COVID-19 in EnM
- Page Path
- HOME > BROWSE ARTICLES > COVID-19 in EnM
Letter
- Thyroid
- Subacute Thyroiditis in the Time of COVID-19
- Hwa Young Ahn
- Endocrinol Metab. 2024;39(1):186-187. Published online February 1, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1928

- 758 View
- 39 Download

Original Articles
- Thyroid
- Beyond Acute COVID-19: Investigating the Incidence of Subacute Thyroiditis in Long COVID-19 in Korea
- Jeongmin Lee, Gi Hyeon Seo, Keeho Song
- Endocrinol Metab. 2023;38(4):455-461. Published online August 8, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1711
- 2,626 View
- 181 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
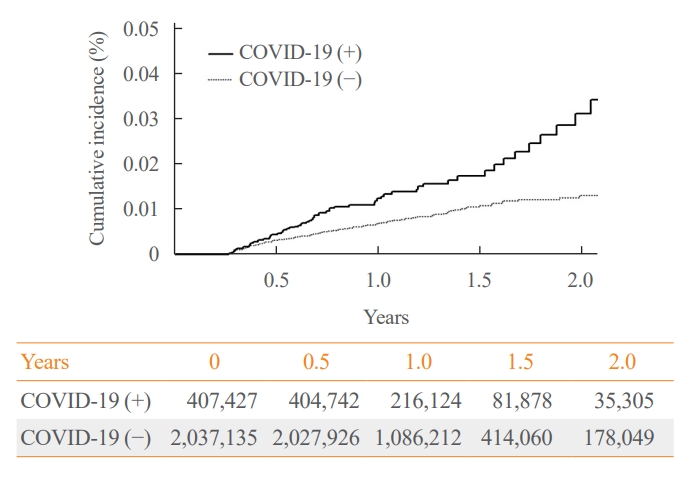
The correlation between acute coronavirus disease 2019 (COVID-19) and subacute thyroiditis (SAT) has not been clearly investigated in “long COVID” patients. We aimed to investigate the incidence of SAT during convalescence and after the acute phase of COVID-19, comparing with that of the general population.
Methods
Data from a total of 422,779 COVID-19 patients and a control group of 2,113,895 individuals were analyzed. The index date was defined as the date 3 months after confirmation of COVID-19. The incidence rate (IR) of SAT and hazard ratios (HRs) were calculated per 100,000 persons. Subgroup analysis included analysis of HRs 90–179 and 180 days post-COVID-19 diagnosis; and additional analysis was conducted according to hospitalization status, sex, and age group.
Results
The IR of SAT was 17.28 per 100,000 persons (95% confidence interval [CI], 12.56 to 23.20) in the COVID-19 group and 8.63 (95% CI, 6.37 to 11.45) in the control group. The HR of COVID-19 patients was 1.76 (95% CI, 1.01 to 3.06; P=0.045). The HR of SAT was 1.39 (95% CI, 0.82 to 2.34; P=0.220) up to 6 months after the index date and 2.30 (95% CI, 1.60 to 3.30; P<0.001) beyond 6 months. The HR for SAT among COVID-19 patients was 2.00 (95% CI, 1.41 to 2.83) in hospitalized patients and 1.76 (95% CI, 1.01 to 3.06) in non-hospitalized patients compared to the control group. The IR of SAT was 27.09 (95% CI, 20.04 to 35.82) for females and 6.47 (95% CI, 3.34 to 11.30) for males. In the 19 to 64 age group, the IR of SAT was 18.19 (95% CI, 13.70 to 23.67), while the IR was 9.18 (95% CI, 7.72 to 10.84) in the 65 to 69 age group.
Conclusion
SAT could be a potential long-term complication of COVID-19. Long-term surveillance for thyroid dysfunction is needed especially in hospitalized, female and young-aged subjects. -
Citations
Citations to this article as recorded by- Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Subacute Thyroiditis in the Time of COVID-19
Hwa Young Ahn
