Selective Agonists of Thyroid Hormone Receptor Beta: Promising Tools for the Treatment of Nonalcoholic Fatty Liver Disease
Article information
Thyroid hormone receptor beta (THR-β) is the primary isoform of the THR found in the liver, and it is primarily responsible for reducing cholesterol levels. Conversely, THR-α is predominantly associated with adverse effects on the heart and bones. Therefore, significant efforts have been made to develop drug candidates with isomer-specific activity, targeting the structure of thyroid hormones to create thyromimetics that selectively target THR-β for application in liver disease [1].
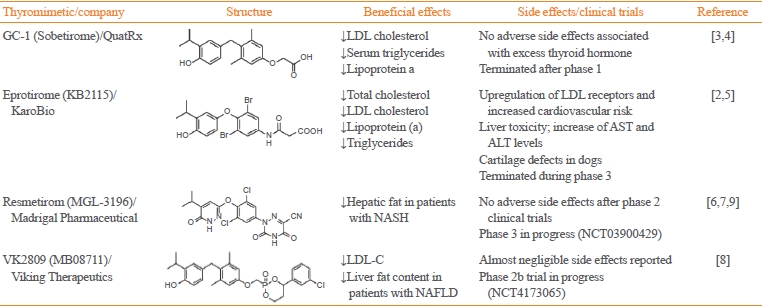
Over the past two decades, several THR-β selective triiodothyronine (T3) analogs, such as sobetirome (GC-1), eprotirome (KB2115), resmetirom (MGL-3196), and the HepDirect prodrug VK2809 (MB07811), have been developed (Table 1) [2-9]. These compounds have shown promising results by mimicking the beneficial effects of T3 without the adverse side effects. For instance, sobetirome and eprotirome advanced to human clinical trials for dyslipidemia, demonstrating positive outcomes without the typical detrimental effects associated with hyperthyroidism [2-5]. However, phase 2 trials for sobetirome have not been conducted, and a phase 3 trial with eprotirome was halted due to observed harmful effects on cartilage in canines (NCT01410383) [5]. Despite setbacks, the liver and THR-β selective agonist VK2809 exhibited positive outcomes in a phase 2 trial involving patients with hypercholesterolemia and nonalcoholic fatty liver disease (NAFLD) and is currently undergoing evaluation in a phase 2b clinical trial in patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) (NCT4173065). Moreover, resmetirom (MGL-3196) successfully completed phase 2 trials, significantly reducing liver fat content in patients with NASH over 12 and 36 weeks of treatment without significant side effects [6]. Most recently, the 52-week results of the ongoing phase 3 MAESTRO-NASH trial (NCT03900429) have been presented [7].
NAFLD is a prevalent condition associated with cirrhosis and hepatocellular carcinoma, affecting around 70% of individuals with obesity or type 2 diabetes [10]. Given the lack of approved pharmacotherapies specifically targeting NAFLD, current guidelines recommend addressing NAFLD by targeting obesity or type 2 diabetes [10-12]. This can be achieved through lifestyle interventions for weight loss and the use of glucagon-like peptide-1 receptor agonists, or by treating type 2 diabetes with insulin sensitizers such as pioglitazone. Understanding the role of thyroid hormone in hepatic glucose and lipid metabolism is crucial, although it is challenging to distinguish its contribution to steatohepatitis from those of insulin resistance, obesity, and type 2 diabetes. THR-β agonists reverse steatosis through various mechanisms, including promoting the hepatic conversion of thyroxine (T4) to T3 and improving mitochondrial function [13,14].
Harrison et al. [7] presented the 52-week results of the ongoing phase 3 MAESTRO-NASH trial in the New England Journal of Medicine. In this study, 966 adults diagnosed with NASH and liver fibrosis were randomly assigned to receive once-daily doses of either 80 or 100 mg of resmetirom, or a placebo. Both doses of resmetirom showed superiority over placebo in achieving the trial’s two primary endpoints: resolution of NASH without worsening fibrosis (25.9%–29.9% of patients on resmetirom vs. 9.7% on placebo) and improvement in fibrosis by at least one stage without worsening of the NAFLD activity score (24.2%– 25.9% of patients on resmetirom vs. 14.2% on placebo). Additionally, resmetirom improved atherogenic dyslipidemia without significantly affecting body weight, insulin resistance, glycemia, heart rate, or blood pressure. The drug’s adverse-event profile was deemed acceptable, although nausea, vomiting, and diarrhea were reported more frequently with resmetirom than with placebo. Notably, no increase was reported in endocrine adverse events. However, attention should be directed toward several changes in the endocrine system in response to resmetirom. In patients with available data, resmetirom led to significant increases in sex hormone-binding globulin, total estradiol, and testosterone levels. While these elevations indicate that resmetirom interacts with THR-β and are linked to treatment response, the long-term consequences of these hormonal alterations remain uncertain. Additionally, resmetirom impacted the pituitary-thyroid hormone axis, resulting in reductions in free T4 and thyrotropin levels. Although mean plasma free T3 levels remained within the normal range, further exploration of individual cases at the lower end of normal or below-normal range is necessary.
In summary, the MAESTRO-NASH trial’s findings mark significant progress in the field. However, it is necessary to remain vigilant about potential adverse effects of the treatment. Close monitoring for endocrine-related adverse events, especially those affecting thyroid, gonadal, or bone health, is crucial. A complete understanding of the long-term safety and effectiveness of resmetirom will only come with the conclusion of the ongoing 54-month trial. The combination of glucagon and T3 as a treatment method, is still in its early stages. However, it offers a promising new approach for diseases that could benefit from T3’s specific actions in tissues and isoforms. In the future, this “combinatorial chemistry” technology is expected to be more widely used to maximize benefits and minimize risks compared to using the components separately.
Notes
CONFLICTS OF INTEREST
Sun Wook Cho is the deputy editor of the journal. However, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.