Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Maternal Thyroid Dysfunction, and Child Autism Spectrum Disorder
Article information
Abstract
Autism spectrum disorder (ASD), with its high economic and societal costs, is a growing public health concern whose prevalence has risen steadily over the last two decades. Although actual increased incidence versus improved diagnosis remains controversial, the increased prevalence of ASD suggests non-inherited factors as likely contributors. There is increasing epidemiologic evidence that abnormal maternal thyroid function during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders. Prenatal exposure to endocrine-disrupting chemicals such as per- and polyfluoroalkyl substances (PFAS) is known to disrupt thyroid function and can affect early brain development; thus, thyroid dysfunction is hypothesized to mediate this relationship. The concept of a potential pathway from prenatal PFAS exposure through thyroid dysfunction to ASD etiology is not new; however, the extant literature on this topic is scant. The aim of this review is to evaluate and summarize reports with regard to potential mechanisms in this pathway.
INTRODUCTION
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by limited interests, repetitive behaviors, and impaired social interaction and communication [1]. ASD is a growing public health concern in part due to its high economic and societal costs, especially in developed countries [2]. Annual costs (direct medical, direct non-medical, and productivity combined) of ASD in 2025 are projected to reach nearly one-half trillion dollars in the United States [3]. The prevalence of ASD has risen steadily in the last two decades [4]; in the United States in 2018, one of every 44 children (3 to 8 years old) was estimated to have ASD [5]. Although actual increased incidence versus improved diagnosis remains controversial [6-8], the rapid rise in ASD prevalence suggests that environmental factors may contribute to ASD etiology [9,10]. In the last decade, environmental research linking modifiable factors to ASD has proliferated, with replication or meta-analysis covering pesticides [11,12], air pollution [13,14], maternal fever during pregnancy [15,16], periconceptional nutrition [17-19], maternal diabetes or obesity [20,21], preeclampsia [22], and interpregnancy interval [23-25].
Thyroid hormones (THs) are essential for brain development and influence brain function throughout life [26,27]. Animal studies have shown that THs regulate crucial processes of brain development in mammals, including proliferation, migration, and differentiation of neuronal cells [28-30]. There is epidemiologic evidence that abnormal maternal thyroid function during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders [31]. In addition, the prevalence of thyroid peroxidase antibody (TPO-Ab), a marker for thyroid autoimmunity, was reported to be higher in families with autism probands than in comparison subjects [32].
Simultaneous with a growing understanding of the importance of maternal thyroid homeostasis for fetal brain development, chemical production volumes have increased 300-fold since the 1970s [33], leading to widespread human exposure to compounds known as endocrine-disrupting chemicals (EDCs) [34-36]. EDCs are defined as exogenous chemicals that interfere with hormone actions, resulting in increased risk of adverse health effects [37]. A wide range of EDCs disrupt thyroid homeostasis in laboratory animal studies [38]. Hundreds of synthetic chemicals interfere with the production, transport, and metabolism of THs [39]. Studies have shown that a broad range of EDCs can bind to TH receptors, may produce complex effects on TH signaling [40-42], and either alone or in combination, act at many levels in the thyroid system [43].
Among a large number of EDCs, this review focuses on per-and polyfluoroalkyl substances (PFAS), a class of synthetic chemicals widely used in consumer (e.g., cookware, dental floss) and industrial (e.g., lining of gas pipes, surfactant) applications [44]. Recently PFAS have received significant public attention due to increasing evidence of their widespread environmental contamination and adverse health effects. As PFAS-containing products are widely used in daily life, many common PFAS compounds have been detected in the blood of most of the United States general population [45]. PFAS have also been detected in cord blood [46,47] and in amniotic fluid [48,49]. Importantly, both animal studies [50-52] and epidemiologic studies [53-55] have shown that prenatal exposure to PFAS disrupts thyroid function and immune systems, which can alter early brain development (Fig. 1). Moreover, there is epidemiologic evidence that PFAS exposure is associated with child neurodevelopmental disorders such as attention-deficit/hyperactivity disorder [56,57], indicating that PFAS may adversely affect child brain development.

Per- and polyfluoroalkyl substances (PFAS) are known to disrupt immune systems and the hypothalamus-pituitary-thyroid axis, either alone or in combination. Thyroid peroxidase antibody (TPO-Ab) and thyroglobulin antibody (Tg-Ab), which are common in autoimmune thyroid disorders, may cause thyroid dysfunction. Primary and secondary pathways discussed in this review are represented in blue and magenta, respectively. TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine.
