Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Original ArticleThyroid Hashimoto Thyroiditis and Mortality in Patients with Differentiated Thyroid Cancer: The National Epidemiologic Survey of Thyroid Cancer in Korea and Meta-Analysis
 Keypoint
Keypoint
This study performed two types of studies to elucidate the prognosis of Hashimoto's thyroiditis for mortality in patients with differentiated thyroid carcinoma, or DTC. In a restrictive cohort study and meta-analysis, the coexistence of Hashimoto’s thyroiditis and DTC was associated with a low risk of advanced DTC and presented a low risk for all-cause and DTC-specific death. -
Injung Yang1
 , Jae Myung Yu2, Hye Soo Chung2, Yoon Jung Kim2, Yong Kyun Roh1, Min Kyu Choi1, Sung-ho Park3
, Jae Myung Yu2, Hye Soo Chung2, Yoon Jung Kim2, Yong Kyun Roh1, Min Kyu Choi1, Sung-ho Park3 , Young Joo Park4,5,6, Shinje Moon2
, Young Joo Park4,5,6, Shinje Moon2
-
Endocrinology and Metabolism 2024;39(1):140-151.
DOI: https://doi.org/10.3803/EnM.2023.1748
Published online: January 3, 2024
1Department of Family Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
2Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
3Department of Obstetrics & Gynecology,Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
4Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
5Genomic Medical Institute Seoul National University Medical Research Center, Seoul, Korea
6Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- Corresponding authors: Shinje Moon. Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, 1 Singil-ro, Yeongdeungpo-gu, Seoul 07441, Korea Tel: +82-2-846-5326, Fax: +82-2-846-4669, E-mail: sinjei82@hanmail.net
- Sung-ho Park. Department of Obstetrics & Gynecology, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, 1 Singil-ro, Yeongdeungpo-gu, Seoul 07441, Korea Tel: +82-2-829-5152, Fax: +82-2-846-4669, E-mail: vth2000@hallym.or.kr
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,009 Views
- 52 Download
ABSTRACT
-
Background
- Many studies have shown that Hashimoto’s thyroiditis (HT) acts as a protective factor in differentiated thyroid cancer (DTC), but little is known about its effects on mortality. Therefore, this study was performed to reveal the prognosis of HT on mortality in patients with DTC.
-
Methods
- This study included two types of research results: retrospective cohort study using the National Epidemiologic Survey of Thyroid cancer (NEST) in Korea and meta-analysis study with the NEST data and eight selected studies.
-
Results
- Of the 4,398 patients with DTC in NEST, 341 patients (7.8%) died during the median follow-up period of 15 years (interquartile range, 12.3 to 15.6). Of these, 91 deaths (2.1%) were related to DTC. HT was associated with a smaller tumor size and less aggressive DTC. In Cox regression analysis after adjusting for age and sex, patients with HT showed a significantly lower risk of all-cause death (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.52 to 0.96) and DTC-related death (HR, 0.33; 95% CI, 0.14 to 0.77). The analysis with inverse probability of treatment weight data adjusted for age, sex, and year of thyroid cancer registration showed similar association. The meta-analysis showed that patients with HT showed a lower risk of all-cause mortality (risk ratio [RR], 0.24; 95% CI, 0.13 to 0.47) and thyroid cancer-related mortality (RR, 0.23; 95% CI, 0.13 to 0.40) in comparison with patients without HT.
-
Conclusion
- This study showed that DTC co-presenting with HT is associated with a low risk of advanced DTC and presents a low risk for all-cause and DTC-related death.
- Over the past 40 years, the incidence of thyroid cancer has increased steadily [1,2]. In general, thyroid cancer more commonly affects women than men, and according to Pizzato et al. [1], the global incidence of thyroid cancer was 10.1 per 100,000 population in women and 3.1 per 100,000 population in men. Thyroid cancer is the most common endocrine malignancy, and papillary thyroid carcinoma (PTC) accounts for 80% to 90% of all thyroid cancer cases [3,4]. PTC is the most common type as well as the type showing the best clinical outcomes [5], and it is categorized as differentiated thyroid cancer (DTC) along with follicular thyroid carcinoma (FTC) [4,6].
- Hashimoto’s thyroiditis (HT) is an autoimmune thyroid disease characterized by lymphocytic infiltration of the thyroid and high levels of thyroid-specific antibodies [7]. The incidence of HT is 0.3–1.5 per 1,000 [8], and similar to PTC, the incidence of HT has been steadily increasing over the past 30 years [9]. The coincidence of HT and DTC has been reported to range from 5% to 85% by previous epidemiological studies [10].
- Since Dailey et al. [11] first proposed the association between HT and PTC in 1955, many studies have suggested that HT is associated with the less invasive and less aggressive clinicopathologic features of PTC [10,12]. Furthermore, reports showing that HT is associated with a low recurrence rate of DTC shed light on the possibility that HT acts as a protective factor against poor prognosis of DTC [6,10,13-15], but the effects of HT on the mortality from DTC have been rarely reported. Metaanalysis reported that HT reduces the risk for all-cause mortality in thyroid cancer [16,17], but no meta-analysis has been conducted on thyroid cancer-related mortality due to lack of data.
- This study aimed to investigate the association between HT and all-cause and DTC-related mortality using data from the National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Additionally, we performed a meta-analysis to provide systematic evidence of the effect of HT on mortality.