Endocrinology and Metabolism.2024; 39(1): 186. CrossRef - Occult endocrine disorders newly diagnosed in patients with post-COVID-19 symptoms
Yasuhiro Nakano, Naruhiko Sunada, Kazuki Tokumasu, Hiroyuki Honda, Yuki Otsuka, Yasue Sakurada, Yui Matsuda, Toru Hasegawa, Daisuke Omura, Kanako Ochi, Miho Yasuda, Hideharu Hagiya, Keigo Ueda, Fumio Otsuka
Scientific Reports.2024;[Epub] CrossRef - rRisk of incident thyroid dysfunction in the post-acute phase of COVID-19: a population-based cohort study in Hong Kong
David Tak Wai Lui, Xi Xiong, Ching‐Lung Cheung, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Lanlan Li, Matthew Shing Hin Chung, Chi Ho Lee, Yu Cho Woo, Kathryn Choon Beng Tan, Carlos
Endocrine Practice.2024;[Epub] CrossRef
- Thyroid dysfunction in COVID-19

- Adrenal gland
Big Data Articles (National Health Insurance Service Database) - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
- Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
- Endocrinol Metab. 2023;38(2):253-259. Published online March 21, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1607
- 2,597 View
- 103 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
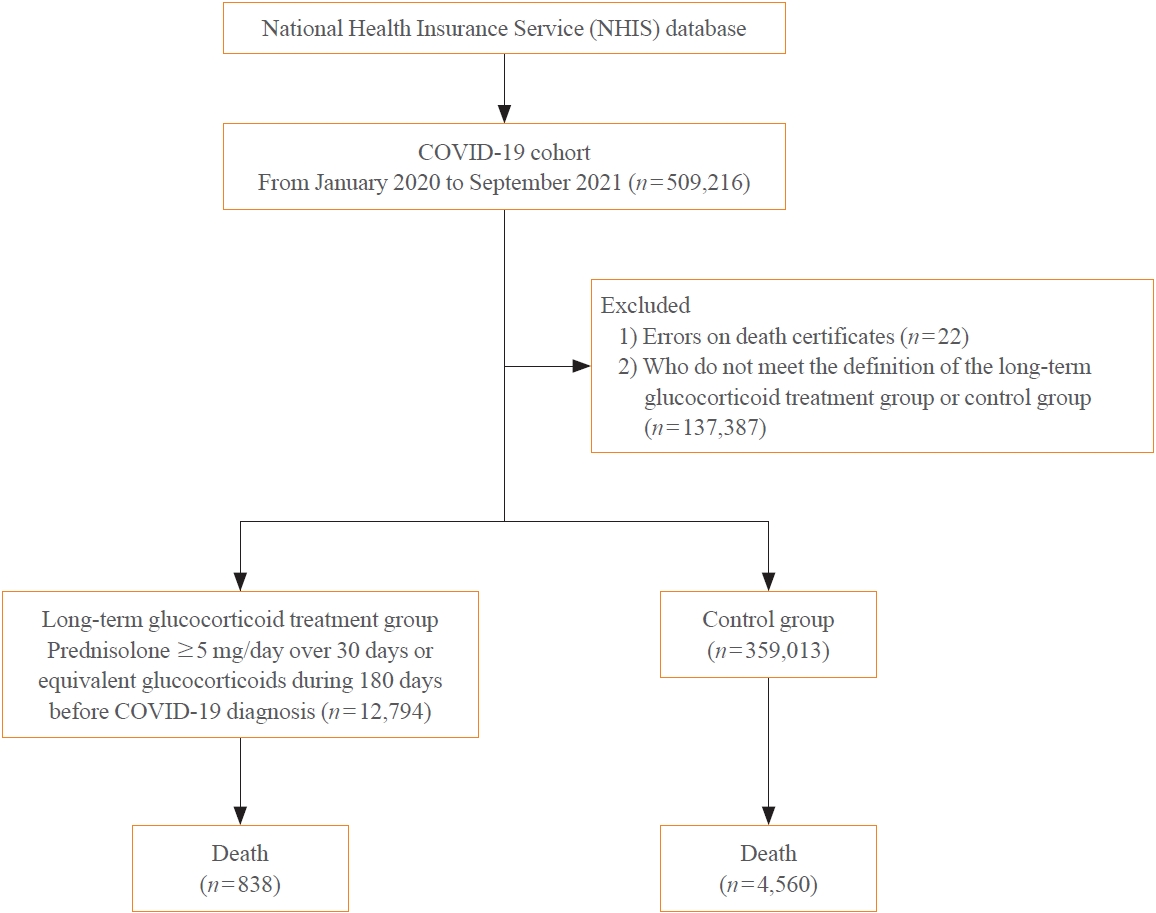
The severity of coronavirus disease 2019 (COVID-19) among patients with long-term glucocorticoid treatment (LTGT) has not been established. We aimed to evaluate the association between LTGT and COVID-19 prognosis.
Methods
A Korean nationwide cohort database of COVID-19 patients between January 2019 and September 2021 was used. LTGT was defined as exposure to at least 150 mg of prednisolone (≥5 mg/day and ≥30 days) or equivalent glucocorticoids 180 days before COVID-19 infection. The outcome measurements were mortality, hospitalization, intensive care unit (ICU) admission, length of stay, and mechanical ventilation.
Results
Among confirmed patients with COVID-19, the LTGT group (n=12,794) was older and had a higher proportion of comorbidities than the control (n=359,013). The LTGT group showed higher in-hospital, 30-day, and 90-day mortality rates than the control (14.0% vs. 2.3%, 5.9% vs. 1.1%, and 9.9% vs. 1.8%, respectively; all P<0.001). Except for the hospitalization rate, the length of stay, ICU admission, and mechanical ventilation proportions were significantly higher in the LTGT group than in the control (all P<0.001). Overall mortality was higher in the LTGT group than in the control group, and the significance remained in the fully adjusted model (odds ratio [OR], 5.75; 95% confidence interval [CI], 5.31 to 6.23) (adjusted OR, 1.82; 95% CI, 1.67 to 2.00). The LTGT group showed a higher mortality rate than the control within the same comorbidity score category.
