Search
- Page Path
- HOME > Search
Original Articles
- Diabetes, obesity and metabolism
- Insulin Preferentially Regulates the Activity of Parasympathetic Preganglionic Neurons over Sympathetic Preganglionic Neurons
- Uisu Hyun, Yoon Young Kweon, Jong-Woo Sohn
- Endocrinol Metab. 2023;38(5):545-556. Published online September 26, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1725
- 1,380 View
- 78 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Insulin is a peptide hormone that regulates post-prandial physiology, and it is well known that insulin controls homeostasis at least in part via the central nervous system. In particular, insulin alters the activity of neurons within the autonomic nervous system. However, currently available data are mostly from unidentified brainstem neurons of the dorsal motor nucleus of the vagus nerve (DMV).
Methods
In this study, we used several genetically engineered mouse models to label distinct populations of neurons within the brainstem and the spinal cord for whole-cell patch clamp recordings and to assess several in vivo metabolic functions.
Results
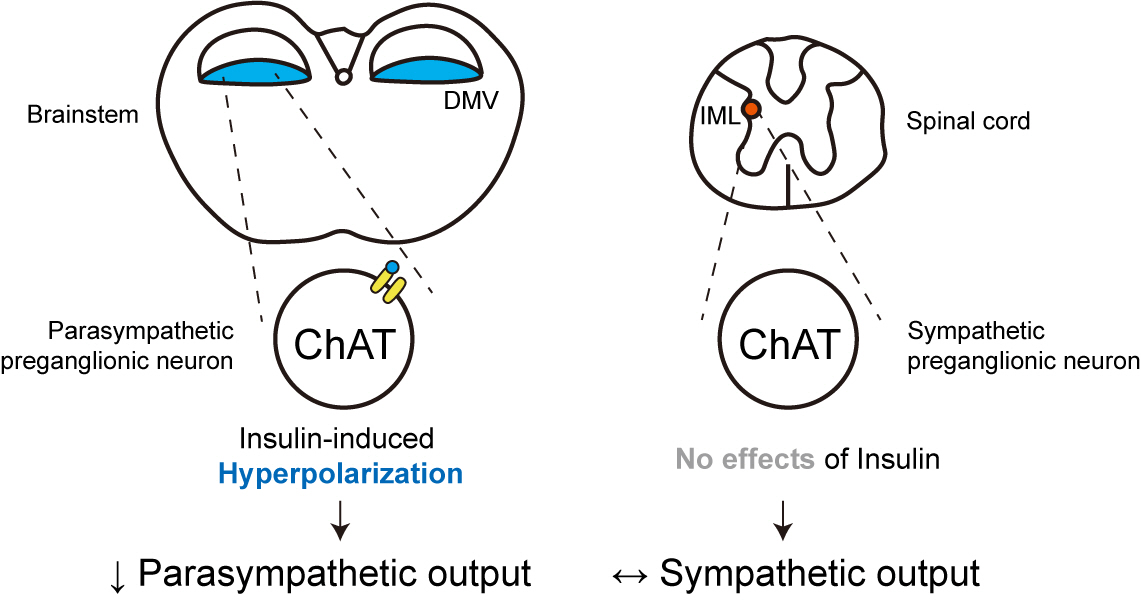
We first confirmed that insulin directly inhibited cholinergic (parasympathetic preganglionic) neurons in the DMV. We also found inhibitory effects of insulin on both the excitatory and inhibitory postsynaptic currents recorded in DMV cholinergic neurons. In addition, GABAergic neurons of the DMV and nucleus tractus solitarius were inhibited by insulin. However, insulin had no effects on the cholinergic sympathetic preganglionic neurons of the spinal cord. Finally, we obtained results suggesting that the insulininduced inhibition of parasympathetic preganglionic neurons may not play a critical role in the regulation of glucose homeostasis and gastrointestinal motility.
Conclusion
Our results demonstrate that insulin inhibits parasympathetic neuronal circuitry in the brainstem, while not affecting sympathetic neuronal activity in the spinal cord.