INTRODUCTION
- NEST study: study design and participants
- The NEST study was a retrospective nationwide study of patients with thyroid cancer. Twenty-four hospitals were selected from the list of hospitals that had admitted patients with thyroid cancer such that at least one hospital from each of the 12 administrative districts in Korea was selected. Thereafter, based on the proportion of patients registered in the sample hospital from the corresponding region, patients with thyroid cancer registered in 1999, 2005, and 2008 were randomly extracted from the Korea National Cancer Incidence Database. A total of 6,846 patients with thyroid cancer were extracted, including 1,103 registered in 1999, 2,785 in 2005, and 2,958 in 2008. Of the 6,846 sampled patients, 5,796 (84.7%) were included in NEST study after excluding 960 patients due to the refusal of two hospitals and 90 patients with missing or inadequate data. Detailed information of NEST was described in the previous study [18].
- Among a total of 5,796 patients in the NEST study, 4,398 with DTC were included in the final analysis of this study after excluding 131 patients due to insufficient data, 1,157 with thyroid diseases other than HT, and 110 with other types of thyroid cancer from DTC.
- Classification of NEST
- The following clinical data were collected from a retrospective review of medical records: comorbidities, histology, tumor, node, metastasis (TNM) stage (defined by the American Joint Committee on Cancer [AJCC] 6th edition) from the postoperative pathology, and treatment. Mortality data, including the date and cause of death recorded as International Classification of Diseases 10th Revision (ICD-10) codes was extracted from the cause of death database of Statistics Korea on December 31, 2020, and linked to the NEST dataset. Thyroid cancer-related deaths were identified by the code C73.
- HT was diagnosed using specimen obtained at surgery. In the NEST study, patients were classified as having HT if their pathology report indicated the presence of HT, Hashimoto’s disease, chronic lymphocytic thyroiditis, lymphocytic thyroiditis, non-specific lymphocytic thyroiditis, autoimmune thyroiditis, or reactive thyroiditis.
- NEST study: ethical considerations
- The NEST data is a publicly open dataset. The Institutional Review Board (IRB) of the National Cancer Center approved the research protocol (No. NCC2017-0070). All procedures of NEST followed the ethical standards outlined by the IRB and the Declaration of Helsinki. Informed consent was not required because all data were fully anonymized before access.
- NEST study: statistical analysis
- Continuous variables were presented as means with standard deviation with P values related to the presence of HT obtained using the t test. Categorical variables were presented as numbers (%) with P values using the chi-square test. We compared the cumulative mortality rates according to HT using the Kaplan-Meier plot with log-rank tests. Multiple logistic regression analysis was performed to assess the odds ratios (ORs) of HT for clinicopathologic characteristics after adjusting for age and sex. Multiple Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-related mortality. We also calculated stabilized inverse probability of treatment weight (IPTW) by inversely weighting propensity scores adjusted for age, sex, and year of thyroid cancer registration according to HT to mitigate possible bias due to age, sex, and year of thyroid cancer registration. This was performed using the “inverse probability weighting (ipw)” R package (R Foundation for Statistical Computing, Vienna, Austria). We reassessed the equilibrium of covariates following the application of IPTW by examining standardized differences. A standardized difference below 0.1 was regarded as indicative of a covariate that was well-balanced. A regression-based causal mediation analysis was conducted to investigate the direct and indirect effect of HT on DTC-related mortality through advanced clinicopathologic status of DTC, using the package “Regmedint” developed by Li et al. [19]. We calculated total effect (TE), total natural direct effect (TNDE), and total natural indirect effect (TNIE) for DTC-related mortality according to HT mediated by advanced clinicopathologic status of DTC. Statistical analysis was performed using International Business Machines (IBM) SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) and R version 3.1.0 (R Foundation for Statistical Computing). P values <0.05 were considered statistically significant.
- Meta-analysis: search strategy
- This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplemental Table S1). The study protocol was registered in the Prospective Register of Systematic Reviews (number CRD: 42023388890). Two investigators (S.M. and I.Y.) refined the data extraction tables prior to data extraction. These two investigators searched citation databases, including PubMed, EMBASE and Cochrane (from inception until January 21, 2023) and extracted data independently using the predefined tables for data extraction. The search terms included combinations of the following: [(Hashimoto) OR (chronic lymphocytic thyroiditis)] AND (thyroid cancer) AND [(mortality) OR (death)].
- Meta-analysis: study selection
- Studies with the following characteristics were included: (1) Population: patients with pathological data of DTC and HT; (2) Intervention: pathological findings by thyroidectomy; (3) Comparators: patients with DTC but without HT in pathological data; (4) Outcomes: all-cause mortality and thyroid cancer-related mortality; and (5) Study design: case-control studies. We excluded studies with the following characteristics: (1) articles on animal studies or in vivo experiments; (2) articles that included only abstracts; (3) non-original articles, including expert opinions or reviews; and (4) studies with insufficient information on the pathology of HT or mortality.
- Meta-analysis: data extraction
- The following variables were independently extracted by the two investigators (S.M. and I.Y.) using the same criteria: first author, publication year, country, number of study participants, number of cases showing coexistence of HT, sex ratio, and clinicopathologic features, including tumor size, extrathyroidal extension (ETE), lymph node metastasis, distant metastasis, TNM stage, recurrence, all-cause mortality, and thyroid cancer-related mortality.
- Meta-analysis: quality assessment
- The Risk of Bias Assessment tool for Non-randomized Studies (RoBANS version 2.0) was used to assess the methodological quality of case-control studies. RoBANS evaluated the following parameters: (1) selection of participants, (2) confounding variables, (3) measurement of intervention, (4) blinding for outcome assessment, (5) incomplete outcome data, and (6) selective outcome reporting. These parameters were independently assessed by two reviewers (S.M. and I.Y.). Any discrepancies were resolved through a discussion with a third investigator (J.M.Y.).