Conclusion
Long-term exposure to glucocorticoids increased the mortality and severity of COVID-19. Prevention and early proactive measures are inevitable in the high-risk LTGT group with many comorbidities. -
Citations
Citations to this article as recorded by- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
Eun-Hee Cho
Endocrinology and Metabolism.2023; 38(2): 223. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy

- Diabetes, obesity and metabolism
Big Data Articles (National Health Insurance Service Database) - Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
- Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
- Endocrinol Metab. 2023;38(2):245-252. Published online April 5, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1662
- 2,252 View
- 120 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
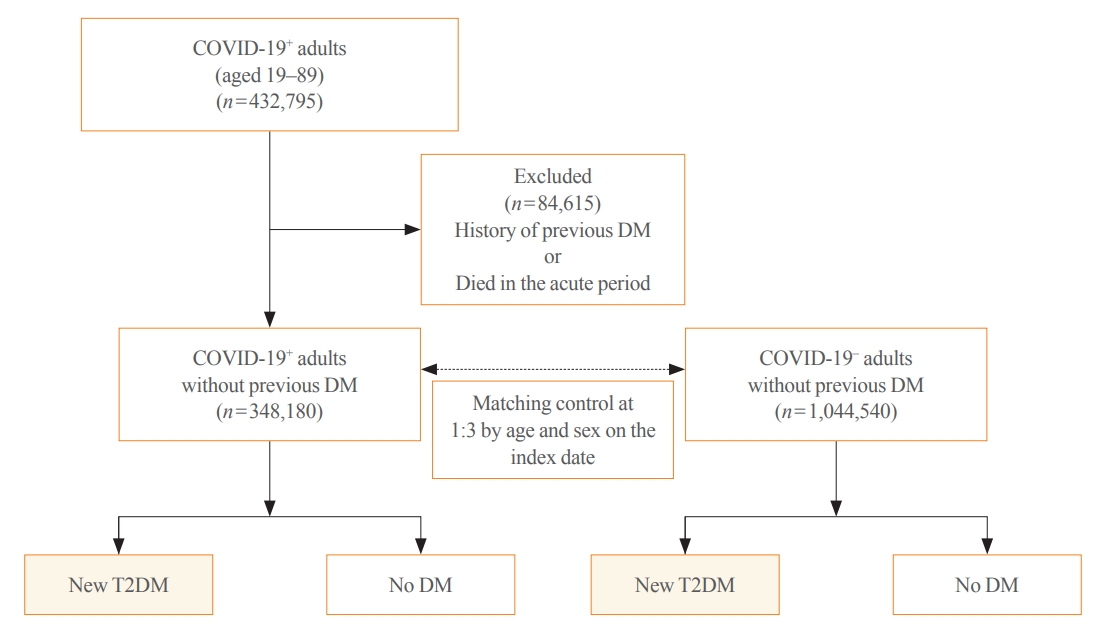
Coronavirus disease 2019 (COVID-19) can cause various extrapulmonary sequelae, including diabetes. However, it is unclear whether these effects persist 30 days after diagnosis. Hence, we investigated the incidence of newly diagnosed type 2 diabetes mellitus (T2DM) in the post-acute phase of COVID-19.
Methods
This cohort study used data from the Health Insurance Review and Assessment Service, a representative national healthcare database in Korea. We established a cohort of 348,180 individuals diagnosed with COVID-19 without a history of diabetes between January 2020 and September 2021. The control group consisted of sex- and age-matched individuals with neither a history of diabetes nor COVID-19. We assessed the hazard ratios (HR) of newly diagnosed T2DM patients with COVID-19 compared to controls, adjusted for age, sex, and the presence of hypertension and dyslipidemia.
Results
In the post-acute phase, patients with COVID-19 had an increased risk of newly diagnosed T2DM compared to those without COVID-19 (adjusted HR, 1.30; 95% confidence interval [CI], 1.27 to 1.33). The adjusted HRs of non-hospitalized, hospitalized, and intensive care unit-admitted patients were 1.14 (95% CI, 1.08 to 1.19), 1.34 (95% CI, 1.30 to 1.38), and 1.78 (95% CI, 1.59 to 1.99), respectively. The risk of T2DM in patients who were not administered glucocorticoids also increased (adjusted HR, 1.29; 95% CI, 1.25 to 1.32).
Conclusion
COVID-19 may increase the risk of developing T2DM beyond the acute period. The higher the severity of COVID-19 in the acute phase, the higher the risk of newly diagnosed T2DM. Therefore, T2DM should be included as a component of managing long-term COVID-19. -
