Obesity and Hyperglycemia in Korean Men with Klinefelter Syndrome: The Korean Endocrine Society Registry
Article information
Abstract
Background
The aim of this study was to investigate the prevalence of obesity in Korean men with Klinefelter syndrome (KS) and the associated risk factors for obesity and hyperglycemia.
Methods
Data were collected retrospectively from medical records from 11 university hospitals in Korea between 1994 and 2014. Subjects aged ≥18 years with newly diagnosed KS were enrolled. The following parameters were recorded at baseline before treatment: chief complaint, height, weight, fasting glucose level, lipid panel, blood pressure, testosterone, luteinizing hormone, follicle-stimulating hormone, karyotyping patterns, and history of hypertension, diabetes, and dyslipidemia.
Results
Data were analyzed from 376 of 544 initially enrolled patients. The rate of the 47 XXY chromosomal pattern was 94.1%. The prevalence of obesity (body mass index ≥25 kg/m2) in Korean men with KS was 42.6%. The testosterone level was an independent risk factor for obesity and hyperglycemia.
Conclusion
Obesity is common in Korean men with KS. Hypogonadism in patients with KS was associated with obesity and hyperglycemia.
INTRODUCTION
Klinefelter syndrome (KS) is the most frequent sex chromosome disorder in men, with a prevalence of 1:500 to 1:1,000 live births [1]. KS is caused by the presence of one or more additional X chromosomes. The 47 XXY karyotype is the most common classical chromosomal abnormality in subjects with KS, although both mosaic patterns and KS variants with supernumerary X or Y chromosomes exist [2]. The classical KS phenotype is a tall, slender man with narrow shoulders, long arms and legs, small testes, micropenis, gynecomastia, a lack of pubic hair, infertility, and mild-to-moderate cognitive deficits [3]. However, many patients do not present with the classical symptoms, and men with KS are associated with a broad spectrum of phenotypes, professions, incomes, and socioeconomic statuses [134]. KS in most men remains undetected, and only 25% of those predicted to have KS are diagnosed [5]. Although it is unknown why so many men with KS go their entire lives undiagnosed, it may be due to the lack of pronounced features, since the genotype does not necessarily imply the presence of any classical phenotypic features [67]. Recent studies reported that KS is associated with abdominal obesity and an increased risk of metabolic syndrome [89]. However, only limited data such as case reports describing obesity and metabolic disorders are available in Korean men with KS [10111213]. Therefore, the current study investigated the prevalence of obesity in Korean men with KS and the associated risk factors for obesity and hyperglycemia.
METHODS
Subjects
Medical record data were collected retrospectively from 11 university hospitals in South Korea willing to participate from 1994 to 2014. Subjects aged ≥18 years with newly diagnosed KS were enrolled. The following parameters were examined at baseline before treatment: chief complaint, height, weight, fasting glucose level, lipid panel, blood pressure, total testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex chromosomal abnormalities, and histories of hypertension, diabetes, and dyslipidemia. Data from 376 of the 544 patients initially enrolled were analyzed. One hundred and sixty-eight patients were excluded due to an inaccurate diagnosis or missing data regarding body weight, height, or testosterone level. The study was approved by the Institutional Review Boards of Ajou University Hospital and each hospital, and it conformed to the ethical guidelines of the Declaration of Helsinki. Patient records and information were anonymized and de-identified before analysis.
Definition
KS was defined based on a karyotype consisting of X-chromosome polysomy and at least one Y chromosome, either as a single lineage or mosaicism. The definition of obesity was a body mass index (BMI) ≥25 kg/m2 [14]. Diabetes was defined as fasting plasma glucose (FPG) ≥126 mg/dL, glycated hemoglobin (HbA1c) ≥6.5%, or current treatment with oral antidiabetes drugs or insulin [15]. Prediabetes was defined as FPG between 100 and 125 mg/dL or an HbA1c of 5.7% to 6.4% and no diagnosis of diabetes [15]. Hyperglycemia was diagnosed if the patient had diabetes, used hypoglycemic agents, or had a FPG ≥100 mg/dL or an HbA1c level ≥5.7%. Hypertension was diagnosed according to a systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or the use of antihypertensive medications [16]. Dyslipidemia was defined as a serum total cholesterol level ≥240 mg/dL, triglyceride level ≥150 mg/dL, low serum high density lipoprotein cholesterol level (<40 mg/dL in men and <50 mg/dL in women), or the use of lipid-lowering agents [17].