- Meta-analysis: statistical methods
- Comparisons of all-cause and thyroid cancer mortality in relation to HT were presented as risk ratios (RRs) and 95% confidence intervals (CIs) using the Mantel-Haenszel method. Pooled RRs were calculated using a random-effects model. The heterogeneity among the studies was tested using Higgins’ I2 statistic, where I2 ≥50% indicated heterogeneity. Publication bias was tested using Egger’s test and a funnel plot. A sensitivity analysis was conducted through repeated meta-analysis after excluding each study to determine the robustness of the outcomes. All statistical analyses and graphical presentations were conducted using the Comprehensive Meta-Analysis software version 3 (Biostat Inc., Englewood, NJ, USA).
METHODS
- NEST study
- In the 4,398 patients with DTC in this study, the mean age was 45.9±12.3 years, and 84.3% of the patients were women. A total of 341 patients (7.8%) died during the median follow-up period of 15 years (interquartile range, 12.3 to 15.6). The median duration from the diagnosis of DTC to the time of death was 8.3 years (interquartile range, 4.0 to 12.3). Of these, 91 deaths (2.1%) were related to DTC. Table 1 shows the baseline characteristics in relation to the presence of HT. The mean proportion of men was higher in patients without HT than in patients with HT. Patients without HT exhibited advanced age and higher prevalence of smoking, drinking, and hypertension. In IPTW weighted data, all variables including age, sex ratio, year of registration and comorbidities were well-balanced (Table 1). The clinical characteristics of DTC were summarized in Table 2. In both unweighted and IPTW weighted data, the proportion of FTC did not differ between patients with and without HT. Patients without HT had larger tumor sizes and a higher incidence of advanced T stage (stages 3–4), ETE, lymph node metastases, and distant metastases than patients with HT (Table 2). Moreover, all-cause mortality and thyroid cancer-related mortality were significantly higher in patients without HT than in patients with HT.
- Compared to patients without HT, those with HT tended to show a smaller tumor size (Table 2) and less aggressive DTC (Table 2). Although the association with advanced stages did not reach statistical significance, HT was inversely associated with advanced T stage, ETE, and lymph node metastasis (Table 3). In addition, none of the patients with HT showed distant metastasis (Table 2). In IPTW weighted data, HT was also associated with less aggressive clinicopathologic characteristics of DTC (Table 3).
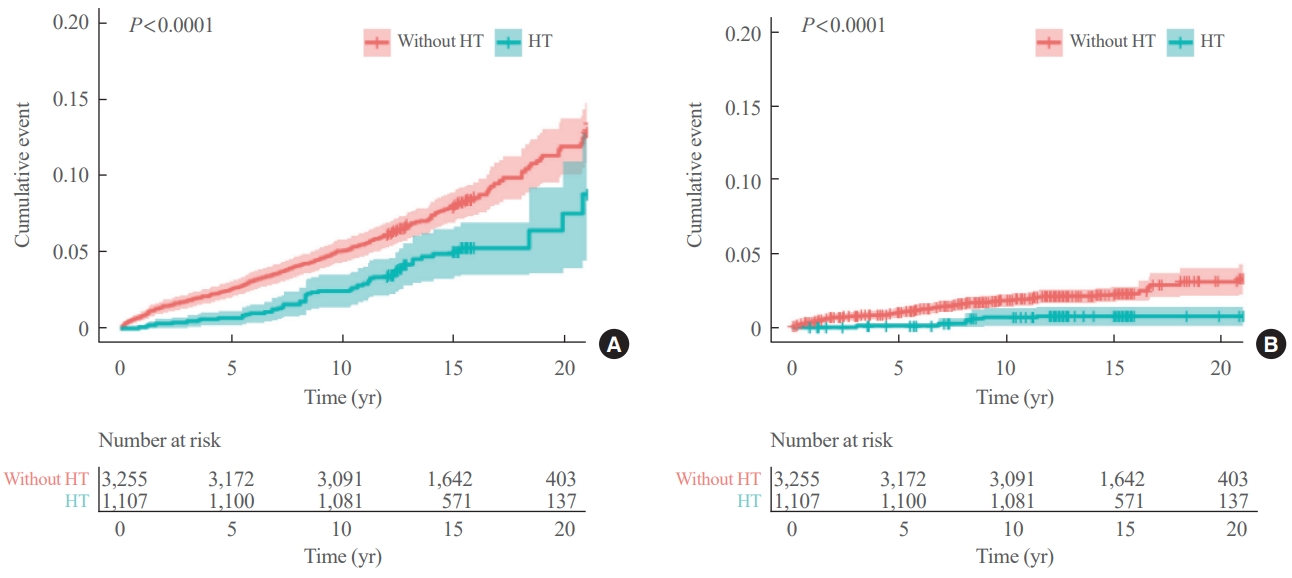
- Kaplan-Meier plot of cumulative mortality revealed a significantly higher rate of all-cause and thyroid cancer-related deaths in patients without HT than in patients with HT (log-rank test, P<0.001) (Fig. 1). In Cox regression analysis, patients with HT showed a significantly lower risk of all-cause mortality (HR, 0.71; 95% CI, 0.52 to 0.96) and DTC-related mortality (HR, 0.33; 95% CI, 0.14 to 0.77) (Table 4). The additional analysis with IPTW weighted data showed similar results (Table 4).