Citations
Citations to this article as recorded by- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review
Anca Pantea Stoian, Ioana-Cristina Bica, Teodor Salmen, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez,
Diabetes Therapy.2024; 15(1): 33. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- New-Onset Diabetes Mellitus in COVID-19: A Scoping Review

Editorial
- Adrenal gland
- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
- Eun-Hee Cho
- Endocrinol Metab. 2023;38(2):223-225. Published online April 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.201
- 1,172 View
- 69 Download

Review Article
- Calcium & Bone Metabolism
- Interplay of Vitamin D and CYP3A4 Polymorphisms in Endocrine Disorders and Cancer
- Siva Swapna Kasarla, Vannuruswamy Garikapati, Yashwant Kumar, Sujatha Dodoala
- Endocrinol Metab. 2022;37(3):392-407. Published online June 3, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1349
- 5,353 View
- 199 Download
- 3 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
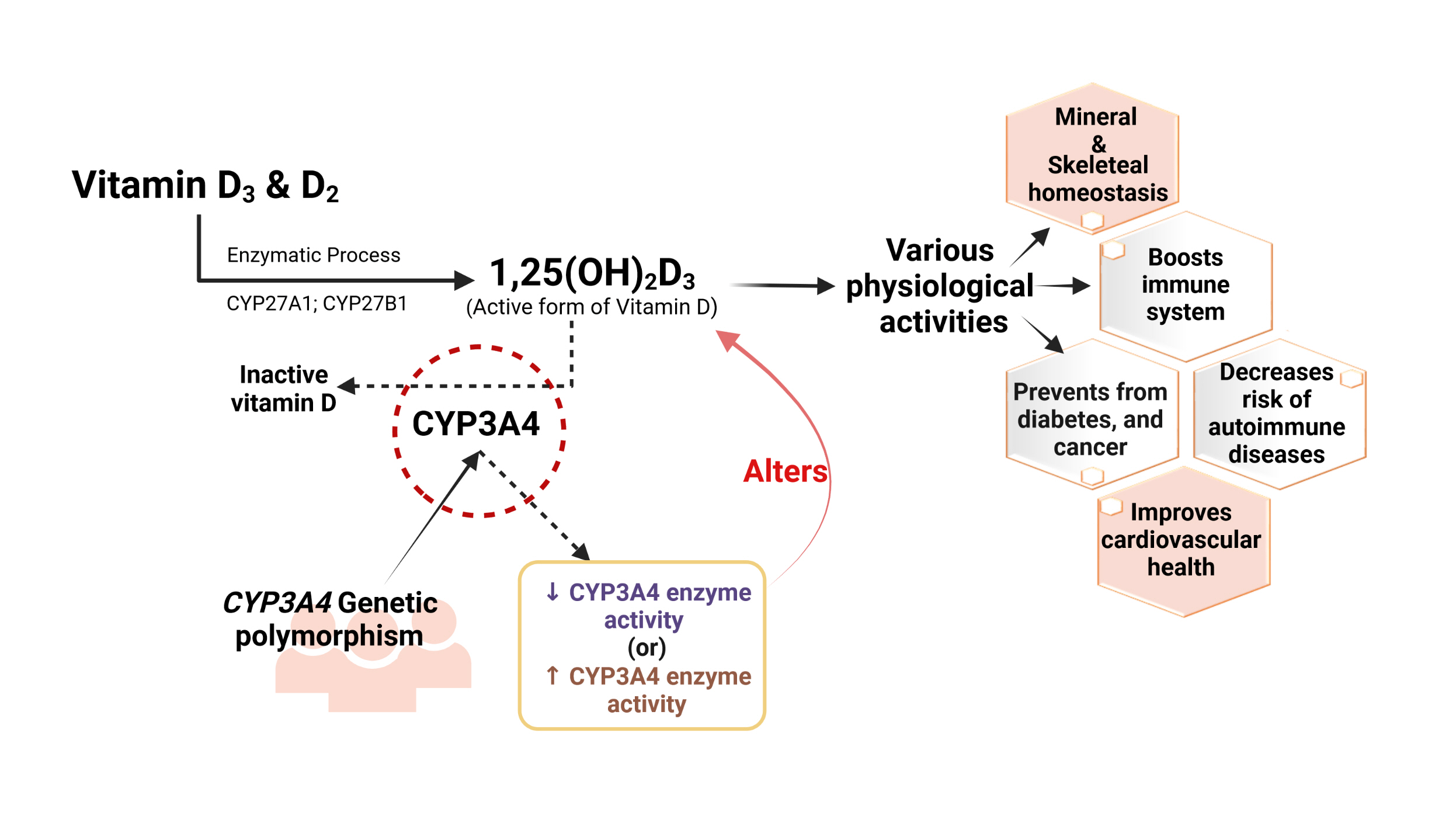
ePub - Vitamin D has received considerable optimistic attention as a potentially important factor in many pathological states over the past few decades. However, the proportion of the active form of vitamin D metabolites responsible for biological activity is highly questionable in disease states due to flexible alterations in the enzymes responsible for their metabolism. For instance, CYP3A4 plays a crucial role in the biotransformation of vitamin D and other drug substances. Food-drug and/or drug-drug interactions, the disease state, genetic polymorphism, age, sex, diet, and environmental factors all influence CYP3A4 activity. Genetic polymorphisms in CYP450-encoding genes have received considerable attention in the past few decades due to their extensive impact on the pharmacokinetic and dynamic properties of drugs and endogenous substances. In this review, we focused on CYP3A4 polymorphisms and their interplay with vitamin D metabolism and summarized the role of vitamin D in calcium homeostasis, bone diseases, diabetes, cancer, other diseases, and drug substances. We also reviewed clinical observations pertaining to CYP3A4 polymorphisms among the aforementioned disease conditions. In addition, we highlighted the future perspectives of studying the pharmacogenetics of CYP3A4, which may have potential clinical significance for developing novel diagnostic genetic markers that will ascertain disease risk and progression.
