Low Iodine Diet for Preparation for Radioactive Iodine Therapy in Differentiated Thyroid Carcinoma in Korea
Article information
Abstract
Preparation for radioactive iodine (RAI) therapy includes an increased serum thyroid stimulating hormone level and a low iodine diet (LID). Because of extremely high iodine intake, some physicians have advocated a more stringent LID for greater than 2 weeks in Korean patients with thyroid cancer prior to RAI therapy; however, it is very difficult to maintain a stringent LID for a longer period of time. According to recent reports in Korea, a nonstringent, simple LID for only 1 week might be enough prior to RAI therapy, if the patients can be educated intensively by specially trained staff. The measurement of simple urinary iodine concentration (UIC; µg/L) may underestimate daily iodine excretion in patients with a urinary volume of more than 1 L/day and can also be affected by dilution status. Simple UIC had a weaker correlation than the iodine/creatinine (I/Cr) ratio. Therefore, the urinary I/Cr ratio can replace 24-hour urine iodine excretion instead of simple UIC, although it may overestimate iodine intake in patients with malnutrition or poor muscle mass. The measurement of serum iodine level might be useful as an adjunct parameter for assessing LID preparation, but its sensitivity and specificity were relatively low compared to the urinary I/Cr ratio.
INTRODUCTION
Preparation for radioactive iodine (RAI) therapy includes an increased serum thyroid stimulating hormone (TSH) level (>25 mU/L) and a low iodine diet (LID). An elevated serum TSH level is traditionally achieved by thyroid hormone withdrawal, which may take 4 to 6 weeks; however, thyroid hormone withdrawal may cause a wide range of hypothyroid symptoms and signs and can have a significant impact on patients' daily activities [1-3]. Recombinant human TSH (rhTSH; Thyrogen, Genzyme, Cambridge, MA, USA) can be used as an alternative to thyroid hormone withdrawal to avoid hypothyroidism [1-3]. Another preparation prior to RAI administration is an LID. Most guidelines describing the management of differentiated thyroid cancer (DTC) recommend an LID less than 50 µg/day for 1 to 2 weeks prior to RAI therapy [1,4-8]. Sawka et al. [9] reported in their meta-analysis that ablation rates were higher in patients following an LID than in controls in one study, but, in another study, there was no significant benefit with an LID. They reported that no studies have examined long-term recurrence or mortality rates [9]. In addition, there have been no published studies regarding the appropriate degree and duration of an LID in an iodine-replete area. Recently, some data concerning an LID for preparation for RAI therapy were reported in Korea.
IS IODINE EXCESS TRUE IN MOST KOREAN POPULATIONS?
According to the World Health Organization epidemiological criteria for assessing iodine nutrition (2007), a median urinary iodine concentration (UIC) of 100 to 199 µg/L indicates adequate iodine intake in men, school-age children (≥6 years), and nonpregnant or lactating women. A median UIC of greater than 300 µg/L indicates excessive iodine intake, which may increase the risk of adverse health consequences [10]. More than 90% of dietary iodine is excreted in the urine, and UIC is considered an index of recent iodine intake. In nonpregnant, nonlactating women, a UIC of 100 µg/L corresponds roughly to a daily iodine intake of approximately 150 µg under steady-state conditions [10].
Kim et al. [11] reported that the average estimated iodine intake in 278 healthy Korean adults was 479 µg/day, and their average urinary iodine/creatinine (I/Cr) ratio was 674 µg/gCr, using an iodide selective electrode. Recently, Chung et al. [3] evaluated median UIC and median urinary I/Cr ratio in 1,072 euthyroid patients with benign thyroid nodules. Their median UIC was 358 µg/L, with a range from 24 to 9,224 µg/L, and the median urinary I/Cr ratio was 341 µg/gCr (data not published). In their study, UIC was measured by inductively coupled plasma mass spectrometry using an Agilent 7500 series instrument (Agilent Technologies Inc., Tokyo, Japan), which has been demonstrated to be extremely accurate in measuring UIC [12]. These levels are higher than those reported in other countries, except some regions in Japan. Because of extremely high iodine intake in most Korean populations, some physicians have advocated a more stringent LID for greater than 2 weeks in Korean DTC patients preparing for RAI therapy [13-15]; however, this is very difficult, and it seems to be impractical to maintain a stringent LID for a longer time prior RAI therapy. Therefore, I am going to discuss the degree and duration of an LID as a preparation for RAI therapy in Korean patients with DTC.