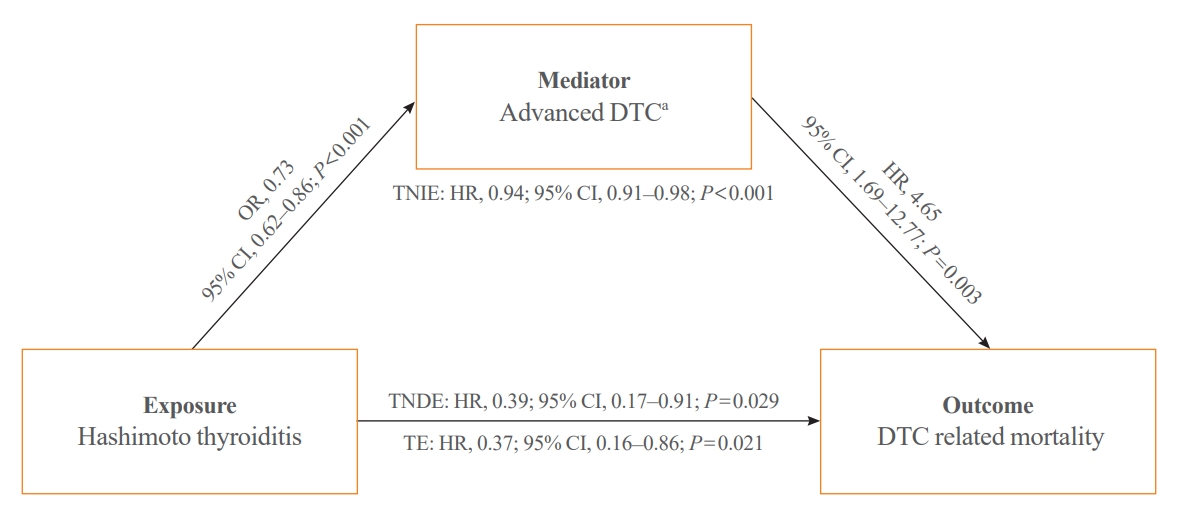
- In the mediation analysis, HT exhibited a significant correlation with a decreased risk of DTC-related death (TE: HR, 0.37; 95% CI, 0.16 to 0.86; P=0.021) (Fig. 2). This association was linked to the lower incidence of advanced DTCs among patients with HT (OR, 0.73; 95% CI, 0.62 to 0.86; P<0.001), leading to a reduction in the risk of DTC-related mortality (TNIE: HR, 0.94; 95% CI, 0.91 to 0.98; P<0.001). Additionally, HT independently showed an association with a reduced risk of DTC-related death, regardless of the lower prevalence of advanced DTCs (TNDE: HR, 0.39; 95% CI, 0.17 to 0.91; P=0.029).
- Meta-analysis
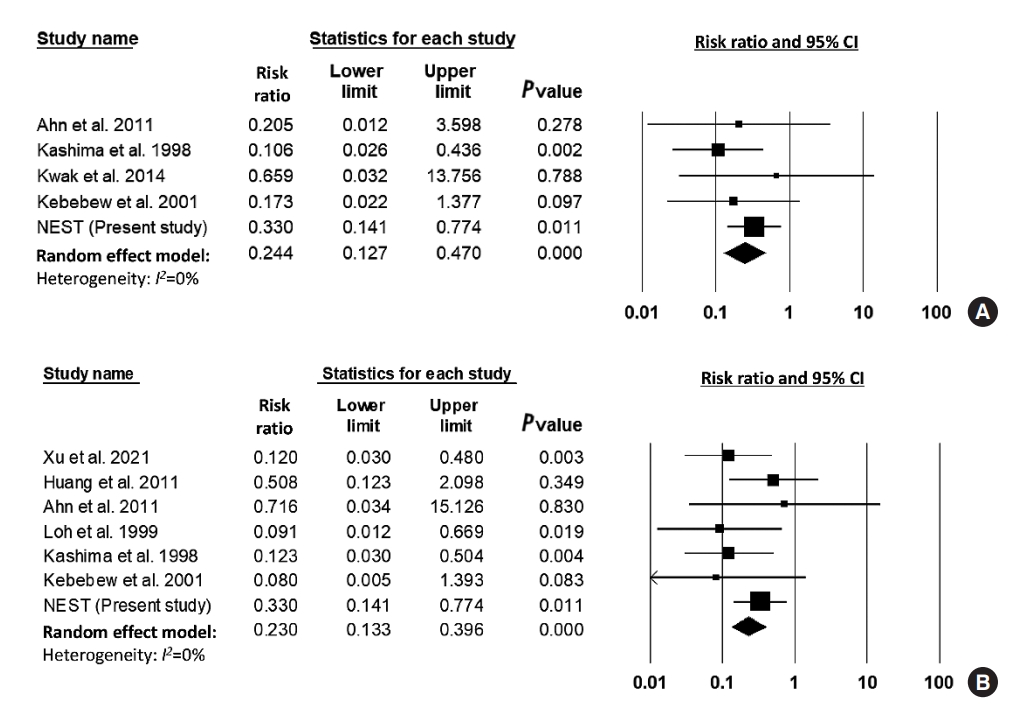
- The literature search yielded 277 studies. After the exclusion of 31 duplicate studies and 238 studies that did not meet the inclusion criteria, seven studies [6,20-25] were finally included in the meta-analysis (Fig. 3). The characteristics of each study are summarized in Table 5. A meta-analysis was performed with the seven included studies and NEST analysis results. A total of 20,119 participants with DTC were enrolled, of which 3,948 (19.6%) had HT. Among the seven studies, four provided longitudinal data for all-cause mortality and six reported the data for thyroid cancer-related mortality. Six studies including NEST were conducted in East Asia and two studies in USA. All studies were conducted in iodine sufficient area.
- The results of the risk of bias assessment using RoBANs are summarized in Supplemental Fig. S1. All studies showed a low risk of bias in selection of participants. One of the seven studies showed a low risk of bias due to confounders while six had a high risk of bias. All of the studies demonstrated a low risk of bias concerning the measurement of the intervention, blinding for outcome assessment, incomplete outcome data, and selective outcome reporting.
