Funded articles
- Page Path
- HOME > BROWSE ARTICLES > Funded articles
Original Articles
- Hypothalamus and pituitary gland
- Preoperative Serum Copeptin Can Predict Delayed Hyponatremia after Pituitary Surgery in the Absence of Arginine Vasopressin Deficiency
- Ho Kang, Seung Shin Park, Yoo Hyung Kim, Hwan Sub Lim, Mi-Kyeong Lee, Kyoung-Ryul Lee, Jung Hee Kim, Yong Hwy Kim
- Endocrinol Metab. 2024;39(1):164-175. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1792
- Funded: Korean Endocrinology Society, Seoul National University
- 1,036 View
- 49 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
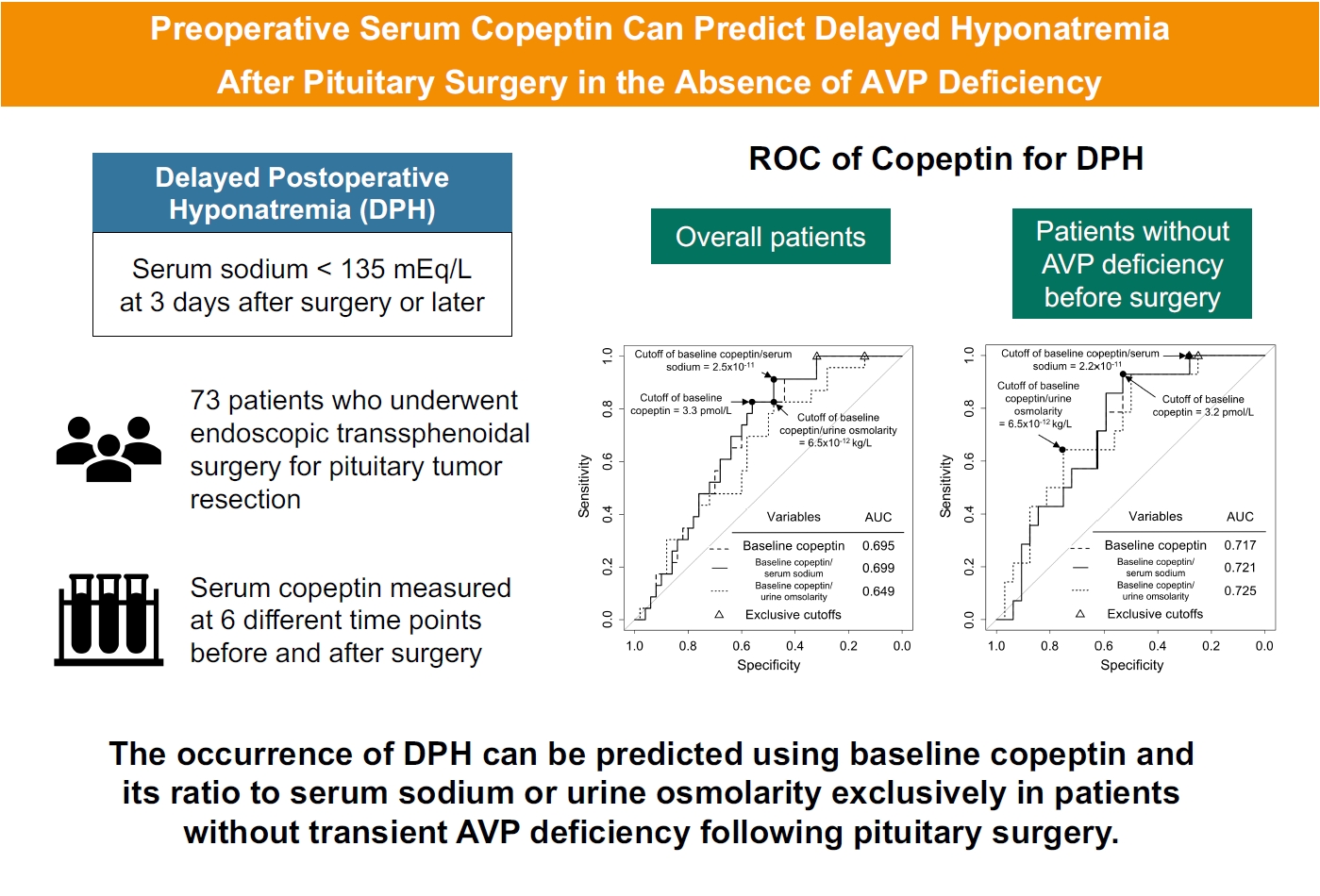
ePub - Background
Delayed postoperative hyponatremia (DPH) is the most common cause of readmission after pituitary surgery. In this study, we aimed to evaluate the cutoff values of serum copeptin and determine the optimal timing for copeptin measurement for the prediction of the occurrence of DPH in patients who undergo endoscopic transsphenoidal approach (eTSA) surgery and tumor resection.
Methods
This was a prospective observational study of 73 patients who underwent eTSA surgery for pituitary or stalk lesions. Copeptin levels were measured before surgery, 1 hour after extubation, and on postoperative days 1, 2, 7, and 90.
Results
Among 73 patients, 23 patients (31.5%) developed DPH. The baseline ratio of copeptin to serum sodium level showed the highest predictive performance (area under the curve [AUROC], 0.699), and its optimal cutoff to maximize Youden’s index was 2.5×10–11, with a sensitivity of 91.3% and negative predictive value of 92.0%. No significant predictors were identified for patients with transient arginine vasopressin (AVP) deficiency. However, for patients without transient AVP deficiency, the copeptin-to-urine osmolarity ratio at baseline demonstrated the highest predictive performance (AUROC, 0.725). An optimal cutoff of 6.5×10–12 maximized Youden’s index, with a sensitivity of 92.9% and a negative predictive value of 94.1%.
Conclusion
The occurrence of DPH can be predicted using baseline copeptin and its ratio with serum sodium or urine osmolarity only in patients without transient AVP deficiency after pituitary surgery.