The aim of this review is to assess evidence for a potential pathway from prenatal PFAS exposure through abnormal thyroid function to ASD etiology. Hypothesizing that maternal thyroid dysfunction mediates a relationship between prenatal PFAS exposure and child ASD, we have focused on potential mechanisms related to thyroid dysfunction. This review also discusses antibody-mediated immune dysregulation that may cause thyroid dysfunction [58,59]. Building on the current report, subsequent research may help set the stage in support of prenatal thyroid treatment and strategies to prevent or reduce PFAS exposure.
THYROID FUNCTION
Thyroid function is assessed with thyroid stimulating hormone (TSH) and free thyroxine (FT4). When TSH is high and FT4 is low or within a normal range, hypothyroidism is diagnosed. According to the American Thyroid Association guidelines, the population-based trimester-specific normal reference range for serum TSH should be used when assessing thyroid function during pregnancy [60]. However, reference ranges which are assay-, laboratory-, cohort-, and population-specific are preferred when available. Some studies have defined maternal abnormal thyroid function based on hospital diagnosis codes for hyperthyroidism or hypothyroidism or on prescriptions for THs or anti-thyroid drugs [61,62].
POTENTIAL MECHANISMS
Abnormal thyroid function and ASD
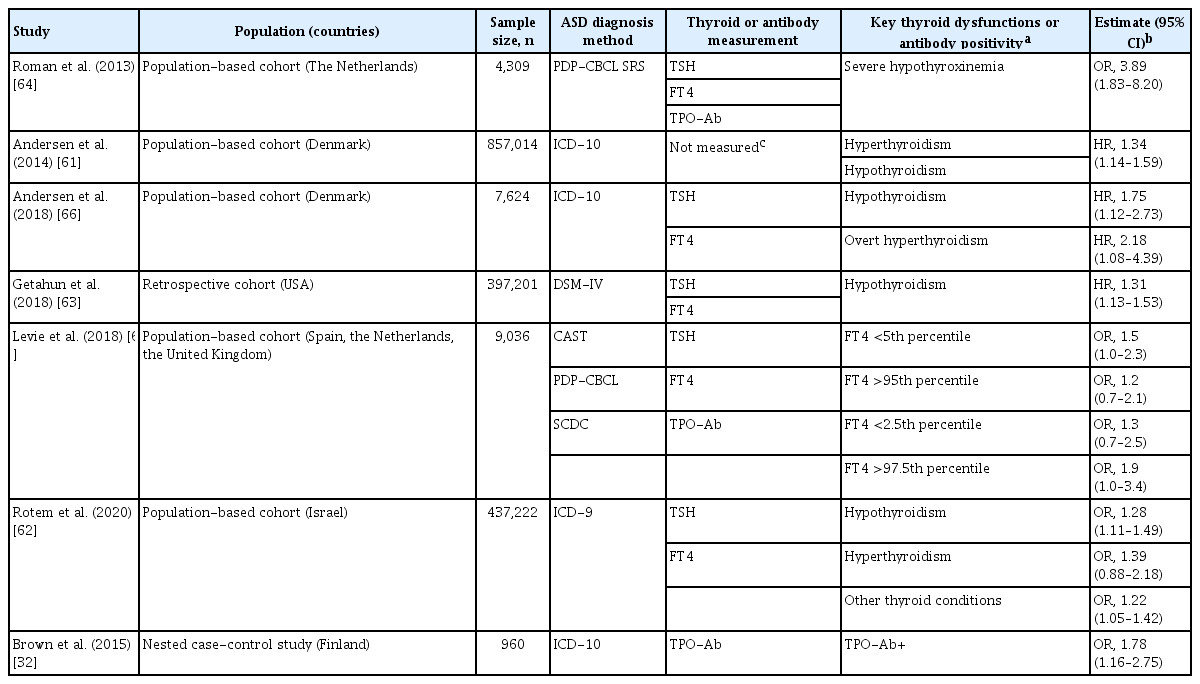
Epidemiologic studies have suggested that maternal gestational hypothyroidism, hyperthyroidism, and hypothyroxinemia were associated with increased risk of ASD in children (Table 1) [61-66]. In addition, low FT4 levels in cord blood were associated with increased ASD risk [67,68]. Overt maternal hypothyroidism is associated with impaired offspring cognition, which reflects that placental transfer of maternal THs to the fetus is essential for the regulation of fetal brain development [69,70]. Severe iodine deficiency (in which inadequate substrate for TH synthesis causes both maternal and fetal TH levels to be low) may cause cretinism, a syndrome of profoundly impaired growth and neurodevelopment. Maternal thyroid dysfunction during pregnancy is also known to be associated with adverse maternal and fetal outcomes such as preterm delivery, preeclampsia, and low birth weight [71-73], which are known risk factors for ASD [22,74-77].