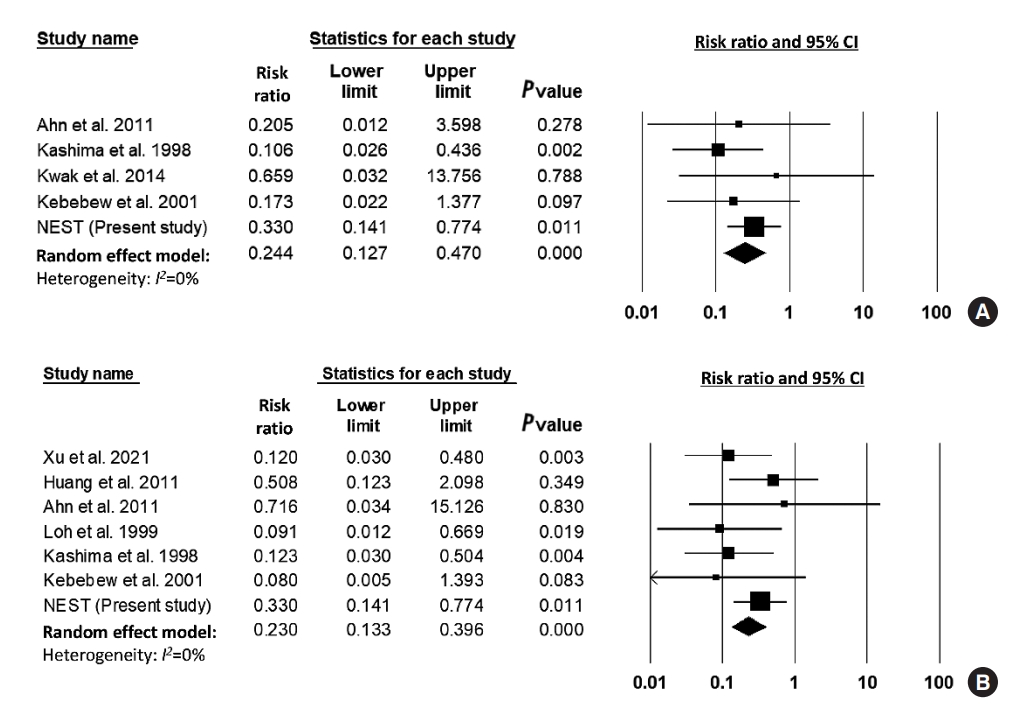
- NEST data and the results of four studies were included for a meta-analysis of the association between HT and all-cause mortality. Compared to patients without HT, those with HT showed a lower risk of all-cause mortality (RR, 0.24; 95% CI, 0.13 to 0.47) (Fig. 4). No significant heterogeneity was found among these studies (I2=0%); the funnel plot was symmetrical; and a significant publication bias was not detected (Egger’s test: P=0.33) (Supplemental Fig. S2). Sensitivity analysis showed robust results by repeated analysis after excluding each study (Supplemental Fig. S3).
- NEST data and the results of six studies were included for a meta-analysis of thyroid cancer-related mortality. Compared to patients without HT, those with HT showed a lower risk of thyroid cancer-related mortality (RR, 0.23; 95% CI, 0.13 to 0.40) (Fig. 4). Heterogeneity was not significant among these studies (I2=0%). The funnel plot analysis and the Egger test revealed no significant publication bias (P=0.481) (Supplemental Fig. S2). Sensitivity analysis showed robust results by repeated analysis after excluding each study (Supplemental Fig. S3). In subgroup analysis with six studies in East Asia showed similar results (RR, 0.23; 95% CI, 0.14 to 0.42; I2=0%).
RESULTS
Baseline characteristics
Association between HT and clinicopathologic characteristics of DTC
Association between the HT and thyroid cancer-related mortality
Study characteristics
Risk of bias assessment
Association between HT and mortality
- The NEST retrospective study showed that patients with DTC and HT had a smaller tumor size and fewer advanced T stage, ETE, and lymphatic metastasis compared to DTC patients without HT. Furthermore, the cumulative mortality for all-cause and thyroid cancer-related death was significantly lower in patients with DTC and HT compared to those with DTC alone. The meta-analysis with a larger group of patients, consisting of 4,024 patients with thyroid cancer and HT and 16,954 patients with thyroid cancer without HT showed co-presentation of HT and DTC was linked to a significantly lower risk of all-cause mortality and thyroid cancer-related mortality.
- Previous studies showed that patients with DTC and HT had a smaller tumor size and fewer advanced features, such as ETE, lymphatic metastasis, and distant metastasis, compared to patients with DTC alone [13,14,26,27]. Our results were consistent with previous studies [13,14,26,27]. Previous studies also reported that patients with DTC and HT had a lower recurrence than with DTC alone [25,26,28,29]. Although we could not conduct the analysis of recurrence due to lack of data, we provided additional evidence that the DTC-coexisting HT patient group showed a lower cancer-related mortality rate. This result is consistent with a recent study [25]. However, the significant difference in age and sex was found according to HT in NEST study, which is similar to the previous studies [13,30] and may bias the study results. To address this potential bias, age- and sex-adjusted analyses and subgroup analysis with age and sex-adjusted propensity score-matching data was performed which showed significant results. In addition, our mediation analysis supports the evidence that HT directly and indirectly reduces the risk of DTC-related death by less advanced clinicopathologic status of DTC.
- The present study was the first meta-analysis on the effect of HT on death from thyroid cancer in DTC patients. While previous meta-analyses have investigated differences in clinicopathological characteristics, recurrence rates or overall mortality between patients with and without HT [13,16], the current study is significant in providing epidemiological evidence of a negative correlation between HT and DTC-related mortality. In addition, although it was not included in the meta-analysis because of unclear diagnosis method for HT, McConahey et al. [31] reported that in a study of 859 patients with PTC, HT was associated with a significantly reduced risk of DTC-related death.