- Thyroid
- Active Surveillance for Low-Risk Papillary Thyroid Carcinoma as an Acceptable Management Option with Additional Benefits: A Comprehensive Systematic Review
- Jee Hee Yoon, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2024;39(1):152-163. Published online January 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1794
- Funded: Korean Thyroid Association, National Cancer Center
- 1,154 View
- 42 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
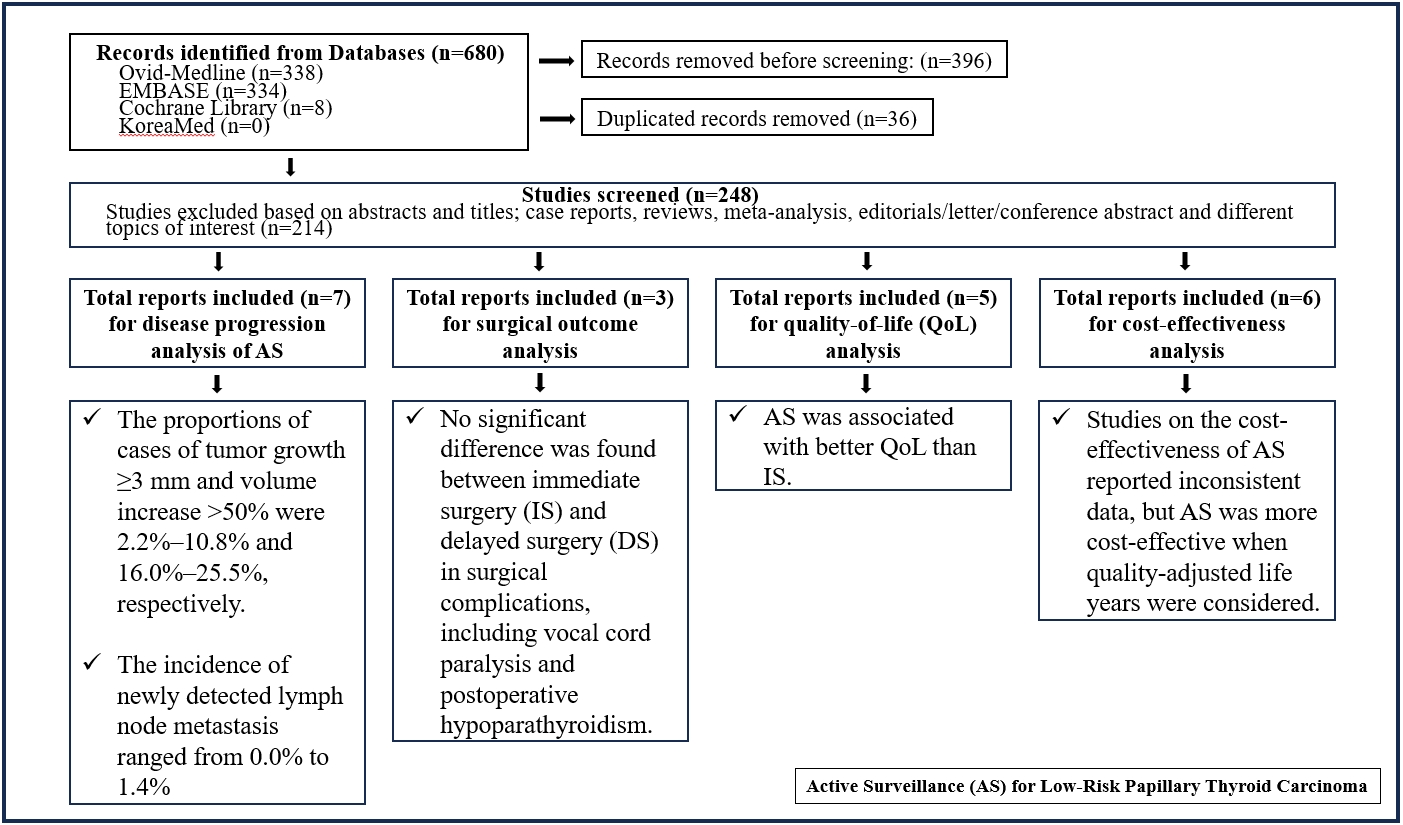
ePub - Background
Active surveillance (AS) has been introduced as a management strategy for low-risk papillary thyroid carcinoma (PTC) due to its typically indolent nature. Despite this, the widespread adoption of AS has encountered several challenges. The aim of this systematic review was to evaluate the safety of AS related to disease progression and its benefits compared with immediate surgery (IS).
Methods
Studies related to AS in patients with low-risk PTC were searched through the Ovid MEDLINE, Embase, Cochrane Library, and KoreaMed databases. Studies on disease progression, surgical complication, quality of life (QoL), and cost-effectiveness were separately analyzed and narratively synthesized.
Results
In the evaluation of disease progression, the proportions of cases with tumor growth ≥3 mm and a volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Newly detected lymph node metastasis was identified in 0.0%–1.4% of patients. No significant difference was found between IS and delayed surgery in surgical complications, including vocal cord paralysis and postoperative hypoparathyroidism. AS was associated with better QoL than IS. Studies on the cost-effectiveness of AS reported inconsistent data, but AS was more cost-effective when quality-adjusted life years were considered.
Conclusion
AS is an acceptable management option for patients with low-risk PTC based on the low rate of disease progression and the absence of an increased mortality risk. AS has additional benefits, including improved QoL and greater QoL-based cost-effectiveness. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

- Thyroid
- Hashimoto Thyroiditis and Mortality in Patients with Differentiated Thyroid Cancer: The National Epidemiologic Survey of Thyroid Cancer in Korea and Meta-Analysis
- Injung Yang, Jae Myung Yu, Hye Soo Chung, Yoon Jung Kim, Yong Kyun Roh, Min Kyu Choi, Sung-ho Park, Young Joo Park, Shinje Moon
- Endocrinol Metab. 2024;39(1):140-151. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1748
- Funded: Hallym University
- 1,004 View
- 52 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
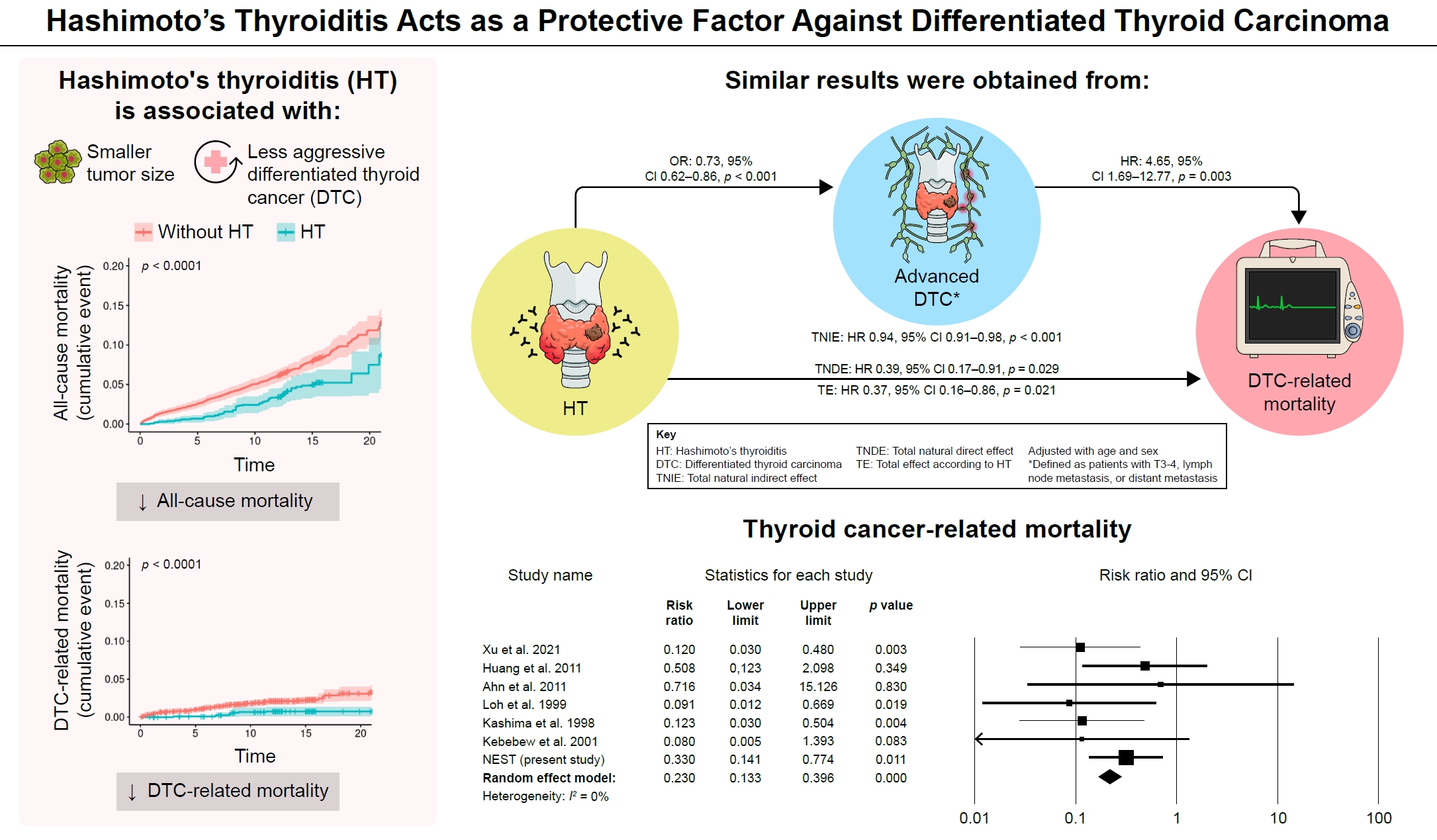
ePub - Background
Many studies have shown that Hashimoto’s thyroiditis (HT) acts as a protective factor in differentiated thyroid cancer (DTC), but little is known about its effects on mortality. Therefore, this study was performed to reveal the prognosis of HT on mortality in patients with DTC.
Methods
This study included two types of research results: retrospective cohort study using the National Epidemiologic Survey of Thyroid cancer (NEST) in Korea and meta-analysis study with the NEST data and eight selected studies.
Results
Of the 4,398 patients with DTC in NEST, 341 patients (7.8%) died during the median follow-up period of 15 years (interquartile range, 12.3 to 15.6). Of these, 91 deaths (2.1%) were related to DTC. HT was associated with a smaller tumor size and less aggressive DTC. In Cox regression analysis after adjusting for age and sex, patients with HT showed a significantly lower risk of all-cause death (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.52 to 0.96) and DTC-related death (HR, 0.33; 95% CI, 0.14 to 0.77). The analysis with inverse probability of treatment weight data adjusted for age, sex, and year of thyroid cancer registration showed similar association. The meta-analysis showed that patients with HT showed a lower risk of all-cause mortality (risk ratio [RR], 0.24; 95% CI, 0.13 to 0.47) and thyroid cancer-related mortality (RR, 0.23; 95% CI, 0.13 to 0.40) in comparison with patients without HT.
Conclusion
This study showed that DTC co-presenting with HT is associated with a low risk of advanced DTC and presents a low risk for all-cause and DTC-related death.