IS A STRICT LOW-IODINE DIET NECESSARY AND REQUIRED FOR SUCCESSFUL RAI THERAPY?
Most guidelines regarding the management of DTC recommend an LID of less than 50 µg/day for 1 or 2 weeks prior to RAI therapy [1,4-8]. The European Association of Nuclear Medicine Therapy Committee (2008) recommends postponement of RAI therapy when the stable UIC is above 150 to 200 µg/L, which is believed to reflect a clinically relevant excess of iodine [5]. The theoretical basis is that low plasma iodide concentrations may increase the expression of the sodium-iodine symporter gene and result in increased RAI uptake in remnant thyroid tissue or cancer cells [16]; however, this recommendation is not based on outcome studies. Although several studies have examined the impact of an LID on success rates of RAI therapy, the results are inconsistent [17-19]. Pluijmen et al. [17] found that low UIC was associated with an improvement in the success rate of ablation. They reported that successful ablation was more frequent in patients who were on an LID than control patients who were on a standard diet (63% vs. 48% with 24-hour urine iodine excretion rates of 26.6 and 158.8 µg/day, respectively) [17]. In contrast, Morris et al. [18] and Tala Jury et al. [19] found no difference in UIC between ablated or nonablated patients. Recently, Sohn et al. [20] reported that the ablation rate was significantly lower in patients who had a UIC >250 µg/gCr at the time of RAI ablation (P<0.05). In a multivariate analysis, a UIC >250 µg/gCr was the only significant variable associated with ablation failure (P=0.001; odds ratio, 4.53 to 4.57; 95% confidence interval [CI], 1.73 to 11.9); however, the ablation rates were not different in patients with a UIC <250 µg/gCr (P for trend <0.05) (Fig. 1). They suggested that a strict LID was not necessary to achieve a successful ablation. Therefore, a nonstringent, simple LID might be adequate preparation for RAI therapy, even in an iodine-replete area such as Korea.
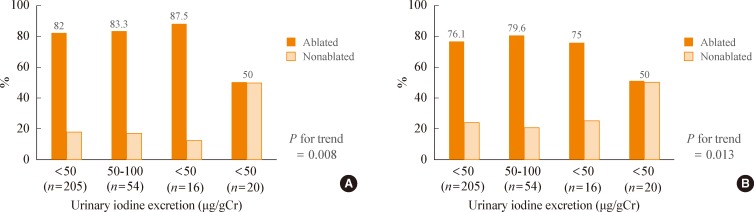
Ablation outcomes. (A) The definition of ablation was negative or faint uptake on a diagnostic whole-body scan. The ablation rate significantly decreased as urinary iodine excretion increased (P for trend=0.008). (B) The definition of ablation was negative or faint uptake on a diagnostic whole-body scan plus a stimulated serum thyroglobulin less than 2 ng/mL. The ablation rate significantly decreased as urinary iodine excretion increased (P for trend=0.013). Adapted from Sohn et al. Thyroid 2013;23:741-7, with permission from Mary Ann Liebert, Inc. [20].
CAN THE I/CR RATIO FROM A SPOT-URINE SAMPLE REPLACE 24-HOUR URINE IODINE EXCRETION TO EVALUATE THE APPROPRIATENESS OF A LOW-IODINE DIET FOR PREPARATION FOR RAI THERAPY?
Most iodine absorbed in the body is eventually excreted in the urine. Therefore, UIC is a good marker of very recent dietary iodine intake. Measurement of UIC in 24-hour urine is considered the gold standard to evaluate the appropriateness of LID [21]; however, 24-hour urine collection is inconvenient and cumbersome for patients [12]. An alternative method is to measure iodine concentration in a random spot-urine, but this is influenced by the time of collection and the degree of urine dilution [22]. There is a circadian rhythm to urinary iodine excretion, and urinary iodine excretion is lowest between 8:00 AM and 11:00 AM and reaches peak levels 4 to 5 hours after main meals [22]. Therefore, a fasting morning urine is recommended to measure the UIC [10]. No simple reliable index in a spot-urine sample has been developed to replace the 24-hour urinary iodine excretion.