Statistical analysis
All continuous variables were expressed as mean±standard deviation except age. Independent t tests were used to analyze continuous data. Age was shown as median and total range and nonparametric test was used to test for differences between groups. Categorical variables are expressed as numbers and percentages. Chi-square tests were implemented for categorical data, as appropriate. Pearson correlation analysis was used to describe correlations between variables, and logistic regression analysis was used to evaluate the factors associated with obesity or hyperglycemia. The data were analyzed using SPSS version 22.0 (IBM Co., Armonk, NY, USA). A two-sided P<0.05 was considered statistically significant.
RESULTS
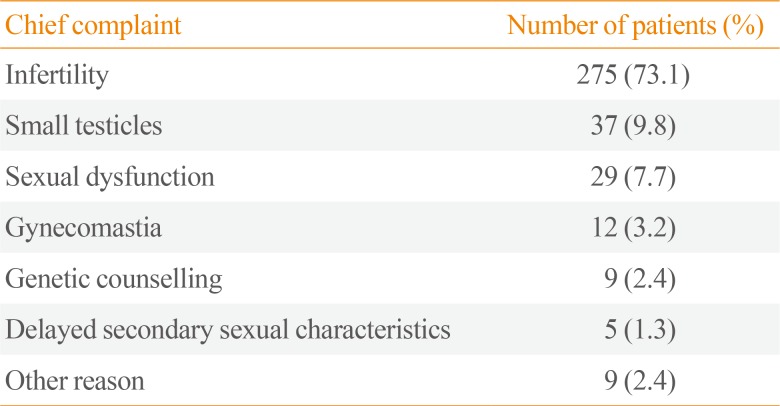
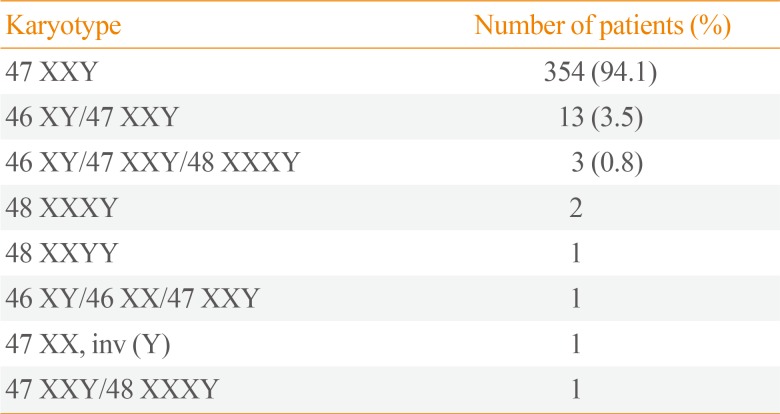
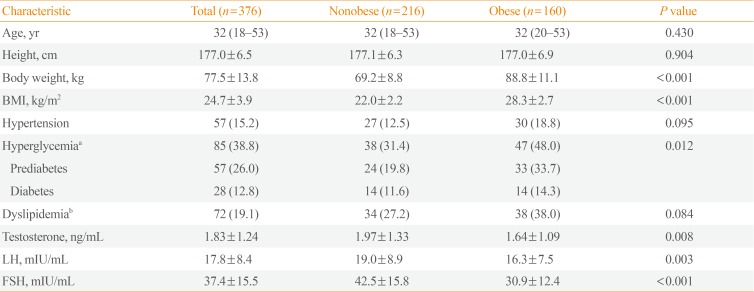
The chief complaints of patients with KS at diagnosis are shown in Table 1. The most common complaints were (in order) infertility, small testicles, sexual dysfunction, and gynecomastia. Table 2 shows the karyotyping results in patients with KS. The proportions of nonmosaic, mosaic, and KS variants were 94.1%, 3.5%, and 2.5%, respectively. The mean patient age and BMI were 32.5±5.9 years and 24.7±3.9 kg/m2, respectively (Table 3). The prevalence of obesity in patients with KS was 42.6%. We found a trend towards an increase in the prevalence of obesity according to age, but there was no significant difference in a subgroup analysis (18 to 29 years [37.5%] vs. 30 to 39 years [43.1%] vs. 40 to 55 years [52.9%], P=0.122). The prevalences of hyperglycemia and dyslipidemia were 38.8% and 19.1%, respectively.