- Diabetes, obesity and metabolism
- FoxO6-Mediated TXNIP Induces Lipid Accumulation in the Liver through NLRP3 Inflammasome Activation
- Mi Eun Kim, Jun Sik Lee, Tae Won Kim, Min Hi Park, Dae Hyun Kim
- Endocrinol Metab. 2024;39(1):127-139. Published online February 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1826
- Funded: National Research Foundation of Korea, Ministry of Education
- 1,110 View
- 44 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
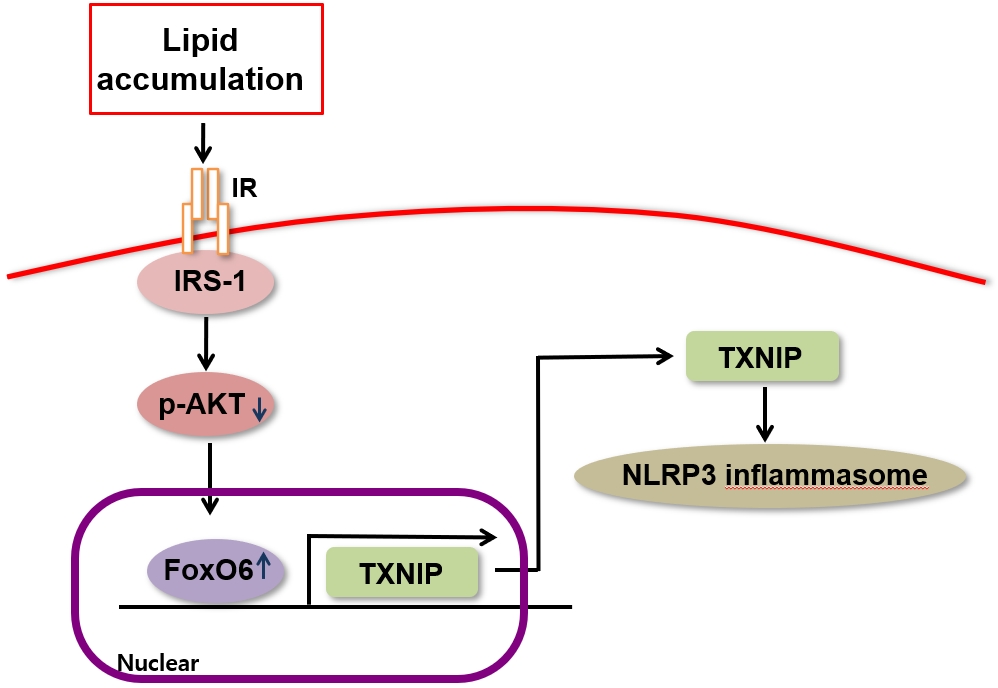
ePub - Background
Hepatic steatosis, which involves the excessive accumulation of lipid droplets in hepatocytes, presents a significant global health concern due to its association with obesity and metabolic disorders. Inflammation plays a crucial role in the progression of hepatic steatosis; however, the precise molecular mechanisms responsible for this process remain unknown.
Methods
This study investigated the involvement of the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NLRP3) inflammasome and the forkhead box O6 (FoxO6) transcription factor in the pathogenesis of hepatic steatosis. We monitored the NLRP3 inflammasome and lipogenesis in mice overexpressing the constitutively active (CA)-FoxO6 allele and FoxO6-null mice. In an in vitro study, we administered palmitate to liver cells overexpressing CA-FoxO6 and measured changes in lipid metabolism.
Results
We administered palmitate treatment to clarify the mechanisms through which FoxO6 activates cytokine interleukin (IL)-1β through the NLRP3 inflammasome. The initial experiments revealed that dephosphorylation led to palmitate-induced FoxO6 transcriptional activity. Further palmitate experiments showed increased expression of IL-1β and the hepatic NLRP3 inflammasome complex, including adaptor protein apoptotic speck protein containing a caspase recruitment domain (ASC) and pro-caspase-1. Furthermore, thioredoxin-interacting protein (TXNIP), a key regulator of cellular redox conditions upstream of the NLRP3 inflammasome, was induced by FoxO6 in the liver and HepG2 cells.
Conclusion
The findings of this study shed light on the molecular mechanisms underpinning the FoxO6-NLRP3 inflammasome axis in promoting inflammation and lipid accumulation in the liver.

- Diabetes, obesity and metabolism
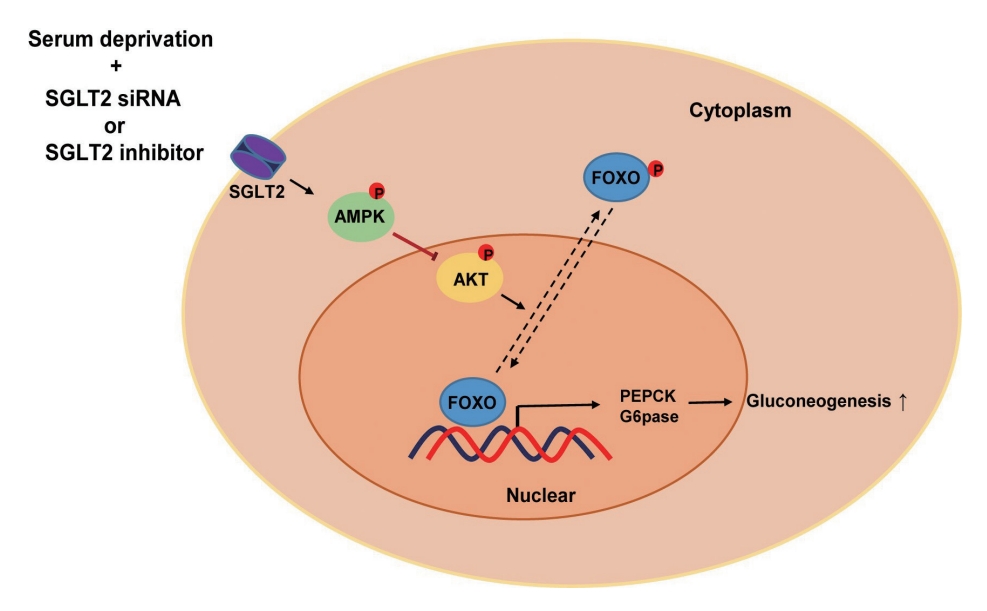
- Inhibition of Sodium-Glucose Cotransporter-2 during Serum Deprivation Increases Hepatic Gluconeogenesis via the AMPK/AKT/FOXO Signaling Pathway
- Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Yu-Mi Lim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
- Endocrinol Metab. 2024;39(1):98-108. Published online January 3, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1786
- Funded: National Research Foundation of Korea
- 1,399 View
- 80 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Sodium-dependent glucose cotransporter 2 (SGLT2) mediates glucose reabsorption in the renal proximal tubules, and SGLT2 inhibitors are used as therapeutic agents for treating type 2 diabetes mellitus. This study aimed to elucidate the effects and mechanisms of SGLT2 inhibition on hepatic glucose metabolism in both serum deprivation and serum supplementation states.
Methods
Huh7 cells were treated with the SGLT2 inhibitors empagliflozin and dapagliflozin to examine the effect of SGLT2 on hepatic glucose uptake. To examine the modulation of glucose metabolism by SGLT2 inhibition under serum deprivation and serum supplementation conditions, HepG2 cells were transfected with SGLT2 small interfering RNA (siRNA), cultured in serum-free Dulbecco’s modified Eagle’s medium for 16 hours, and then cultured in media supplemented with or without 10% fetal bovine serum for 8 hours.
Results
SGLT2 inhibitors dose-dependently decreased hepatic glucose uptake. Serum deprivation increased the expression levels of the gluconeogenesis genes peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α), glucose 6-phosphatase (G6pase), and phosphoenolpyruvate carboxykinase (PEPCK), and their expression levels during serum deprivation were further increased in cells transfected with SGLT2 siRNA. SGLT2 inhibition by siRNA during serum deprivation induces nuclear localization of the transcription factor forkhead box class O 1 (FOXO1), decreases nuclear phosphorylated-AKT (p-AKT), and p-FOXO1 protein expression, and increases phosphorylated-adenosine monophosphate-activated protein kinase (p-AMPK) protein expression. However, treatment with the AMPK inhibitor, compound C, reversed the reduction in the protein expression levels of nuclear p- AKT and p-FOXO1 and decreased the protein expression levels of p-AMPK and PEPCK in cells transfected with SGLT2 siRNA during serum deprivation.
Conclusion
These data show that SGLT2 mediates glucose uptake in hepatocytes and that SGLT2 inhibition during serum deprivation increases gluconeogenesis via the AMPK/AKT/FOXO1 signaling pathway.