-
Citations
Citations to this article as recorded by- Revealing the association between vitamin D metabolic pathway gene variants and lung cancer risk: a systematic review and meta-analysis
Mohamed I. Elsalahaty, Samar Sami Alkafaas, Aya O. Bashir, Khaled A. El-Tarabily, Mohamed T. El-Saadony, Eman H. Yousef
Frontiers in Genetics.2024;[Epub] CrossRef - Vitamin D in Melanoma: Potential Role of Cytochrome P450 Enzymes
Mohamed Ben-Eltriki, Erysa J. Gayle, Jhoanne M. Paras, Louisa Nyame-Addo, Manik Chhabra, Subrata Deb
Life.2024; 14(4): 510. CrossRef - Heat stress as a potential risk factor for vitamin D deficiency
Martina Balducci, Letizia Pruccoli, Andrea Tarozzi
Medical Hypotheses.2023; 176: 111085. CrossRef - Association and Haplotype Analysis of the PON1, ITGB3 and CYP3A4 Genes, Strong Candidates for Familial Coronary Artery Disease Susceptibility
Faruk SAYDAM, İrfan DEĞİRMENCİ, Alparslan BİRDANE, Cansu ÖZBAYER, Taner ULUS, Mahmut ÖZDEMİR, Necmi ATA, Hasan Veysi GÜNEŞ
Online Türk Sağlık Bilimleri Dergisi.2023; 8(1): 81. CrossRef - Association of flame retardants, polybrominated diethyl ethers (PBDEs), with vitamin D in female subjects
Alexandra E. Butler, Edwina Brennan, Daniel S. Drage, Thozhukat Sathyapalan, Stephen L. Atkin
Chemosphere.2023; 338: 139488. CrossRef - Genetic variations of CYP3A4 on the metabolism of itraconazole in vitro
Sai-li Xie, Xiayan Zhu, Nanyong Gao, Qianmeng Lin, Chaojie Chen, Yun-jun Yang, Jian-ping Cai, Guo-xin Hu, Ren-ai Xu
Food and Chemical Toxicology.2023; 181: 114101. CrossRef
- Revealing the association between vitamin D metabolic pathway gene variants and lung cancer risk: a systematic review and meta-analysis

Brief Reports
- Diabetes, Obesity and Metabolism
- Year-Long Trend in Glycated Hemoglobin Levels in Patients with Type 2 Diabetes during the COVID-19 Pandemic
- Jonghwa Jin, Seong Wook Lee, Won-Ki Lee, Jae-Han Jeon, Jung-Guk Kim, In-Kyu Lee, Yeon-Kyung Choi, Keun-Gyu Park
- Endocrinol Metab. 2021;36(5):1142-1146. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1154
- 3,872 View
- 148 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
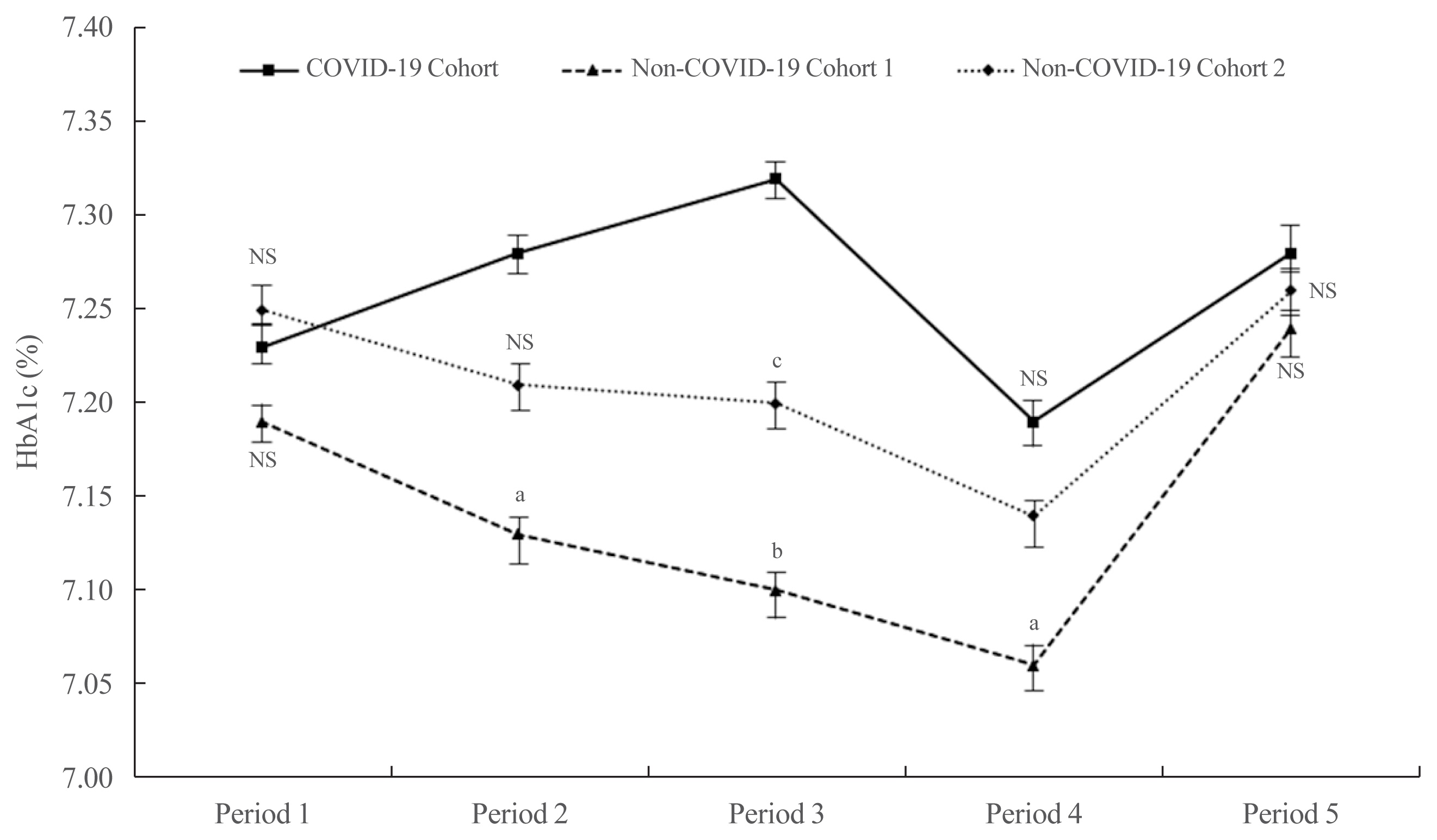
ePub - It has been suggested that the coronavirus disease 2019 (COVID-19) pandemic has had a negative impact on glycemic control in patients with type 2 diabetes mellitus (T2DM). However, no study has examined yearly trends in glycated hemoglobin (HbA1c) levels after the start of the COVID-19 outbreak. Here, we performed a retrospective analysis of HbA1c concentrations during the early period of the COVID-19 outbreak (COVID-19 cohort) and then compared the yearly trend in the mean HbA1c level, along with fluctuations in HbA1c levels, with those during previous years (non-COVID-19 cohorts). We observed that the mean HbA1c level in patients with T2DM increased during the first 6 months of the COVID-19 outbreak. After 6 months, HbA1c levels in the COVID-19 cohort returned to levels seen in the non-COVID-19 cohorts. The data suggest that vulnerable patients with T2DM should be monitored closely during the early period of a pandemic to ensure they receive appropriate care.