Kim et al. [23] demonstrated that the I/Cr ratio from a spot-urine sample could serve as a useful and reliable alternative to 24-hour urine collection to evaluate the appropriateness of an LID prior to RAI therapy. They enrolled 193 patients and collected 24-hour urine samples (µg/day) and fasting spot-urine samples (µg/L and µg/gCr). There were statistically significant correlations between the 24-hour urinary iodine excretion and the two spot-urine-based indices (correlation coefficient, 0.539 for simple UIC; 0.773 for I/Cr ratio, P<0.001) (Fig. 2). When a 24-hour urinary iodine excretion of >150 µg/day was defined as a poor LID and receiver operating characteristic analysis was performed, the cutoff of the I/Cr ratio for poor LID was >66.2 µg/gCr (sensitivity 96.4%, specificity 83.6%, positive predictive value 50.0%, and negative predictive value 99.3%). A simple UIC has been proposed as a marker for iodine status in epidemiological studies [24-26]; however, this measurement could underestimate daily iodine excretion in patients with a urinary volume of more than 1 L/day and be affected by dilution status. Kim et al. [23] found that a simple UIC had a weaker correlation than the I/Cr ratio; however, the I/Cr ratio has a limitation in that it may overestimate iodine intake in patients with malnutrition or poor muscle mass because of low Cr levels.
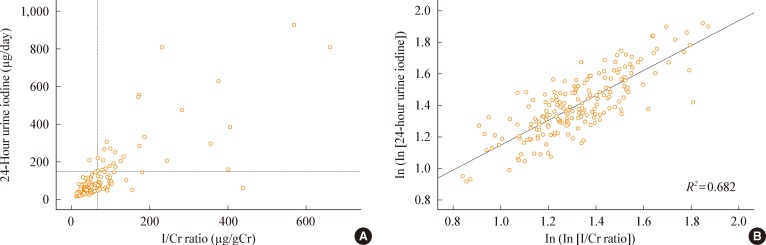
(A) The relationship between the iodine/creatinine (I/Cr) ratio from a spot-urine and iodine excretion from a 24-hour urine collection. The horizontal dotted line denotes the cutoff for the poor status of a low-iodine-diet preparation for radioiodine therapy based on a 24-hour urine iodine excretion, which was set at 150 µg/day. The vertical dotted line is the proposed cutoff of the 'I/Cr ratio' from a spoturine, which was set at 66.2 µg/gCr. (B) The correlation after log-log transformation of the data. R2 was computed by regression analysis. Adapted from Kim et al. Clin Endocrinol (Oxf) 2010;73:114-8, with permission from Blackwell Publishing [23].
OPTIMAL LENGTH OF A LOW-IODINE DIET FOR PREPARATION OF RAI THERAPY: 1 WEEK VS. 2 WEEKS
The American Thyroid Association and Korean Thyroid Association recommend an LID for 1 to 2 weeks for patients undergoing RAI remnant ablation, particularly for those patients with high iodine intake [1,8]. In contrast, the European Thyroid Association recommends an LID for 3 weeks prior to RAI therapy [4]. Several protocols exist for an LID, but there are no standardized guidelines for the duration of an LID prior to RAI therapy. Iodine intake varies depending on the region and patients' diet patterns. The reduction by 1 week for an LID has a large impact on the patients' quality of life. A longer duration of an LID tries their patience, and some patients reach the end of endurance. A longer duration of LID can even affect patients negatively.