Review Articles
- Thyroid
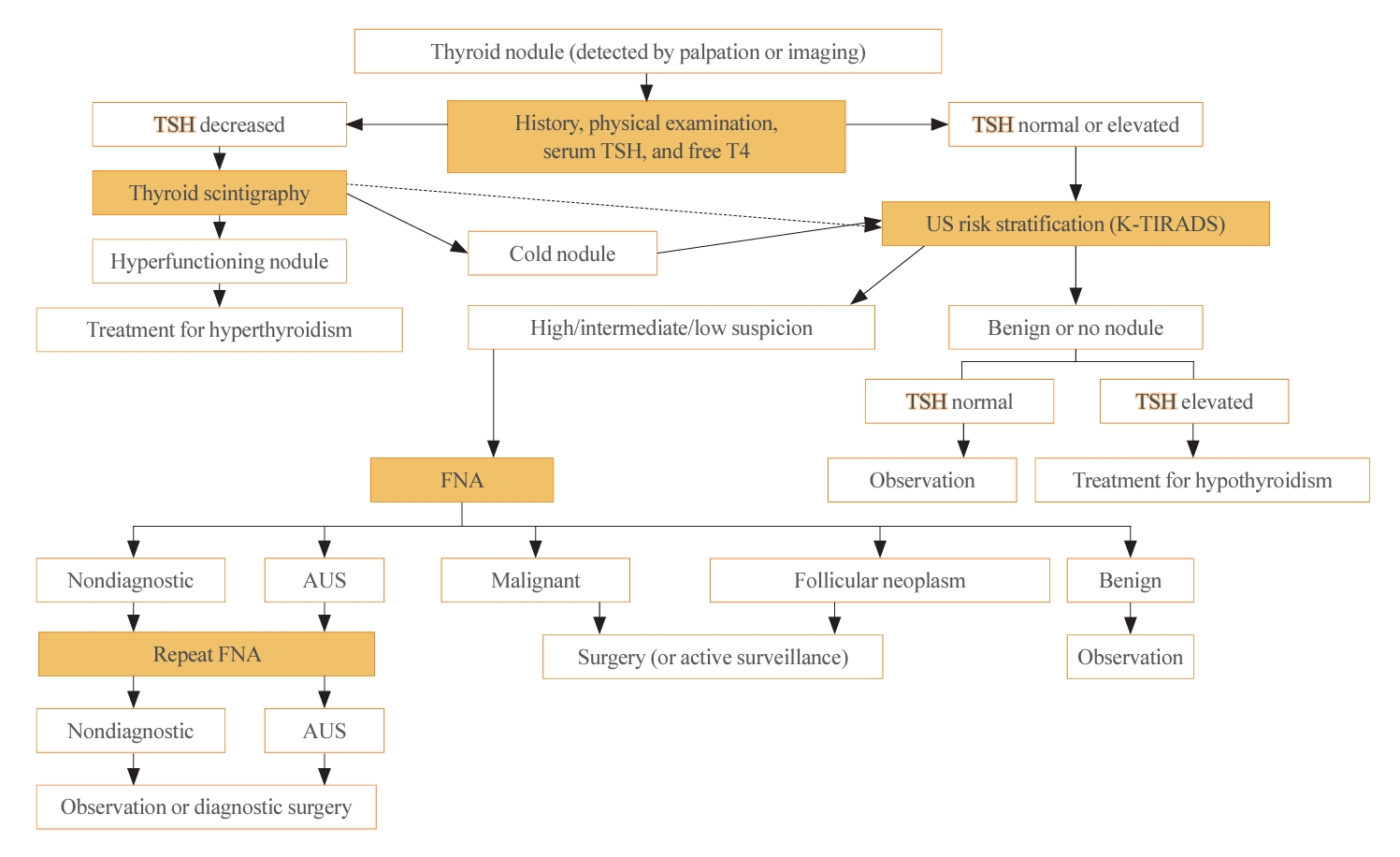
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
- Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
- Endocrinol Metab. 2024;39(1):61-72. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1938
- Funded: National Cancer Center, Ministry of Health and Welfare
- 1,507 View
- 95 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The 2023 Korean Thyroid Association (KTA) Management Guideline for Patients with Thyroid Nodules constitute an update of the 2016 KTA guideline for thyroid nodules and cancers that focuses specifically on nodules. The 2023 guideline aim to offer updated guidance based on new evidence that reflects the changes in clinical practice since the 2016 KTA guideline. To update the 2023 guideline, a comprehensive literature search was conducted from January 2022 to May 2022. The literature search included studies, reviews, and other evidence involving human subjects that were published in English in MEDLINE (PubMed), Embase, and other relevant databases. Additional significant clinical trials and research studies published up to April 2023 were also reviewed. The limitations of the current evidence are discussed, and suggestions for areas in need of further research are identified. The purpose of this review is to provide a summary of the 2023 KTA guideline for the management of thyroid nodules released in May 2023 and to give a balanced insight with comparison of recent guidelines from other societies.

- Thyroid
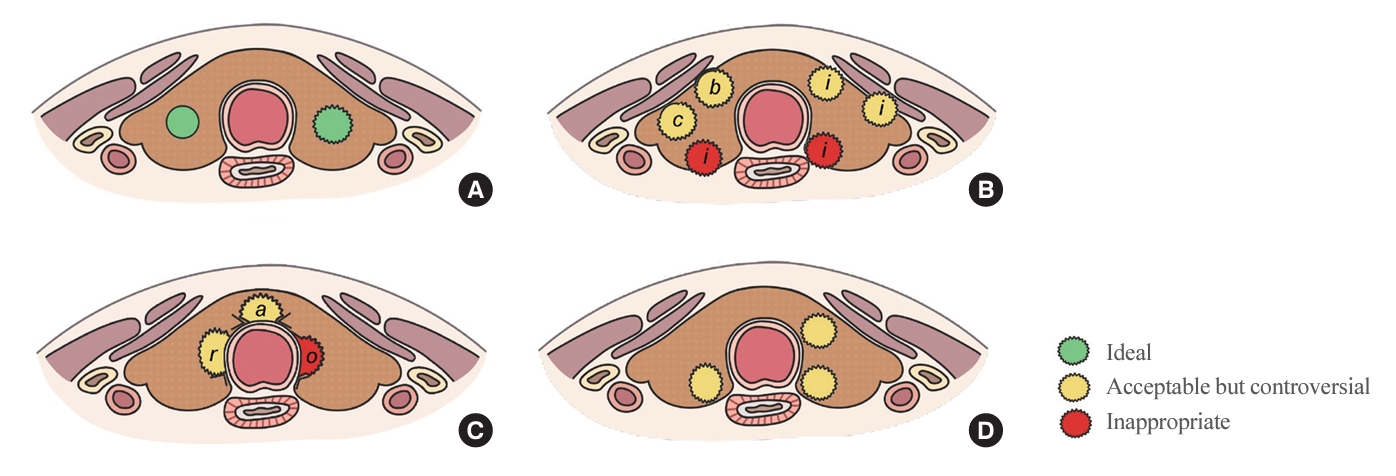
- Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Min Joo Kim, Jae Hoon Moon, Eun Kyung Lee, Young Shin Song, Kyong Yeun Jung, Ji Ye Lee, Ji-hoon Kim, Kyungsik Kim, Sue K. Park, Young Joo Park
- Endocrinol Metab. 2024;39(1):47-60. Published online February 15, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1937
- Funded: Ministry of Health and Welfare, National Research Foundation of Korea, Ministry of Education
- 1,898 View
- 173 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.