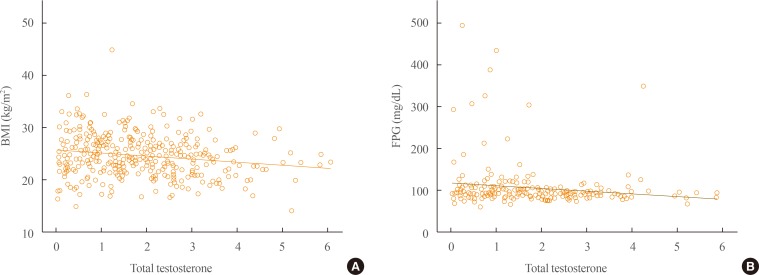
Obese subjects with KS had a high incidence of hyperglycemia and low testosterone level and elevated LH and FSH levels compared with nonobese patients with KS. Testosterone levels were negatively correlated with BMI (r=−0.178, P=0.001) and FPG levels (r=−0.147, P=0.030) (Fig. 1). In a logistic regression model using obesity as the dichotomous variable, testosterone was the only independent risk factor after adjusting for age and karyotype (classical vs. nonclassical KS; odds ratio, 0.797; 95% confidence interval, 0.671 to 0.948; P=0.010). In a model using hyperglycemia as the dependent variable, testosterone level, BMI, and age were independent risk factors after exclusion of the karyotyping pattern.
DISCUSSION
The present study revealed that the prevalence of obesity in Korean men with KS is 42.6%, and that testosterone level is an independent risk factor for obesity and hyperglycemia. Previous studies reported that even though patients with KS have abdominal adiposity, their BMI is often in the normal range due to decreased muscle mass and increased body fat [89]. Although we were unable to compare patients with KS with age-matched healthy controls, the prevalence of obesity, based on the criterion of a BMI ≥25 kg/m2, in Korean men with KS was higher than that in the general Korean male population. According to statistical data from Korea between 1998 and 2014, the prevalence of obesity in men aged ≥19 years was 25.1% to 37.7% [18]. Considering the mean age in the current study population was 32.5 years, the prevalence of obesity in KS seems rather higher than that of the general population. In Argentina, 62.5% of patients with KS had a BMI ≥25 kg/m2 [19].
Testosterone levels were an independent risk factor for obesity and glucose intolerance in the current study. This result is consistent with previous studies, which showed that testosterone deficiency is a predictor of abdominal obesity and metabolic syndrome [8920]. Testosterone may play a central role in metabolic syndrome and type 2 diabetes by increasing skeletal muscle mass and decreasing abdominal obesity and free fatty acids; therefore, it improves insulin resistance in men with a normal karyotype [21]. However, testosterone treatment in patients with KS does not fully correct the unfavorable body composition, although it is unclear whether adequate levels of testosterone were administered in these studies [822]. Furthermore, increased body fat mass is already present before puberty in boys with KS, which suggests that both genetic abnormalities and testosterone deficiency influence body fat in patients with KS [22].
Insulin resistance is the major pathogenesis of glucose intolerance in patients with KS [823]. Lee et al. [10] used euglycemic hyperinsulinemic clamps to demonstrate impaired peripheral insulin resistance as the mechanism underlying impaired glucose tolerance in Korean patients with KS. Recent both epidemiology and clinical studies showed clear evidence of a dramatically increased risk of diabetes and metabolic syndrome in KS worldwide [7]. In particular, In Korea, rapid economic development and a westernized lifestyle have resulted in increased rates of obesity [24]. Thus these factors can affect phenotype of KS.
The median total testosterone level in the current study was in the lower-to-normal range, whereas LH and FSH levels were increased, consistent with previous studies [1891925]. This suggests that hypogonadism in KS is relative rather than absolute [4]. Among patients with KS, 45.1% to 56.5% have low total testosterone levels [1925].
Less than 10% of the expected number of KS cases are diagnosed during childhood, and 15% to 20% of male KS cases are identified via investigations into the underlying cause of infertility [2627]. The current data are consistent with these previous results, since 73% of patients with KS complained of infertility at the first consultation. Therefore, most Korean men with KS are not diagnosed until adulthood.
The current study has the strength of including a relatively large sample size. It is also the first report focusing on obesity in Korean patients with KS. Nevertheless, several limitations need to be considered. First, the retrospective design of the study did not allow any missing data to be collected. Relatively few physicians tend to measure metabolic parameters in patients with KS. Second, the present study was cross-sectional in nature, which prevents defining causal relationships. Third, the study was based on data from patients with KS from 11 university hospitals; therefore, the results need to be interpreted with caution before generalizing to the entire Korean population.
In conclusion, the current study describes the prevalence of obesity in Korean patients with KS for the first time. Hypogonadism is a risk factor for obesity and hyperglycemia in patients with KS.
ACKNOWLEDGMENTS
This study was funded by a research grant from the Korean Endocrine Society.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.