- Hypothalamus and Pituitary Gland
- Heart Rate Variability in Postoperative Patients with Nonfunctioning Pituitary Adenoma
- Jeonghoon Ha, Hansang Baek, Chaiho Jeong, Minsoo Yeo, Seung-Hwan Lee, Jae Hyoung Cho, Ki-Hyun Baek, Moo Il Kang, Dong-Jun Lim
- Endocrinol Metab. 2021;36(3):678-687. Published online June 10, 2021
- DOI: https://doi.org/10.3803/EnM.2021.978
- 4,567 View
- 109 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Decreased heart rate variability (HRV) has been reported to be associated with cardiac autonomic dysfunction. Hypopituitarism in nonfunctioning pituitary adenoma (NFPA) is often linked to increased cardiovascular mortality. We therefore hypothesized that postoperative NFPA patients with hormone deficiency have an elevated risk of HRV alterations indicating cardiac autonomic dysfunction.
Methods
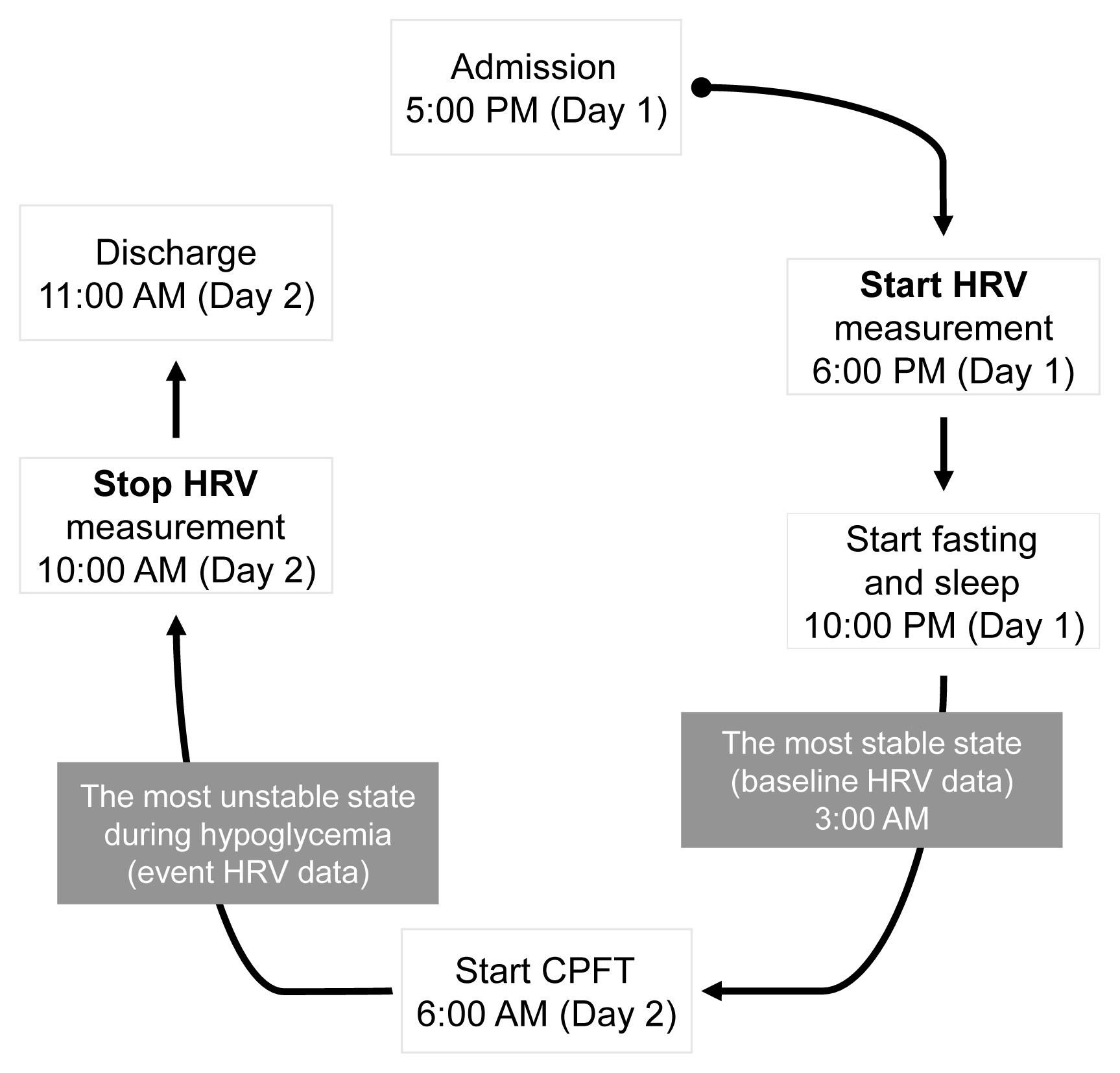
A total of 22 patients with NFPA were enrolled in the study. Between 3 and 6 months after surgery, a combined pituitary function test (CPFT) was performed, and HRV was measured. The period of sleep before the CPFT was deemed the most stable period, and the hypoglycemic period that occurred during the CPFT was defined as the most unstable period. Changes in HRV parameters in stable and unstable periods were observed and compared depending on the status of hormone deficiencies.
Results
In patients with adrenocorticotropic hormone (ACTH) deficiency with other pituitary hormone deficiencies, the low frequency to high frequency ratio, which represents overall autonomic function and is increased in the disease state, was higher (P=0.005). Additionally, the standard deviation of the normal-to-normal interval, which decreases in the autonomic dysfunction state, was lower (P=0.030) during the hypoglycemic period. In panhypopituitarism, the low frequency to high frequency ratio during the hypoglycemic period was increased (P=0.007).
Conclusion
HRV analysis during CPFT enables estimation of cardiac autonomic dysfunction in patients with NFPA who develop ACTH deficiency with other pituitary hormone deficiencies or panhypopituitarism after surgery. These patients may require a preemptive assessment of cardiovascular risk. -
Citations
Citations to this article as recorded by- Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination
Maria Bernadette Cilona, Filippo D’Amico, Chiara Asperti, Giuseppe Alvise Ramirez, Stefano Turi, Giovanni Benanti, Shai Marc Bohane, Serena Nannipieri, Rosa Labanca, Matteo Gervasini, Federica Russetti, Naomi Viapiana, Martina Lezzi, Giovanni Landoni, Lor
Vaccines.2023; 11(3): 567. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Effect of a 16-Session Qigong Program in Non-Hodgkin Lymphoma Survivors: A Randomized Clinical Trial
Keyla Vargas-Román, Emilia I. De la Fuente-Solana, Jonathan Cortés-Martín, Juan Carlos Sánchez-García, Christian J. González-Vargas, Lourdes Díaz-Rodríguez
Journal of Clinical Medicine.2022; 11(12): 3421. CrossRef
- Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination


 KES
KES

 First
First Prev
Prev