There are four articles dealing with the duration of an LID (1 week vs. 2 weeks) prior to RAI therapy. Two articles reported in Korea suggested that an LID for 1 week was enough to achieve an acceptable reduction of UIE [15,27]. On the contrary, another two articles reported in the USA and Japan suggested an LID for 2 weeks was superior to 1 week to achieve the targeted reduction in UIC [13,14]. Kim et al. [27] suggested that a 1-week strict LID was enough to decrease the level of UIC in preparation for RAI therapy, even in an iodine-sufficient area such as Korea. They prospectively designed and enrolled 19 patients with DTC preparing for RAI therapy. Patients went on a strict LID for 2 weeks prior to RAI administration. The daily first spot-urine in the morning was collected and stored at -20℃ for 2 weeks from the first day of the LID to the day of RAI administration. Patients were educated by specially trained nurses and dieticians for 2 hours before the start of the LID. They were informed about the foods that were allowed and not allowed. They were given a 3-day sample menu specifically designed for patients living in Korea and the contact information for dietary questions. The UIC (µg/gCr) decreased significantly from day 0 (576.9±825.5) to day 7 (26.7±16.2) and day 14 (19.5±8.8) (P<0.05) (Fig. 3). On day 6, the UIC decreased below the cutoff level (66.2 µg/gCr) by both 95% CI (0 to 60.8 µg/gCr) and 90th percentile (51.9 µg/gCr). Roh et al. [15] also demonstrated that a stringent LID for 1 week resulted in a better UIC (I/Cr ratio) compared with a less stringent LID for 2 weeks; however, they cautiously suggested that a stringent LID for 2 weeks is expected to be more desirable. On the contrary, Park and Hennessey. [13] suggested that an acceptable reduction (<100 µg/gCr) of UIC was achieved in 41% of patients with a 1-week LID and in 71% patients with a 2-week LID. They reported that the mean UIC (I/Cr ratio) was significantly lower in 24 patients who followed a 2-week LID (84.7±44.0) compared to 21 patients on a 1-week LID (199.9±169.5, P<0.01). Tomoda et al. [14] also suggested that a targeted reduction of UIC (<100 µg/gCr) was achieved in 26% of patients with a 1-week and in 70% with a 2-week LID. They reported that UIC (I/Cr ratio) was lower after 2 weeks of LID (17 patients; 63.1±38.7) than after 1 week of LID treatment (15 patients; 119.4±55.9) when the patients followed a stringent LID. In both studies, LID was explained to the patients after surgery and they were sent home with a simple, one-page list of dietary recommendations. A possible explanation for this discrepancy between Kim (Korea), Park (USA), and Tomoda (Japan) might be the intensity and compliance to the LID by patients. In conclusion, LID for 1 week may be enough prior to RAI therapy even in an iodine-replete area, if the patients can be educated intensively by specially trained staffs and their compliance can be regularly checked and reinforced.
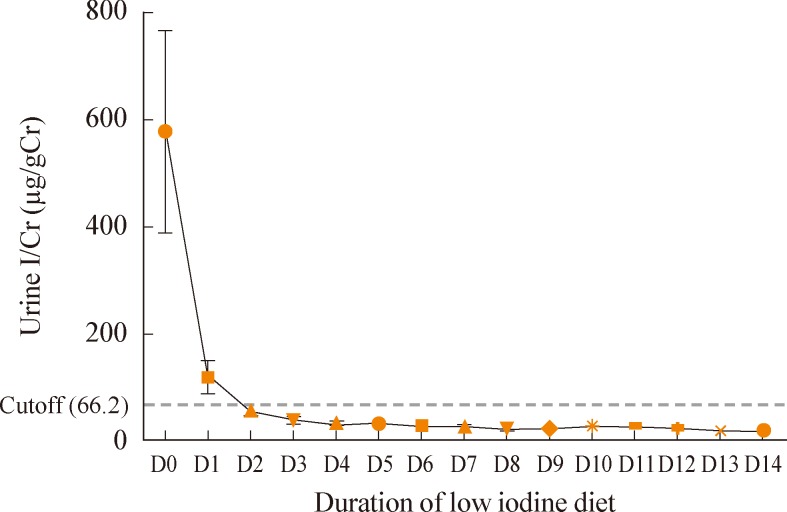
Changes in daily urine iodine excretion by the iodine/creatinine (I/Cr) ratio during a low iodine diet in preparation for radioiodine therapy among differentiated thyroid cancer patients. The I/Cr ratio decreased significantly from day 0 (576.9±825.5) to day 7 (26.7±16.2) and day 14 (19.5±8.8) (P<0.05). On day 6, the I/Cr ratio decreased below the cutoff level (66.2 µg/gCr) by both 95% confidence interval (0 to 60.8 µg/gCr) and 90th percentile (51.9 µg/gCr). Adapted from Kim et al. Clin Endocrinol (Oxf) 2011;75:851-6, with permission from Blackwell Publishing [27].