- Thyroid
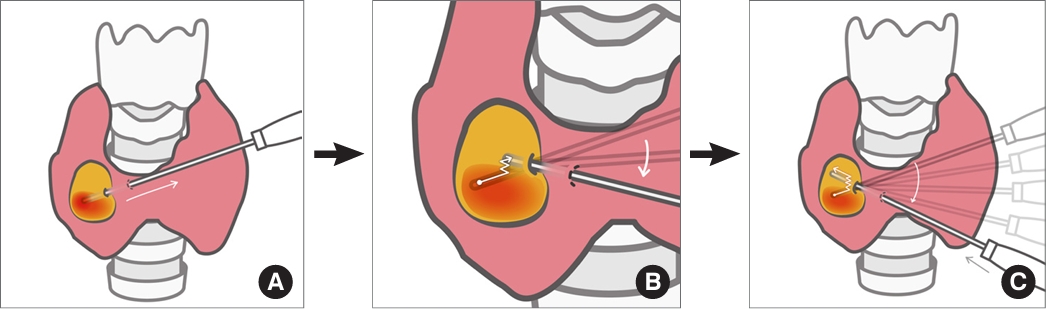
- Novel and Advanced Ultrasound Techniques for Thyroid Thermal Ablation
- Wai-Kin Chan, Jui-Hung Sun, Miaw-Jene Liou, Chia-Jung Hsu, Yu-Ling Lu, Wei-Yu Chou, Yan-Rong Li, Feng-Hsuan Liu
- Endocrinol Metab. 2024;39(1):40-46. Published online February 13, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1917
- Funded: Chang Gung Medical Research Project
- 1,708 View
- 113 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Thyroid radiofrequency ablation and microwave ablation are widely adopted minimally invasive treatments for diverse thyroid conditions worldwide. Fundamental skills such as the trans-isthmic approach and the moving shot technique are crucial for performing thyroid ablation, and advanced techniques, including hydrodissection and vascular ablation, improve safety and efficacy and reduce complications. Given the learning curve associated with ultrasound-guided therapeutic procedures, operators need training and experience. While training models exist, limited attention has been given to ultrasound maneuvers in ablation needle manipulation. This article introduces two essential maneuvers, the zigzag moving technique and the alienate maneuver, while also reviewing the latest ultrasound techniques in thyroid ablation, contributing valuable insights into this evolving field.

- Diabetes, obesity and metabolism
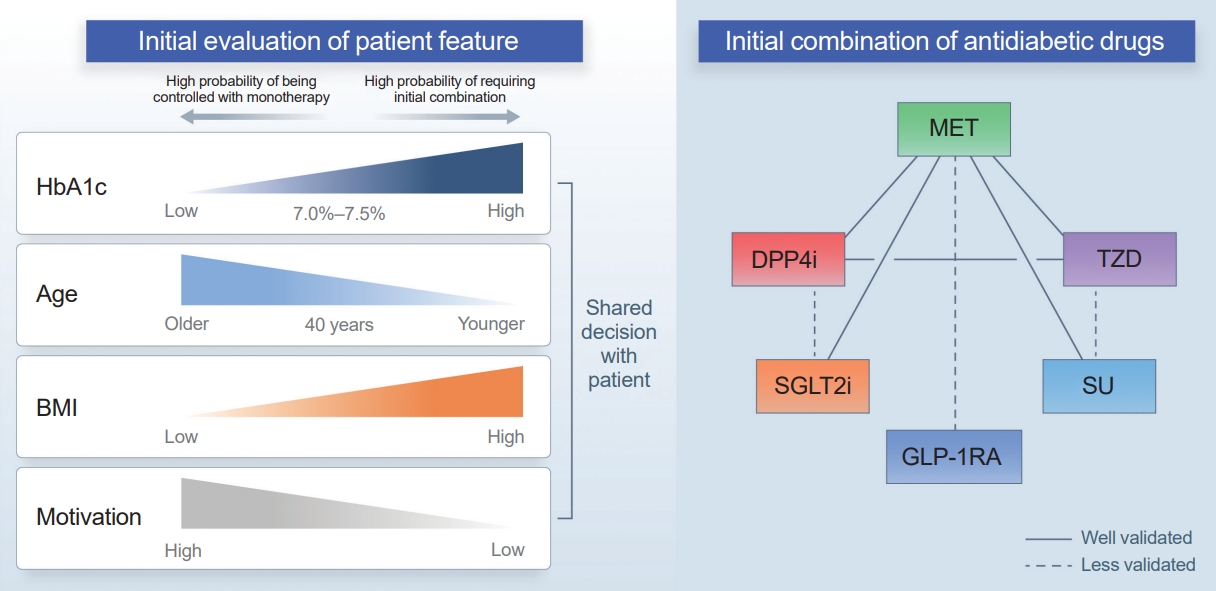
- Initial Combination Therapy in Type 2 Diabetes
- Ji Yoon Kim, Nam Hoon Kim
- Endocrinol Metab. 2024;39(1):23-32. Published online November 30, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1816
- Funded: Korean Endocrine Society
- 2,059 View
- 249 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Type 2 diabetes (T2D) is a progressive disease in which it is challenging to achieve long-term durable glycemic control. However, intensive glycemic control is crucial for preventing diabetes-related complications. Previous studies showed that monotherapy with a stepwise add-on approach was seldom effective for long-term durable glycemic control. Combination therapy, which refers to the use of two or more drugs to control hyperglycemia, has multiple benefits, including the ability to target a variety of pathophysiological processes underlying hyperglycemia. In clinical trials, initial combination therapy showed better glycemic control than monotherapy or a stepwise approach. Emerging evidence indicates that initial combination therapy is associated with preserved β-cell function and fewer complications in T2D. However, cost-effectiveness and adverse events with combination therapy are issues that should be considered. Therefore, initial combination therapy is an important option for patients with T2D that clinicians should consider with a view toward balancing benefits and potential harms. In this review, we summarize the literature addressing initial combination therapy in T2D, and we suggest optimal strategies based on clinical situations and patient characteristics.

- Diabetes, obesity and metabolism
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
- Agnieszka Jakubowska, Carel W. le Roux, Adie Viljoen
- Endocrinol Metab. 2024;39(1):12-22. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1942
- Funded: Amarin, Amgen, Astra Zeneca, Boehringer Ingleheim, Daiichi-Sankyo, Eli Lilly Japan, Menarini, NewAmsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron Pharmaceuticals, Sanofi, Takeda Foundation, Tosoh, Irish Research Council, Science Foundation Ireland, Anabio, Health Research Board

- 2,593 View
- 207 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Obesity is the fifth leading risk factor for global deaths with numbers continuing to increase worldwide. In the last 20 years, the emergence of pharmacological treatments for obesity based on gastrointestinal hormones has transformed the therapeutic landscape. The successful development of glucagon-like peptide-1 (GLP-1) receptor agonists, followed by the synergistic combined effect of glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists achieved remarkable weight loss and glycemic control in those with the diseases of obesity and type 2 diabetes. The multiple cardiometabolic benefits include improving glycemic control, lipid profiles, blood pressure, inflammation, and hepatic steatosis. The 2023 phase 2 double-blind, randomized controlled trial evaluating a GLP-1/GIP/glucagon receptor triagonist (retatrutide) in patients with the disease of obesity reported 24.2% weight loss at 48 weeks with 12 mg retatrutide. This review evaluates the current available evidence for GLP-1 receptor agonists, dual GLP-1/GIP receptor co-agonists with a focus on GLP-1/GIP/glucagon receptor triagonists and discusses the potential future benefits and research directions.
-
Citations
Citations to this article as recorded by- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
Jorge E. Jalil, Luigi Gabrielli, María Paz Ocaranza, Paul MacNab, Rodrigo Fernández, Bruno Grassi, Paulina Jofré, Hugo Verdejo, Monica Acevedo, Samuel Cordova, Luis Sanhueza, Douglas Greig
International Journal of Molecular Sciences.2024; 25(8): 4407. CrossRef
- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review