-
Citations
Citations to this article as recorded by- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey
Abdulbari Bener, Murat Atmaca, Abdulla O. A. A. Al-Hamaq, Antonio Ventriglio
Brain Sciences.2024; 14(4): 377. CrossRef - A Hybrid Model of In-Person and Telemedicine Diabetes Education and Care for Management of Patients with Uncontrolled Type 2 Diabetes Mellitus: Findings and Implications from a Multicenter Prospective Study
Ayla M. Tourkmani, Turki J. Alharbi, Abdulaziz M. Bin Rsheed, Azzam F. Alotaibi, Mohammed S. Aleissa, Sultan Alotaibi, Amal S. Almutairi, Jancy Thomson, Ahlam S. Alshahrani, Hadil S. Alroyli, Hend M. Almutairi, Mashael A. Aladwani, Eman R. Alsheheri, Hyfa
Telemedicine Reports.2024; 5(1): 46. CrossRef - The indirect impact of the COVID-19 pandemic on people with type 2 diabetes mellitus and without COVID-19 infection: Systematic review and meta-analysis
Zhuoran Hu, Hin Moi Youn, Jianchao Quan, Lily Luk Siu Lee, Ivy Lynn Mak, Esther Yee Tak Yu, David Vai-Kiong Chao, Welchie Wai Kit Ko, Ian Chi Kei Wong, Gary Kui Kai Lau, Chak Sing Lau, Cindy Lo Kuen Lam, Eric Yuk Fai Wan
Primary Care Diabetes.2023; 17(3): 229. CrossRef - Evaluating Effects of Virtual Diabetes Group Visits in Community Health Centers During the COVID-19 Pandemic
Tracy Dinh, Erin M Staab, Daisy Nuñez, Mengqi Zhu, Wen Wan, Cynthia T Schaefer, Amanda Campbell, Michael Quinn, Arshiya A Baig
Journal of Patient Experience.2023;[Epub] CrossRef - Cardiovascular-related health behavior changes: lessons from the COVID-19 pandemic and post-pandemic challenges
Inha Jung, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2023; 5(4): 99. CrossRef
- Physical and Mental Health Characteristics of Hospitalized COVID-19 Patients with and without Type 2 Diabetes Mellitus in Turkey

- Diabetes, Obesity and Metabolism
- Dipeptidyl Peptidase-4 Inhibitors and COVID-19-Related Deaths among Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Observational Studies
- Dimitrios Patoulias, Michael Doumas
- Endocrinol Metab. 2021;36(4):904-908. Published online July 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1048
- 11,027 View
- 216 Download
- 17 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
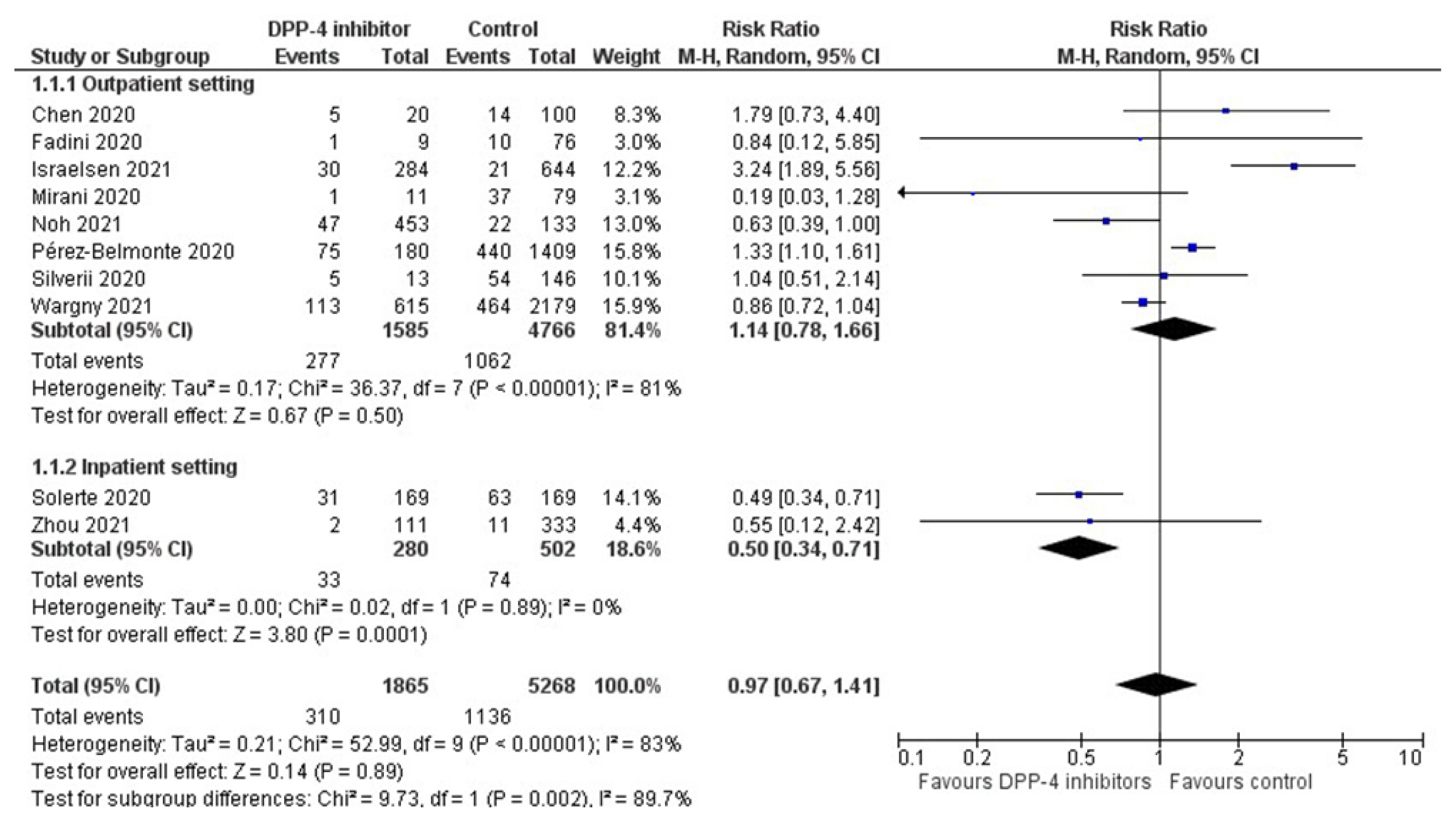
ePub - The coronavirus disease 2019 (COVID-19) pandemic remains an unbeaten enemy. Unfortunately, no targeted treatment option is available. Patients with type 2 diabetes mellitus (T2DM) have increased odds for severe or fatal disease, as demonstrated in recent observational studies. There is an ongoing discussion regarding the impact of different antidiabetic drug classes on outcomes of interest among affected subjects. Dipeptidyl peptidase-4 (DPP-4) inhibitors have been placed at the epicenter, since the DPP-4 enzyme seems to be implicated in the disease pathogenesis. Herein we present an updated meta-analysis of observational studies addressing the risk of COVID-19 death among patients with T2DM on prior DPP-4 inhibitor treatment. We pooled data from 10 observational studies, showing that DPP-4 inhibitors produce a non-significant decrease in the risk for COVID-19-related death. However, when administered in the inpatient setting, DPP-4 inhibitors decrease the risk for COVID-19-related death by 50%. Ongoing randomized controlled trials will shed further light.
-
Citations
Citations to this article as recorded by- Noninsulin‐based antihyperglycemic medications in patients with diabetes and COVID‐19: A systematic review and meta‐analysis