Levothyroxine might be a significant source of dietary iodine in patients undergoing rhTSH-stimulated RAI therapy. Park and Hennessey [13] reported in a previous study that the urinary iodine excretion (I/Cr ratio) after a 2-week LID was different between patients without levothyroxine (59.6±76.0) and with levothyroxine (84.7±44.0, P<0.01).
CAN THE MEASUREMENT OF THE SERUM IODINE LEVEL REPLACE URINE IODINE EXCRETION IN THE EVALUATION OF THE APPROPRIATENESS OF A LOW-IODINE DIET FOR PREPARATION FOR RAI THERAPY?
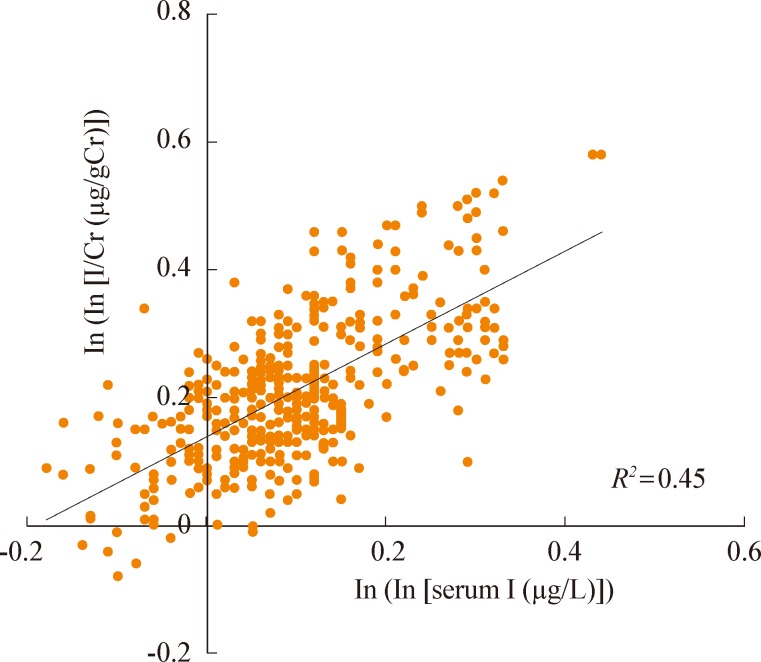
Sohn et al. [28] suggested that the measurement of the serum iodine level might be useful as an adjunct parameter for assessing LID preparation, but its sensitivity and specificity were relatively low compared to the urine I/Cr ratio. They found significant correlations between the serum iodine level and the spot UIC (µg/L) or I/Cr ratio (µg/gCr) (correlation coefficients, 0.51 for UIC; 0.62 for I/Cr ratio; P<0.001) (Figs. 4, 5). The cutoff value for the serum iodine level was 20.4 µg/L (sensitivity 79.3%, specificity 81.5%) for the evaluation of an appropriate LID.

The relationship between serum iodine and urine iodine levels. (A) Serum iodine levels were significantly correlated with spoturine iodine levels (r=0.51, P<0.001). (B) Serum iodine levels were significantly correlated with the spot-urine iodine/creatinine (I/Cr) ratio (r=0.62, P<0.001). Adapted from Sohn et al. J Korean Thyroid Assoc 2012;5:143-7 [28].
CONCLUSIONS
A nonstringent, simple LID for only 1 week might be enough prior to RAI therapy, if the patients can be educated intensively. The urinary I/Cr ratio can replace 24-hour urine iodine excretion instead of simple UIC, The measurement of serum iodine level might be useful as an adjunct parameter for assessing LID preparation, but its sensitivity and specificity were relatively low compared to the urinary I/Cr ratio.
ACKNOWLEDGMENTS
I would like to express my sincere appreciation to Dr. Hee Kyung Kim, Dr. Seo Young Sohn, Dr. Na Kyung Kim, and Professor Sun Wook Kim for discussions regarding their raw data.
Notes
No potential conflict of interest relevant to this article was reported.