Namgok Lecture 2023
- Diabetes, obesity and metabolism
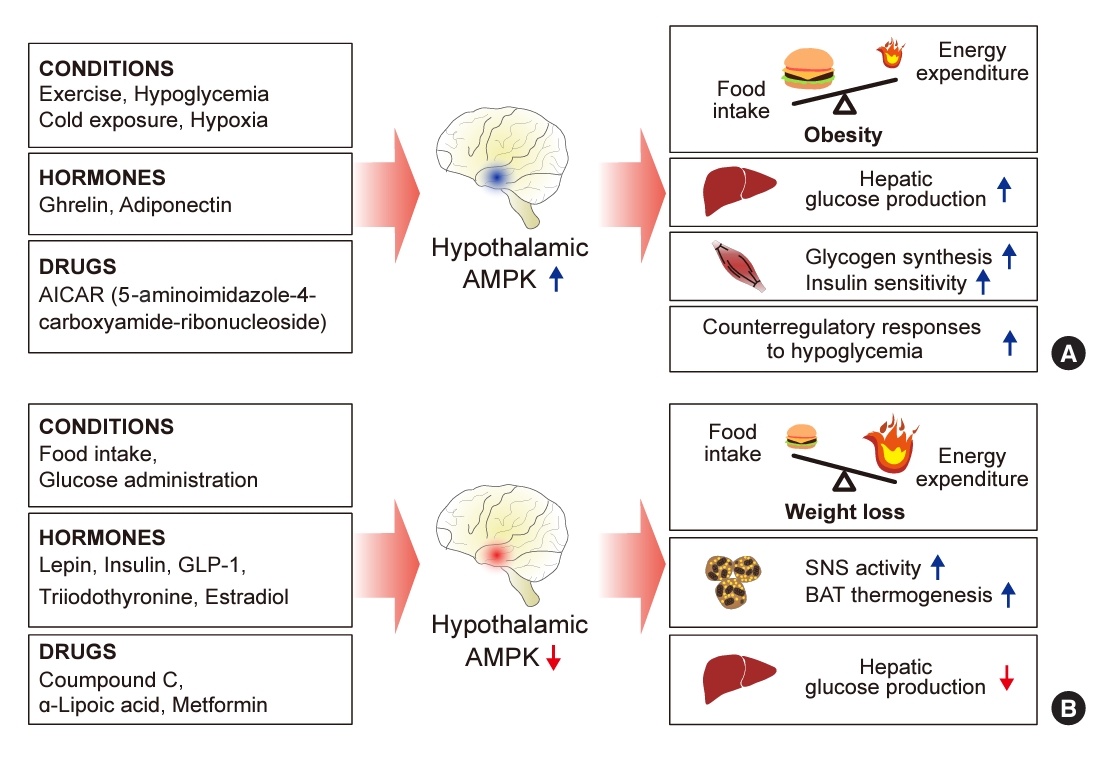
- Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator
- Se Hee Min, Do Kyeong Song, Chan Hee Lee, Eun Roh, Min-Seon Kim
- Endocrinol Metab. 2024;39(1):1-11. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1922
- Funded: National Research Foundation of Korea, Ministry of Science and ICT
- 1,883 View
- 74 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - 5´-Adenosine monophosphate (AMP)-activated protein kinase (AMPK), a cellular energy sensor, is an essential enzyme that helps cells maintain stable energy levels during metabolic stress. The hypothalamus is pivotal in regulating energy balance within the body. Certain neurons in the hypothalamus are sensitive to fluctuations in food availability and energy stores, triggering adaptive responses to preserve systemic energy equilibrium. AMPK, expressed in these hypothalamic neurons, is instrumental in these regulatory processes. Hypothalamic AMPK activity is modulated by key metabolic hormones. Anorexigenic hormones, including leptin, insulin, and glucagon-like peptide 1, suppress hypothalamic AMPK activity, whereas the hunger hormone ghrelin activates it. These hormonal influences on hypothalamic AMPK activity are central to their roles in controlling food consumption and energy expenditure. Additionally, hypothalamic AMPK activity responds to variations in glucose concentrations. It becomes active during hypoglycemia but is deactivated when glucose is introduced directly into the hypothalamus. These shifts in AMPK activity within hypothalamic neurons are critical for maintaining glucose balance. Considering the vital function of hypothalamic AMPK in the regulation of overall energy and glucose balance, developing chemical agents that target the hypothalamus to modulate AMPK activity presents a promising therapeutic approach for metabolic conditions such as obesity and type 2 diabetes mellitus.

Brief Report
- Diabetes, obesity and metabolism
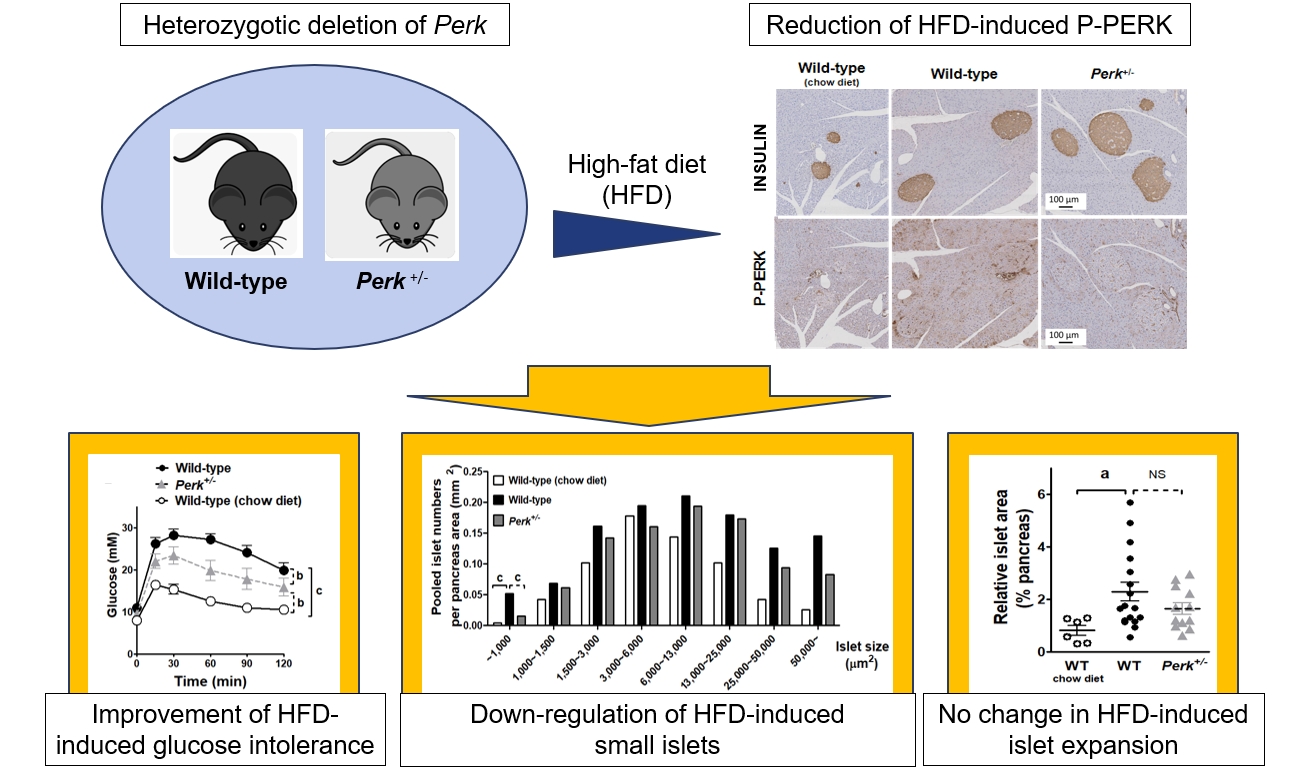
- Partial Deletion of Perk Improved High-Fat Diet-Induced Glucose Intolerance in Mice
- Jooyeop Lee, Min Joo Kim, Seoil Moon, Ji Yoon Lim, Kyong Soo Park, Hye Seung Jung
- Endocrinol Metab. 2023;38(6):782-787. Published online November 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1738
- Funded: National Research Foundation of Korea, Ministry of Science and ICT, Seoul National University Hospital
- 721 View
- 45 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Although pancreatic endoplasmic reticulum kinase (PERK) is indispensable to beta cells, low-dose PERK inhibitor improved glucose- stimulated insulin secretion (GSIS) and hyperglycemia in diabetic mice. Current study examined if partial deletion of Perk (Perk+/-) recapitulated the effects of PERK inhibitor, on the contrary to the complete deletion. Perk+/- mice and wild-type controls were fed with a high-fat diet (HFD) for 23 weeks. Glucose tolerance was evaluated along with serum insulin levels and islet morphology. Perk+/- mice on normal chow were comparable to wild-type mice in various metabolic features. HFD-induced obesity was not influenced by Perk reduction; however, HFD-induced glucose intolerance was significantly improved since 15-week HFD. HFD-induced compromises in GSIS were relieved by Perk reduction, accompanied by reductions in phosphorylated PERK and activating transcription factor 4 (ATF4) in the islets. Meanwhile, HFD-induced islet expansion was not significantly affected. In summary, partial deletion of Perk improved glucose tolerance and GSIS impaired by diet-induced obesity, without changes in body weights or islet mass.