- Although the current study and previous studies have provided important epidemiological evidence [13,14,32-34], the precise mechanisms through which HT may exert its protective effect on DTC remain unclear. There are several hypotheses to explain that HT acts as a protective factor to DTC. One of the proposed hypotheses states that HT produces auto-antibodies through an immunological response that primarily target thyroid peroxidase, which is involved in the production of thyroid hormones, and thyroglobulin, which is a thyroid-related protein produced in follicular cells, ultimately destroying the PTC cells [7,15,35]. Moreover, the lymphocytes that infiltrate the thyroid in HT may act as specific cytotoxic T-cells and thereby destroy cancer cells [11,36]. In 2020, Sulaieva et al. [37] confirmed that CD8+ cells were increased in DTC tissue and normal thyroid tissue when HT coexisted. Previous findings [38,39] may explain the protective effect of HT on DTCs, as an increase in CD8+ cell numbers is believed to promote CD8+ cell differentiation, recruitment and elimination of tumor cells through the process of activation of the anti-tumor response. These studies can explain the protective effect of HT on PTC growth and progression. However, further studies are still needed to fully understand the mechanism between HT and DTC.
- The strength of the NEST study lies in that it uses well-designed nationwide dataset on a large scale that shows low bias. As a result of meta-analysis of the NEST data and of eight studies [6,20-25,31], significant results were obtained which revealed the link between HT and low risk of DTC-related mortality.
- There are several limitations in this study. First, NEST data include small number of DTC-related deaths because of low mortality risk of DTC. Second, in the meta-analysis study, the possibility of improper bias cannot be ruled out because six of the seven studies had high risk of bias due to confounders. Especially, most studies did not present HRs adjusted for age and gender. Last, almost all of the patients included in the meta-analysis underwent surgical resection, such as total or lobectomy, but it was not clearly specified in some studies [20,23,31] whether radioactive iodine ablation-therapy was performed after surgery. Hence this may have caused bias.
- In conclusion, we showed that in comparison with DTC without HT, DTC co-presenting with HT is associated with the less aggressive clinicopathologic features of DTC and presents a low risk for all-cause and DTC-related death. These results support the possibility that HT is a protective factor against DTC progression and is a less invasive and good prognostic factor. However, further studies are needed to gain clarity of the mechanism through which HT slows down the progression of DTC.
DISCUSSION
Supplementary Material
Supplemental Table S1.
Supplemental Fig. S2.
Supplemental Fig. S3.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: I.Y., S.M. Acquisition, analysis, or interpretation of data: I.Y., S.P., S.M. Drafting the work or revising: I.Y., S.P., S.M. Final approval of the manuscript: J.M.Y., H.S.C., Y.J.K., Y.K.R., M.K.C., S.P., Y.J.P., S.M.
Article information
-
Acknowledgements
- This study was supported by Hallym University Research Fund 2022 (HURF-2022-34).
| Characteristic |
Unweighted |
IPTW weighted |
||
|---|---|---|---|---|
| Age and sex-adjusted OR (95% CI) | P value | OR (95% CI) | P value | |
| T3–4 | 0.82 (0.71–0.94) | 0.005 | 0.80 (0.69–0.92) | 0.001 |
| Extrathyroidal extension | 0.83 (0.73–0.96) | 0.011 | 0.81 (0.70–0.93) | 0.003 |
| Lymph node metastasis | 0.68 (0.58–0.79) | <0.001 | 0.68 (0.59–0.79) | <0.001 |
| TNM stage III–IV | 0.83 (0.66–1.05) | 0.125 | 1.06 (0.90–1.24) | 0.507 |
| Advanced DTCa | 0.73 (0.62–0.86) | <0.001 | 0.72 (0.62–0.85) | <0.001 |
OR of clinical detection group compared to patients without Hashimoto thyroiditis; IPTW: with age, sex and year of thyroid cancer registration.
IPTW, inverse probability of treatment weight; OR, odds ratio; CI, confidence interval; TNM, tumor, node, metastasis; DTC, differentiated thyroid cancer.
a Defined as patients with T3-4, lymph node metastasis, or distant metastasis.
| Variable | HR (95% CI) | P value |
|---|---|---|
| Total dataset | ||
| All-cause mortality | ||
| Model 1 | 0.71 (0.52–0.96) | 0.028 |
| Model 2 | 0.75 (0.52–1.09) | 0.131 |
| TC-mortality | ||
| Model 1 | 0.33 (0.14–0.77) | 0.010 |
| Model 3 | 0.42 (0.18–0.92) | 0.045 |
| IPTW data | ||
| All-cause mortalitya | 0.60 (0.43–0.83) | 0.002 |
| Model 4 | 0.53 (0.36–0.77) | <0.001 |
| TC-mortalitya | 0.32 (0.14–0.76) | <0.001 |
| Model 5 | 0.44 (0.18–1.04) | 0.061 |
Model 1: analysis adjusted for age and sex; Model 2: analysis adjusted for age, sex, year of thyroid cancer registration, smoking status, alcohol consumption, diabetes and hypertension; Model 3: analysis adjusted for age, sex and initial treatment for differentiated thyroid cancer (DTC); Model 4: analysis of IPTW data with additional adjustment for smoking status, alcohol consumption, diabetes and hypertension; Model 5: analysis of IPTW data with additional adjustment for initial treatment for DTC.