Antibody-mediated immune dysregulation, ASD, and thyroid dysfunction
There is substantial evidence that autoimmunity and immune system dysfunction likely play a role in the development of ASD [78]. A body of epidemiologic evidence has shown that autoimmune disorders are significantly more frequent in families of autism probands than in those of comparison subjects [79-81]. More mothers of children with ASD had ASD-specific autoantibodies to proteins in the developing brain, compared with mothers of typically developing children [82-87]. In addition, a higher prevalence of maternal ASD-specific autoantibodies during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders [88-90].
In a case-control Finnish study, maternal TPO-Ab positivity during pregnancy was associated with increased risk of child ASD (odds ratio, 1.78; 95% confidence interval, 1.16 to 2.75) (Table 1) [32], implying that there is a potential role of thyroid autoimmunity in ASD etiology, although in that analysis maternal FT4 and TSH levels were not independently associated with ASD. It has been demonstrated that women with TPO-Ab positivity have a blunting of the typical thyroidal response to human chorionic gonadotropin in early gestation [91,92], resulting in lower serum FT4 levels and higher serum TSH levels. Thus, thyroid autoimmunity (high TPO-Ab and/or thyroglobulin antibody [Tg-Ab]) may be a secondary intermediate outcome (1) between PFAS exposure and ASD or (2) between PFAS exposure and thyroid dysfunction (Fig. 1).
Prenatal PFAS exposure and ASD
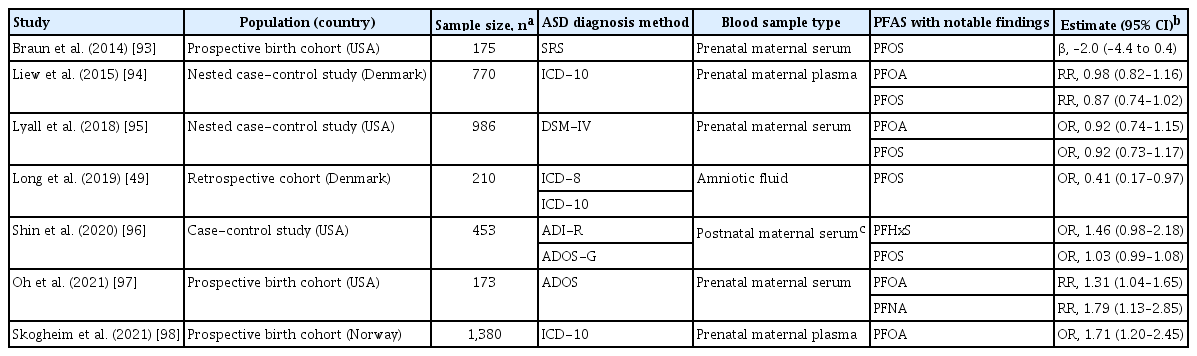
To our knowledge, seven epidemiologic studies to date have examined associations between maternal PFAS exposure and child ASD [49,93-98]. Although results differed, three studies showed that higher prenatal exposure to perfluorohexane sulfonate, perfluorononanoate, perfluorooctanoate (PFOA), or perfluorooctane sulfonate was associated with increased risk of child ASD (Table 2) [96-98]. Potential reasons for inconsistent results among these studies include differences in timing of exposure measures in pregnancy, characteristics of study populations, methods of identification or confirmation of ASD cases, and genetic factors. In addition, because PFAS were moderately correlated with each other and one PFAS may confound another, consideration of a single compound in the model may explain, at least in part, the inconsistent findings. The number of ASD cases is relatively small in three prospective birth cohorts [49, 93,97], potentially resulting in inadequate power to detect associations.