Original Articles
- Diabetes, obesity and metabolism
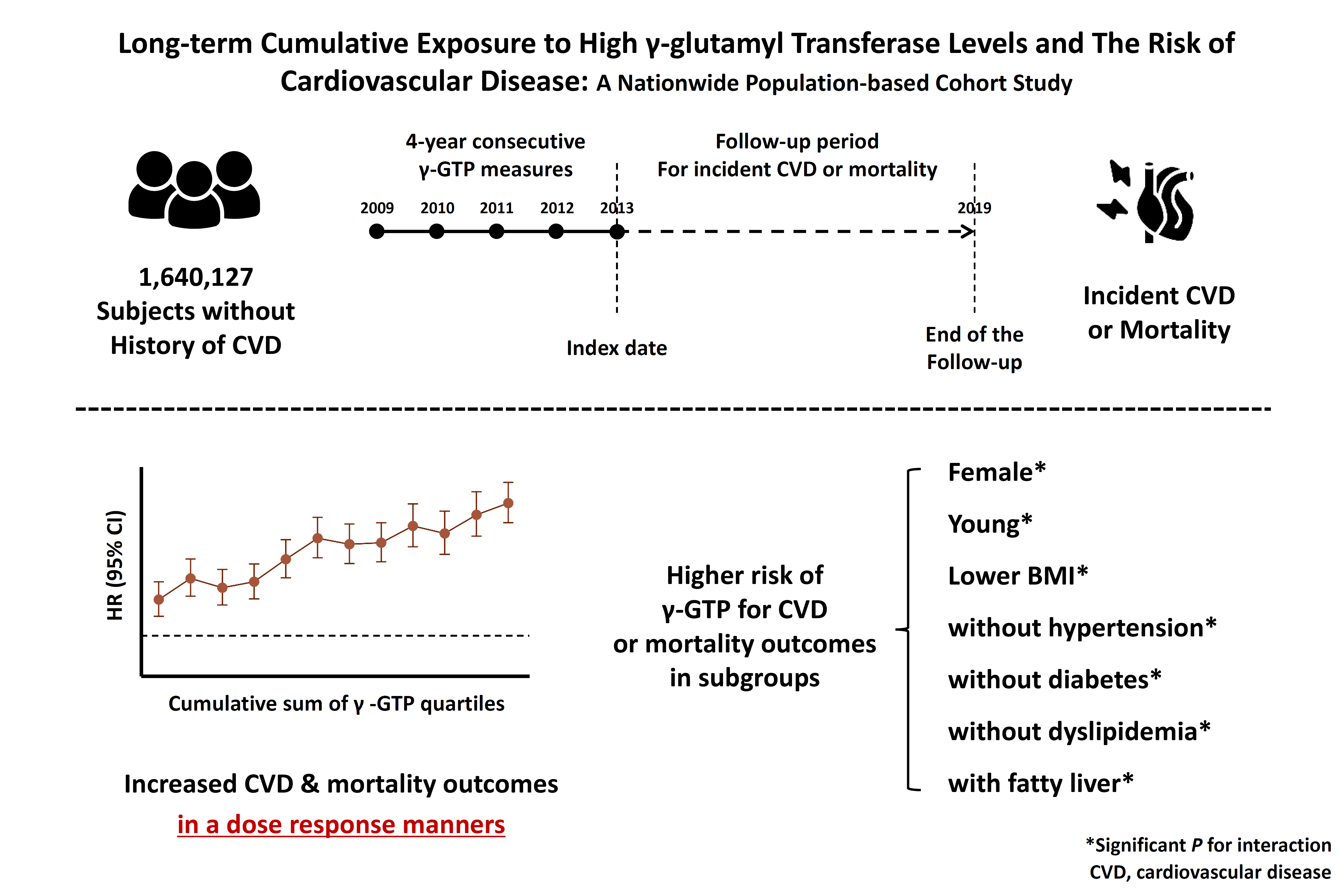
Big Data Articles (National Health Insurance Service Database) - Long-Term Cumulative Exposure to High γ-Glutamyl Transferase Levels and the Risk of Cardiovascular Disease: A Nationwide Population-Based Cohort Study
- Han-Sang Baek, Bongseong Kim, Seung-Hwan Lee, Dong-Jun Lim, Hyuk-Sang Kwon, Sang-Ah Chang, Kyungdo Han, Jae-Seung Yun
- Endocrinol Metab. 2023;38(6):770-781. Published online November 6, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1726
- Funded: National Research Foundation of Korea, Ministry of Science and Information and Communication Technologies
- 1,113 View
- 48 Download
- 1 Web of Science
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Elevated γ-glutamyl transferase (γ-GTP) levels are associated with metabolic syndrome. We investigated the association of cumulative exposure to high γ-GTP with the risk of cardiovascular disease (CVD) in a large-scale population.
Methods
Using nationally representative data from the Korean National Health Insurance system, 1,640,127 people with 4 years of consecutive γ-GTP measurements from 2009 to 2012 were included and followed up until the end of 2019. For each year of the study period, participants were grouped by the number of exposures to the highest γ-GTP quartile (0–4), and the sum of quartiles (0–12) was defined as cumulative γ-GTP exposure. The hazard ratio for CVD was evaluated using the Cox proportional hazards model.
Results
During the 6.4 years of follow-up, there were 15,980 cases (0.97%) of myocardial infarction (MI), 14,563 (0.89%) of stroke, 29,717 (1.81%) of CVD, and 25,916 (1.58%) of death. Persistent exposure to high γ-GTP levels was associated with higher risks of MI, stroke, CVD, and death than those without such exposure. The risks of MI, stroke, CVD, and mortality increased in a dose-dependent manner according to total cumulative γ-GTP (all P for trend <0.0001). Subjects younger than 65 years, with a body mass index <25 kg/m2, and without hypertension or fatty liver showed a stronger relationship between cumulative γ-GTP and the incidence of MI, CVD, and death.
Conclusion
Cumulative γ-GTP elevation is associated with CVD. γ-GTP could be more widely used as an early marker of CVD risk, especially in individuals without traditional CVD risk factors.