HR, hazard ratio; CI, confidence interval; TC, thyroid cancer; IPTW, inverse probability of treatment weight.
a Inverse probability treatment weighting data with age, sex and year of thyroid cancer registration.
| Study | Region | Participants according to HT | Female sex | Age, yr | No. of thyroid cancer type | No. of extent of surgery (LT/TT) | RAIT | Tumor size mean, cm | ETE | LN metastasis | Distant metastasis | No. of TNM stage (Ⅰ/Ⅱ/Ⅲ/Ⅳ) | Follow-up duration, yr | Recurrence | All-cause death | Thyroid cancer-related death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xu et al. (2021) [25] | China | HT absent, 7,459 (81) | 5,263 (71) | 44.1±12 (SD) | All PTC | 4,718/2,741 | 1,674 (23) | 1.5±1.2 (SD) | 3,228 (43) | 3,682 (50) | 80 (1.1) | 6,560/653/213/33 | 7.1 (1–12) | 633 (8.5) | NA | 129 (1.7) |
| HT present, 1,751 (19) | 1,609 (92) | 41.6±11 (SD) | All PTC | 1,059/692 | 412 (24) | 1.2±0.7 (SD) | 658 (38) | 844 (50) | 2 (0.1) | 1,620/105/25/1 | 7 (2.5–12) | 100 (5.7) | NA | 2 (0.1) | ||
| Huang et al. (2011) [6] | Taiwan | HT absent, 1,904 (95.3) | 1,512 (79.4) | 40.8±14.2 (SD) | PTC: 1,703 | 201/1,703 | 158 (8.3) | 2.3±0.04 (SD) | 322 (18.9) | 236 (14) | 61 (3.6) | 1,278/140/76/205 | 9.7±0.2 (SD) | 238 (14) | NA | 79 (4.6) |
| FTC: 201 | ||||||||||||||||
| HT present, 93 (4.7) | 91 (97.8) | 39.9±12.8 (SD) | PTC: 85 | 0/93 | 5 (5.9) | 2.2±0.2 (SD) | 10 (11.8) | 17 (20) | 1 (1.2) | 75/5/9/4 | 8.7±0.6 (SD) | 4 (4.3) | NA | 2 (2.2) | ||
| FTC: 8 | ||||||||||||||||
| Ahn et al. (2011) [21] | Korea | HT absent, 211 (78.4) | 170 (80.6) | 48.3±14.4 (SD) | All PTC | 178/33 | NA | 1.8±1.5 (SD) | 125 (59.2) | 63 (29.9) | NA | 127 (Ⅰ)/84 (Ⅱ–Ⅳ) | 5.2±0.2 (SD) | 35 (16.6) | 8 (3.8) | 2 (1.0) |
| HT present, 58 (21.6) | 55 (94.8) | 42.8±12.7 (SD) | All PTC | 11/47 | NA | 1.6±1.0 (SD) | 36 (62.1) | 10 (12.2) | NA | 35 (Ⅰ)/23 (Ⅱ–Ⅳ) | 4.9±2.1 (SD) | 4 (6.9) | 0 | 0 | ||
| Loh et al. (1999) [24] | USA | HT absent, 503 (79.7) | 336 (67) | 40.6±0.8 (SE) | PTC: 439 | NA | NA | NA | 117 (23.3) | 218 (43.3) | 24 (4.8) | 367/42/77/17 | 11±0.3 (SE) | 121 (24.1) | NA | 40 (8) |
| FTC: 64 | ||||||||||||||||
| HT present, 128 (20.3) | 109 (85) | 38±1.2 (SE) | PTC: 125 | NA | NA | NA | 10 (7.8) | 33 (25.8) | 0 | 111/6/11/0 | 11±0.4 (SE) | 8 (6.3) | NA | 1 (0.8) | ||
| FTC: 3 | ||||||||||||||||
| Kashima et al. (1998) [20] | Japan | HT absent, 1,252 (81.7) | 1,123 (89.7) | 48.6 | All PTC | NA | NA | 2.82 (median 2.5) | 706 (56.4) | 1,078 (86.1) | NA | NA | 6.9±4.7 (SD) | 109 (8.7) | 79 (6.3) | 69 (5) |
| HT present, 281 (18.3) | 279 (99.3) | 42.6 | All PTC | NA | NA | 2.42 (median 2.2) | 131 (46.6) | 232 (82.6) | NA | NA | 10.6±6.0 (SD) | 13 (4.6) | 2 (0.7) | 2 (0.7) | ||
| Kwak et al. (2015) [23] | Korea | HT absent, 1,493 (76.8) | 1,187 (79.5) | 46.1±12.1 (SD) | All PTC | NA | NA | 0.9±0.7 (SD) | 728 (48.8) | 371 (24.8) | 0 | 648/9/736/100 | 1.7 (0.8–4.6) | 25 (1.7) | 2 (0.1) | NA |
| HT present, 452 (23.2) | 412 (91.2) | 45.3±11.6 (SD) | All PTC | NA | NA | NA | 186 (41.2) | 119 (26.3) | 0 | 229/2/205/16 | 4 (0.9) | 0 | NA | |||
| Kebebew et al. (2001) [22] | USA | HT absent, 95 (69.9) | 61 (64.2) | 54 | All PTC | 31/64 | 33 (34) | NA | NA | NA | NA | 61/12/13/5 | 4.4 | 11 (11.6) | 12 (12.6) | 12 (12.6) |
| Unknown: 4 | ||||||||||||||||
| HT present, 41 (30.1) | 34 (82.9) | 45.5 | All PTC | 15/26 | 11 (27) | NA | NA | NA | NA | 27/6/6/0 | 4 (9.8) | 1 (2.4) | 0 | |||
| Unknown: 2 |
Values are expressed as number (%), mean±standard deviation (SD) or standard error (SE), or median (range).