Prenatal PFAS exposure, thyroid dysfunction, and immune dysregulation
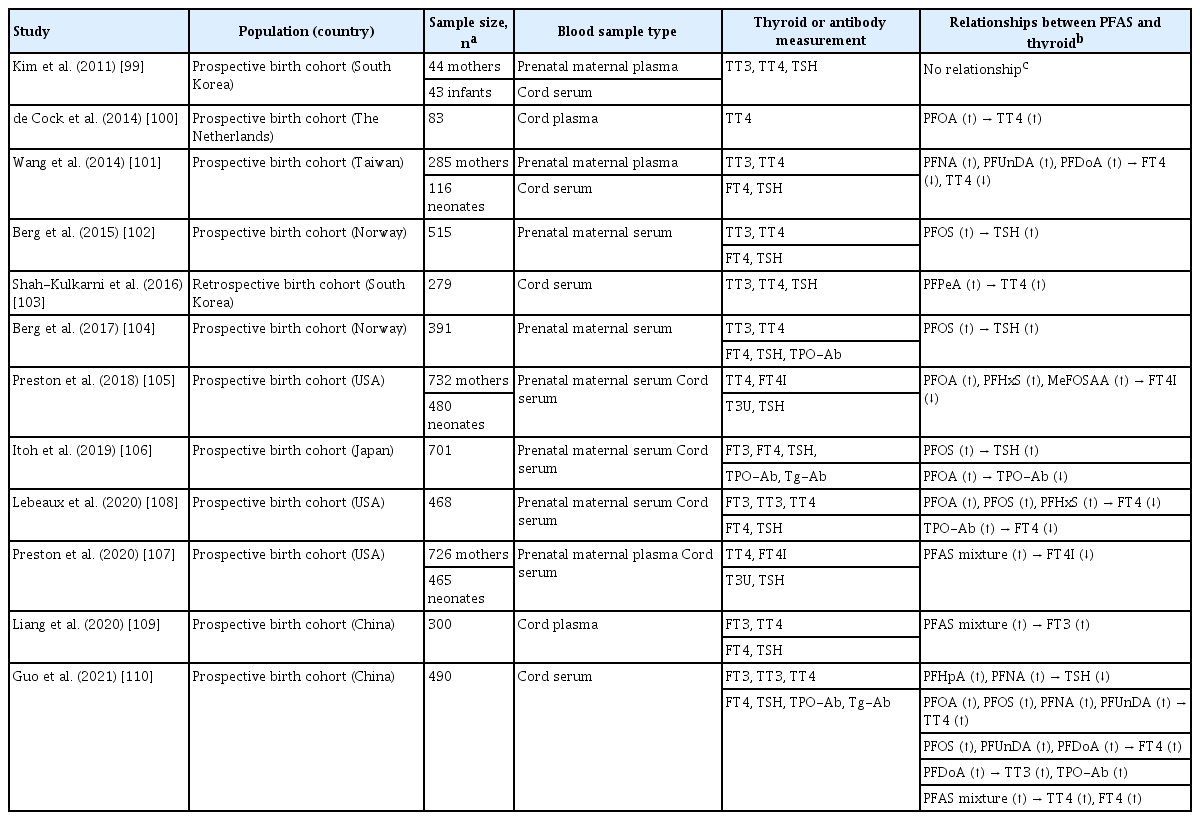
Many epidemiologic studies have shown that higher prenatal PFAS concentrations in maternal blood are associated with altered TH levels in maternal blood or cord blood (Table 3) [99-110]. Although study results are not entirely consistent, increases or decreases in THs indicate that prenatal PFAS exposure may disrupt maternal or neonatal thyroid homeostasis. In addition, three studies have reported that higher exposure to a mixture of PFAS was associated with increased or decreased THs [107,109,110], implying that PFAS can disrupt thyroid either alone or in combination. Some PFAS levels were significantly higher in infants with congenital hypothyroidism compared with healthy infants [111]. Two studies reported the relationship between prenatal PFAS exposure and thyroid autoimmunity; PFOA was inversely associated with TPO-Ab [106], whereas perfluorododecanoic acid was positively associated with TPO-Ab [110].
Studies have shown that PFAS exposure alone was not associated with TH levels among those with normal TPO-Ab but was associated with increases and decreases in THs among those with high TPO-Ab levels or low iodine concentrations. In a prospective birth cohort study, higher prenatal PFAS levels were associated with increased TSH levels only among pregnant women with high TPO-Ab (≥9 IU/mL) [112]. Another prospective birth cohort showed that higher prenatal PFOA levels were associated with lower prevalence of TPO-Ab in maternal blood and that PFAS-induced thyroid disruption and susceptibility may vary by the presence of two maternal TPO-Ab and Tg-Ab [106]. In a subset of United States adults, PFAS exposure was more likely to be associated with thyroid disruption in individuals with both TPO-Ab positivity and a urinary iodine concentration (UIC) <100 µg/L than in individuals with TPO-Ab positivity or low UIC alone, or TPO-Ab negative individuals with UIC ≥100 µg/L [113].
CONCLUSIONS
To date, no studies have examined a potential pathway from prenatal PFAS exposure through thyroid dysfunction and/or thyroid autoimmunity to ASD etiology within a well-characterized ASD population. Iodine deficiency is associated with increased risk of hypothyroidism [114] and known to cause brain damage [65,115]. However, most studies included in Tables 1, 3 have failed to measure important biomarkers that might affect maternal thyroid function, such as iodine status or thyroid antibodies. Thus, this review highlights that more rigorous studies are needed to yield robust and generalizable information about this potential pathway. Moreover, the evidence on mechanisms of this pathway summarized in this review suggests that thyroid dysfunction could mediate a relationship between prenatal PFAS exposure and child ASD, and this potential mediation effect could help explain significant findings from only three of the seven studies on an association between PFAS exposure and child ASD [96-98]. Therefore, future studies need to carefully disentangle the relationships among all potential mechanisms through mediation analysis [116-118] to help explain the underlying mechanism of any relationship between PFAS exposure and child ASD.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the grant from the National Institute of Environmental Health Sciences (R21-ES033389).