- Miscellaneous
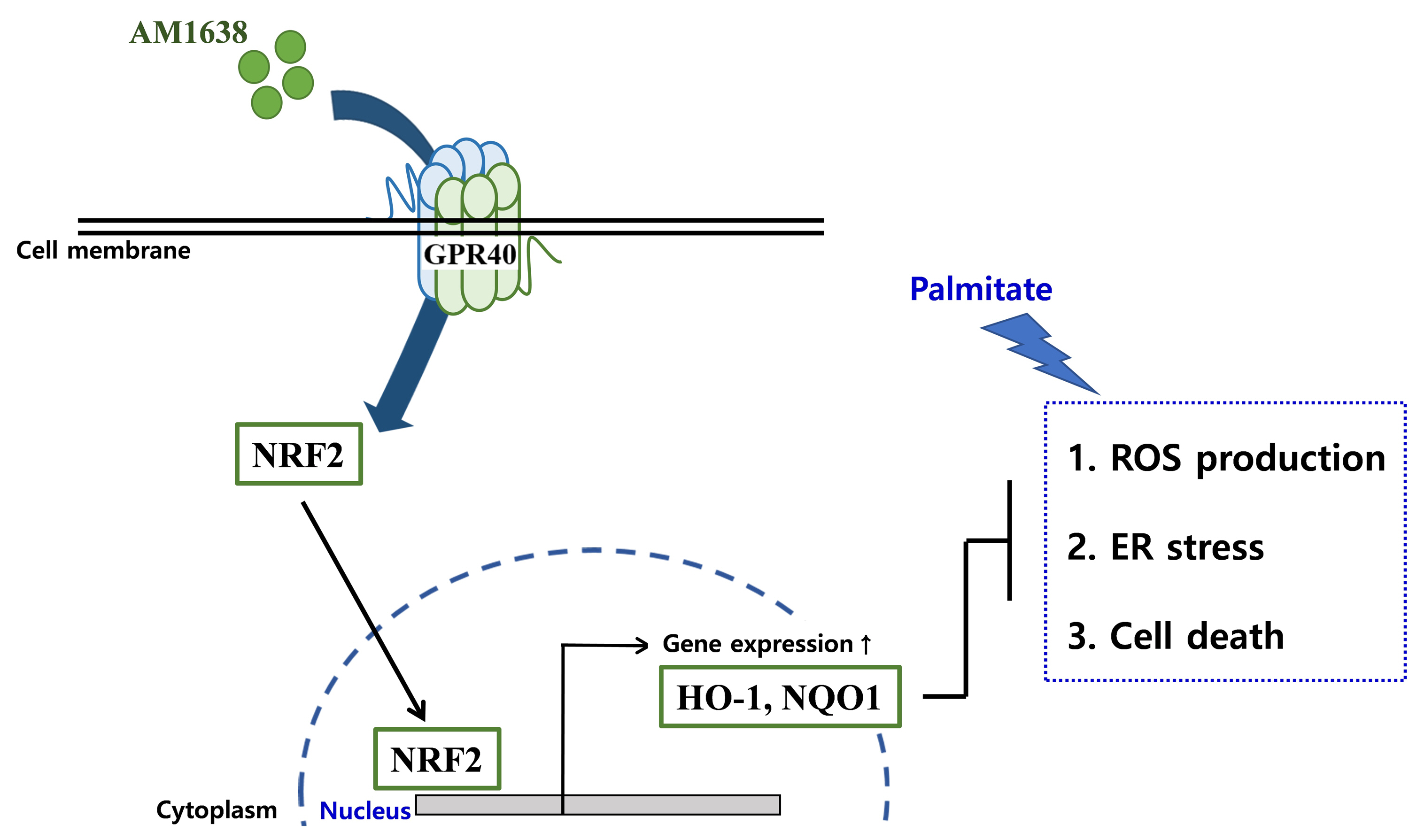
- AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Hwan-Jin Hwang, Joo Won Kim, SukHwan Yun, Min Jeong Park, Eyun Song, Sooyeon Jang, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
- Endocrinol Metab. 2023;38(6):760-769. Published online November 2, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1774
- Funded: National Research Foundation of Korea, Ministry of Education, Korean Endocrine Society
- 1,193 View
- 85 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
G protein-coupled receptor 40 (GPR40) is a key molecule in diabetes and fatty liver, but its role in endothelial dysfunction remains unclear. Our objective in this study was to determine whether GPR40 agonists protect endothelial cells against palmitatemediated oxidative stress.
Methods
Human umbilical vein endothelial cells (HUVECs) were used to investigate effects of various GPR40 agonists on vascular endothelium.
Results
In HUVECs, AM1638, a GPR40-full agonist, enhanced nuclear factor erythroid 2–related factor 2 (NRF2) translocation to the nucleus and heme oxygenase-1 (HO-1) expression, which blocked palmitate-induced superoxide production. Those antioxidant effects were not detected after treatment with LY2922470 or TAK875, GPR40-partial agonists, suggesting that GPR40 regulates reactive oxygen species (ROS) removal in a ligand-dependent manner. We also found that palmitate-induced CCAAT/enhancer‐binding protein homologous protein expression; X-box binding protein-1 splicing, nuclear condensation, and fragmentation; and caspase-3 cleavage were all blocked in an NRF2-dependent manner after AM1638 treatment. Both LY2922470 and TAK875 also improved cell viability independent of the NRF2/ROS pathway by reducing palmitate-mediated endoplasmic reticulum stress and nuclear damage. GPR40 agonists thus have beneficial effects against palmitate in HUVECs. In particular, AM1638 reduced palmitate-induced superoxide production and cytotoxicity in an NRF2/HO-1 dependent manner.
Conclusion
GPR40 could be developed as a good therapeutic target to prevent or treat cardiovascular diseases such as atherosclerosis.

- Miscellaneous
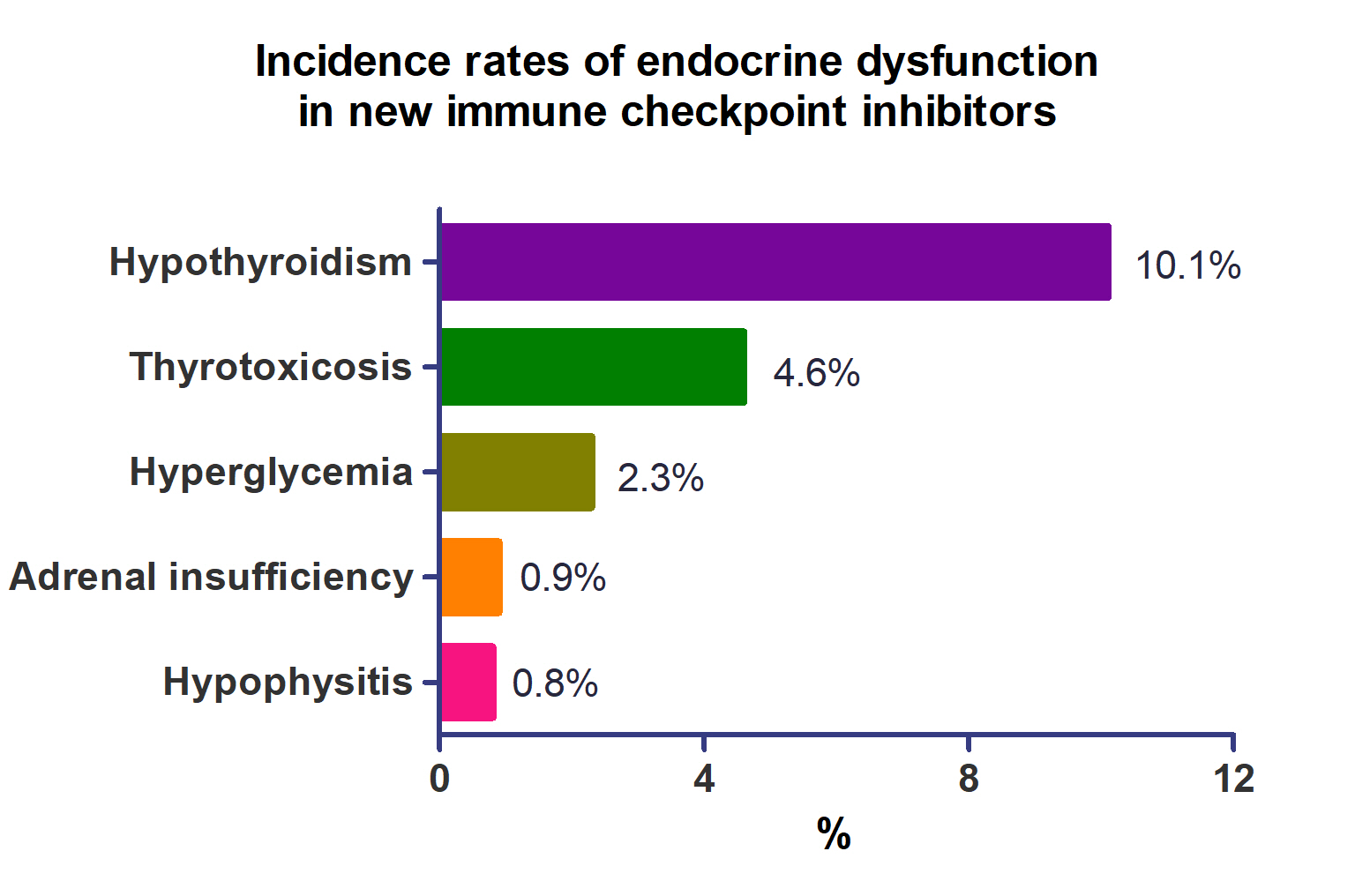
- Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
- Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
- Endocrinol Metab. 2023;38(6):750-759. Published online November 13, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1785
- Funded: Korean Endocrine Society, Ministry of Health and Welfare, National Cancer Center
- 1,443 View
- 121 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigated the incidence of endocrine immune-related adverse events (irAEs) for recently developed immune checkpoint inhibitor (ICI) drugs.
Methods
We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through January 31, 2023. Among ICI drugs, nivolumab, pembrolizumab, and ipilimumab were excluded from the new ICI drugs because many papers on endocrine-related side effects have already been published.
Results
A total of 44,595 patients from 177 studies were included in this analysis. The incidence of hypothyroidism was 10.1% (95% confidence interval [CI], 8.9% to 11.4%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%). Hypothyroidism and thyrotoxicosis occurred most frequently with programmed cell death protein-1 (PD-1) inhibitors (13.7% and 7.5%, respectively). The rate of endocrine side effects for the combination of a programmed death-ligand 1 inhibitor (durvalumab) and cytotoxic T lymphocyte-associated antigen 4 inhibitor (tremelimumab) was higher than that of monotherapy. In a meta-analysis, the combination of tremelimumab and durvalumab had a 9- to 10-fold higher risk of pituitary and adrenal-related side effects than durvalumab alone.
Conclusion
Newly developed PD-1 inhibitors had a high incidence of thyroid-related irAEs, and combined treatment with durvalumab and tremelimumab increased the risk of pituitary- and adrenal-related irAEs. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.


 KES
KES

 First
First Prev
Prev