HT, Hashimoto’s thyroiditis; LT, lobectomy; TT, total thyroidectomy; RAIT, radioactive iodine treatment; ETE, extrathyroidal extension; LN, lymph node; TNM, tumor, node, metastasis; PTC, papillary thyroid cancer; NA, not available; FTC, follicular thyroid cancer.
- 1. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol 2022;10:264–72.ArticlePubMed
- 2. Lamartina L, Leboulleux S, Borget I, Schlumberger M. Global thyroid estimates in 2020. Lancet Diabetes Endocrinol 2022;10:235–6.ArticlePubMed
- 3. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer 2009;115:3801–7.ArticlePubMed
- 4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338–48.ArticlePubMedPMC
- 5. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016;12:646–53.ArticlePubMedPMCPDF
- 6. Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol 2011;22:144–9.ArticlePubMedPDF
- 7. Ehlers M, Schott M. Hashimoto’s thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metab 2014;25:656–64.ArticlePubMed
- 8. Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto’s thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid 2013;23:142–50.ArticlePubMedPMC
- 9. Ott J, Meusel M, Schultheis A, Promberger R, Pallikunnel SJ, Neuhold N, et al. The incidence of lymphocytic thyroid infiltration and Hashimoto’s thyroiditis increased in patients operated for benign goiter over a 31-year period. Virchows Arch 2011;459:277–81.ArticlePubMedPDF
- 10. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol 2013;168:343–9.ArticlePubMed
- 11. Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 1955;70:291–7.ArticlePubMed
- 12. Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of coexistent Hashimoto’s thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2018;88:123–8.ArticlePubMedPDF
- 13. Moon S, Chung HS, Yu JM, Yoo HJ, Park JH, Kim DS, et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: a meta-analysis of observational studies. Endocrinol Metab (Seoul) 2018;33:473–84.ArticlePubMedPMCPDF
- 14. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab 2013;98:2409–14.ArticlePubMed
- 15. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:581–6.ArticlePubMed
- 16. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery 1999;126:1070–7.ArticlePubMed
- 17. Tang Q, Pan W, Peng L. Association between Hashimoto thyroiditis and clinical outcomes of papillary thyroid carcinoma: a meta-analysis. PLoS One 2022;17:e0269995.ArticlePubMedPMC
- 18. Oh CM, Kong HJ, Kim E, Kim H, Jung KW, Park S, et al. National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Epidemiol Health 2018;40:e2018052.ArticlePubMedPMC
- 19. Li Y, Yoshida K, Kaufman JS, Mathur MB. A brief primer on conducting regression-based causal mediation analysis. Psychol Trauma 2023;15:930–8.ArticlePubMedPMC
- 20. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid 1998;8:197–202.ArticlePubMed
- 21. Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH, Park JY, et al. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncol 2011;50:1228–34.ArticlePubMed
- 22. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg 2001;25:632–7.ArticlePubMedPDF
- 23. Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, et al. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol 2015;20:463–73.ArticlePubMedPDF
- 24. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 1999;84:458–63.ArticlePubMed
- 25. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of Hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open 2021;4:e2118526.ArticlePubMedPMC
- 26. Battistella E, Pomba L, Costantini A, Scapinello A, Toniato A. Hashimoto’s thyroiditis and papillary cancer thyroid coexistence exerts a protective effect: a single centre experience. Indian J Surg Oncol 2022;13:164–8.ArticlePubMedPMCPDF
- 27. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol 2014;140:1021–6.ArticlePubMedPDF
- 28. Ryu YJ, Yoon JH. Chronic lymphocytic thyroiditis protects against recurrence in patients with cN0 papillary thyroid cancer. Surg Oncol 2020;34:67–73.ArticlePubMed
- 29. Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, et al. Hashimoto’s thyroiditis: a “double-edged sword” in thyroid carcinoma. Front Endocrinol (Lausanne) 2022;13:801925.ArticlePubMedPMC
- 30. Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, et al. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg 2013;148:396–402.ArticlePubMedPDF
- 31. McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc 1986;61:978–96.ArticlePubMed
- 32. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, et al. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci 2012;27:883–9.ArticlePubMedPMCPDF
- 33. Borowczyk M, Janicki A, Dworacki G, Szczepanek-Parulska E, Danieluk M, Barnett J, et al. Decreased staging of differentiated thyroid cancer in patients with chronic lymphocytic thyroiditis. J Endocrinol Invest 2019;42:45–52.ArticlePubMedPMCPDF
- 34. Graceffa G, Patrone R, Vieni S, Campanella S, Calamia S, Laise I, et al. Association between Hashimoto’s thyroiditis and papillary thyroid carcinoma: a retrospective analysis of 305 patients. BMC Endocr Disord 2019;19(Suppl 1):26.ArticlePubMedPMCPDF
- 35. Latrofa F, Ricci D, Grasso L, Vitti P, Masserini L, Basolo F, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab 2008;93:591–6.ArticlePubMedPDF
- 36. Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerstrom G, Rastad J, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol 1996;27:1329–35.ArticlePubMed
- 37. Sulaieva O, Selezniov O, Shapochka D, Belemets N, Nechay O, Chereshneva Y, et al. Hashimoto’s thyroiditis attenuates progression of papillary thyroid carcinoma: deciphering immunological links. Heliyon 2020;6:e03077.ArticlePubMedPMC
- 38. Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol 2013;3:231.ArticlePubMedPMC
- 39. Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J 2018;17:1–13.ArticlePubMedPMC
References
Figure & Data
References
Citations


 KES
KES

 PubReader
PubReader ePub Link
ePub Link Cite
Cite