Mahmoud Nassar, Hazem Abosheaishaa, Awadhesh Kumar Singh, Anoop Misra, Zachary Bloomgarden
Journal of Diabetes.2023; 15(2): 86. CrossRef - Effects of novel glucose-lowering drugs on the COVID-19 patients with diabetes: A network meta-analysis of clinical outcomes
Yang Yang, Ling Zhao, Yeying Wang, Chengjiang Liu, Tingyu Ke
International Journal of Diabetes in Developing Countries.2023;[Epub] CrossRef - COVID-19 and metabolic syndrome
Harsha Dissanayake
Best Practice & Research Clinical Endocrinology & Metabolism.2023; 37(4): 101753. CrossRef - Current management of diabetes patients with COVID-19
Arup Kumar Misra, Gaurav Rangari, Madhavrao C, Sushil Sharma
Expert Review of Endocrinology & Metabolism.2023; 18(2): 199. CrossRef - Current management of diabetes patients with COVID-19
Arup Kumar Misra, Gaurav Rangari, Madhavrao C, Sushil Sharma
Expert Review of Endocrinology & Metabolism.2023; : 1. CrossRef - Dipeptidyl Peptidase-4 Inhibitors, Glucagon-like Peptide-1 Receptor Agonists, and Sodium-Glucose Cotransporter-2 Inhibitors and COVID-19 Outcomes
Andreana Foresta, Luisa Ojeda-Fernandez, Giulia Macaluso, Maria Carla Roncaglioni, Mauro Tettamanti, Ida Fortino, Olivia Leoni, Stefano Genovese, Marta Baviera
Clinical Therapeutics.2023; 45(4): e115. CrossRef - DPP-4 Inhibitors as a savior for COVID-19 patients with diabetes
Snehasish Nag, Samanwita Mandal, Oindrila Mukherjee, Suprabhat Mukherjee, Rakesh Kundu
Future Virology.2023; 18(5): 321. CrossRef - DrugRep-HeSiaGraph: when heterogenous siamese neural network meets knowledge graphs for drug repurposing
Zahra Ghorbanali, Fatemeh Zare-Mirakabad, Najmeh Salehi, Mohammad Akbari, Ali Masoudi-Nejad
BMC Bioinformatics.2023;[Epub] CrossRef - Immunomodulatory activity of dipeptidyl peptidase‐4 inhibitors in immune‐related diseases
Marija Drakul, Miodrag Čolić
European Journal of Immunology.2023;[Epub] CrossRef - Dipeptidyl peptidase-4 (DPP-IV) inhibitor was associated with mortality reduction in COVID-19 — A systematic review and meta-analysis
Ahmad Fariz Malvi Zamzam Zein, Wilson Matthew Raffaello
Primary Care Diabetes.2022; 16(1): 162. CrossRef - Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis
Nam Nhat Nguyen, Dung Si Ho, Hung Song Nguyen, Dang Khanh Ngan Ho, Hung-Yuan Li, Chia-Yuan Lin, Hsiao-Yean Chiu, Yang-Ching Chen
Metabolism.2022; 131: 155196. CrossRef - The Association Between Antidiabetic Agents and Clinical Outcomes of COVID-19 Patients With Diabetes: A Bayesian Network Meta-Analysis
Yidan Chen, Xingfei Lv, Sang Lin, Mohammad Arshad, Mengjun Dai
Frontiers in Endocrinology.2022;[Epub] CrossRef - Type 2 Diabetes Mellitus and COVID-19: A Narrative Review
Cristina Rey-Reñones, Sara Martinez-Torres, Francisco M. Martín-Luján, Carles Pericas, Ana Redondo, Carles Vilaplana-Carnerero, Angela Dominguez, María Grau
Biomedicines.2022; 10(9): 2089. CrossRef - Role of Dipeptidyl Peptidase-4 (DPP4) on COVID-19 Physiopathology
Alba Sebastián-Martín, Belén G. Sánchez, José M. Mora-Rodríguez, Alicia Bort, Inés Díaz-Laviada
Biomedicines.2022; 10(8): 2026. CrossRef - Non-Insulin Novel Antidiabetic Drugs Mechanisms in the Pathogenesis of COVID-19
Teodor Salmen, Valeria-Anca Pietroșel, Bianca-Margareta Mihai, Ioana Cristina Bica, Claudiu Teodorescu, Horia Păunescu, Oana Andreia Coman, Doina-Andrada Mihai, Anca Pantea Stoian
Biomedicines.2022; 10(10): 2624. CrossRef - Antidiabetic treatment and COVID-19 Outcomes: A population-based cohort study in primary health care in Catalonia during the first wave of the pandemic
Dan Ouchi, Carles Vilaplana-Carnerero, Vanessa de Dios, Maria Giner-Soriano, Rosa Morros
