exposure, especially during pregnancy, has been found to alter thyroid gland weight or to change thyroid histology [3536].
In thyroid hormone synthesis, iodine enters thyrocytes via the sodium iodide symporter (NIS), is oxidized by TPO, and is incorporated into tyrosyl residues of thyroglobulin (Tg). BPA exposure has been found to change the expression of genes involved in these processes, such as Slc5a5 (NIS), Tpo, and Tg. For example, BPA treatment increased Tg and Slc5a5 gene expression in zebrafish experiments [333738] and Tshr, Slc5a5, Tpo, and Tg gene expression in FRTL5 cells [373940]. BPA treatment decreased iodide uptake in FRTL5 cells and TPO activity in isolated rat thyroid microsomes [40]. In rats, BPA treatment decreased thyroid iodide uptake and TPO activity [36]. These findings suggest that BPA can inhibit thyroid hormone synthesis.
Regulation by the hypothalamus and pituitary gland
Little is known about BPA-associated changes in the hypothalamus and pituitary gland. BPA exposure (0.1 to 1 µM) did not change Crh or Tshβ gene expression in zebrafish experiments [33]. However, BPA treatment (10 µM) decreased Tshβ, Trα, Trβ, and deiodinase 2 (Dio2) expression in GH3 pituitary cells [39]. Dong and Wade [41] reported that BPA can inhibit thyroid hormone uptake via the thyroid hormone transporter monocarboxylate transporter 8 (MCT8) in the brain.
Thyroid hormone transport
In the blood, thyroid hormone is transported in conjunction with proteins such as thyroxine-binding globulin (TBG) and transthyretin (TTR). BPA can bind TTR [42]. Competitive binding with thyroid hormone transport proteins interferes with thyroid hormone. However, the affinity of BPA for TBG and TTR is weak. Instead, derivatives of BPA such as tetrachlorinate BPA (TCBPA) or tetrabrominated BPA (TBBPA) have a stronger affinity [43]. In addition, the BPA concentrations commonly found in humans are insufficient to interfere with thyroid hormone transport [42].
Thyroid hormone metabolism
Deiodination catalyzed by DIO is important in thyroid hormone metabolism. In rats, BPA treatment reduced hepatic DIO1 activity [24]. BPA exposure (0.1 to 1 µM) increased the expression of Dio1 gene and Ugt1ab gene encoding UDP glucuronosyltrasferase in zebrafish [33].
Thyroid hormone receptor
The structure of BPA and its analogues resembles that of T3 (Fig. 2). BPA can bind TR, particularly the beta isoform of TR (TRβ), and acts as an antagonist [2644], as confirmed in a cell-based reporter gene assay [4546]. TR was inhibited by BPA treatment (10 to 100 µM), where TRβ was at a lower concentration (0.001 to 0.1 µM). BPA was found to inhibit TR-mediated transcription of T3-response genes [47]. These findings suggest that BPA can disrupt the action of thyroid hormone. It is thought that the TR-antagonistic effect of BPA may be the main mechanism through which it disrupts thyroid function.
OTHER BISPHENOLS AND THYROID FUNCTION
Since concerns have been raised regarding BPA from a public health perspective, several BPA substitutes, such as bisphenol F (BPF) and bisphenol S (BPS), have become used with increasing frequency. Because their structures are similar to that of BPA (Fig. 2), it is possible that these bisphenols disrupt thyroid function. However, since these bisphenols are only starting to be used, little research has been conducted on their role in thyroid disruption.
Like BPA, BPF and BPS can bind TRβ and exert antagonistic activity [4849]. In zebrafish, BPF exposure altered T4, T3, and TSH levels and changed the expression of genes including Tg, Ttr, and Ugt1ab [3350].
In zebrafish, BPS exposure decreased T4 and T3 levels and increased TSH levels [5152]. Furthermore, in zebrafish, BPS treatment increased the expression of genes including Ttr and Ugt1ab [3351].
In human, some epidemiological studies have investigated associations between non-BPA bisphenols and thyroid hormone levels, but only in pregnant women. Urinary BPF concentrations were associated with higher free T3 [13] or free T4 levels [53]. Aker et al. [53] reported that urinary BPS concentrations were associated with lower corticotropin-releasing hormone levels, but other studies found no association between BPS and thyroid hormone levels [1354].
BPA AND THYROID NODULES
As BPA became known as a thyroid-disrupting chemical, the association between BPA and thyroid nodules or thyroid cancer emerged as a topic of interest. In case-control studies conducted in China, urinary BPA concentrations in patients with thyroid nodules or thyroid cancer were significantly higher than in the control groups (Table 1) [5556]. However, Andrianou et al. [9] reported that BPA exposure was inversely associated with thyroid nodules. In animal experiments, BPA treatment in F344 rats did not induce thyroid cancer stimulated by N-bis(2-hydroxypropyl) nitrosamine (DHPN) [57]. However, BPA treatment enhanced the susceptibility of thyroid cancer stimulated by DHPN and iodine excess in rats [58]. BPA can induce the proliferation of thyroid cancer cells [59]. Taken together, a link may possibly exist between BPA and thyroid nodules or cancer, but there is a lack of evidence that BPA can induce thyroid nodules or thyroid cancer.
CONCLUSIONS
Here, we reviewed the associations between bisphenols and thyroid function. Several previous studies indicate that BPA affects thyroid hormone action. Considering the results of studies in pregnant women and experiments on perinatal exposure, the effects of BPA on thyroid hormone are thought to be more critical and harmful in the early stages of life. BPA may affect thyroid function through several possible mechanisms of action. First, the main mechanism of action is thought to be binding of BPA to TR and interference with thyroid hormone. However, this review also suggests that BPA can interfere with thyroid hormone synthesis, transport, and metabolism. Recently, this thyroid-disrupting effect was identified for other bisphenols, as well as BPA. Although they were not the primary focus of this review, BPA derivatives such as TCBPA and TBBPA resulting from BPA degradation processes have increasingly been investigated as thyroid-disrupting chemicals [60]. Therefore, attention should be paid to the effects of bisphenols, including BPA, on the thyroid.
ACKNOWLEDGMENTS
The authors would like to thank Jin Hyoung Pyo (Seoul National University Hospital Healthcare System Gangnam Center) for his assistance in drawing the figure. This research was supported by a grant (18182MFDS65) from Ministry of Food and Drug Safety in 2018.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.