Primary Care Diabetes.2022; 16(6): 753. CrossRef - Immunotropic effects of hypoglycemic agents on coronavirus infection: a view from the perspective of pharmacogenetics
Konstantin G. Gurevich, Yulia A. Sorokina, Alexander L. Urakov, Snezhana D. Sinyushkina, Maria I. Pryazhnikova, Alyona V. Gorinova, Lyubov V. Lovtsova, Olga V. Zanozina
Reviews on Clinical Pharmacology and Drug Therapy.2022; 20(3): 269. CrossRef - Dipeptidyl peptidase 4 inhibitors in COVID-19: Beyond glycemic control
Niya Narayanan, Dukhabandhu Naik, Jayaprakash Sahoo, Sadishkumar Kamalanathan
World Journal of Virology.2022; 11(6): 399. CrossRef - Improvement of glycemic control and reduction of major cardiovascular events in 18 cardiovascular outcome trials: an updated meta-regression
Maria Ida Maiorino, Miriam Longo, Lorenzo Scappaticcio, Giuseppe Bellastella, Paolo Chiodini, Katherine Esposito, Dario Giugliano
Cardiovascular Diabetology.2021;[Epub] CrossRef
- Noninsulin‐based antihyperglycemic medications in patients with diabetes and COVID‐19: A systematic review and meta‐analysis

Original Articles
- Diabetes, Obesity and Metabolism
- High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study
- Sung-Woo Kim, Jae-Han Jeon, Jun Sung Moon, Mi Kyung Kim
- Endocrinol Metab. 2021;36(4):800-809. Published online August 20, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1040
- 5,060 View
- 175 Download
- 6 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Based on recent evidence on the importance of the presence of diabetes mellitus (DM) and fibrosis-4 (FIB-4) index in coronavirus disease 2019 (COVID-19) mortality, we analyzed whether these factors could additively predict such mortality.
Methods
This multicenter observational study included 1,019 adult inpatients admitted to university hospitals in Daegu. The demographic and laboratory findings, mortality, prevalence of severe disease, and duration of quarantine were compared between patients with and without DM and/or a high FIB-4 index. The mortality risk and corresponding hazard ratio (HR) were analyzed using the Kaplan-Meier method and Cox proportional hazard models.
Results
The patients with DM (n=217) exhibited significantly higher FIB-4 index and mortality compared to those without DM. Although DM (HR, 2.66; 95% confidence interval [CI], 1.63 to 4.33) and a high FIB-4 index (HR, 4.20; 95% CI, 2.21 to 7.99) were separately identified as risk factors for COVID-19 mortality, the patients with both DM and high FIB-4 index had a significantly higher mortality (HR, 9.54; 95% CI, 4.11 to 22.15). Higher FIB-4 indices were associated with higher mortality regardless of DM. A high FIB-4 index with DM was more significantly associated with a severe clinical course with mortality (odds ratio, 11.24; 95% CI, 5.90 to 21.41) than a low FIB-4 index without DM, followed by a high FIB-4 index alone and DM alone. The duration of quarantine and hospital stay also tended to be longer in those with both DM and high FIB-4 index.
Conclusion
Both DM and high FIB-4 index are independent and additive risk factors for COVID-19 mortality. -
Citations
Citations to this article as recorded by- COVID-19 and hepatic injury: Diversity and risk assessment
Fares E M Ali, Mostafa K Abd El-Aziz, Mahmoud M Ali, Osama M Ghogar, Adel G Bakr
World Journal of Gastroenterology.2023; 29(3): 425. CrossRef - Differential Effects of COVID-19 Hospitalization on the Trajectory of Liver Disease Progression
Dilara Hatipoğlu, Connor Mulligan, Jeffrey Wang, Juan Peticco, Reid Grinspoon, Sanjay Gadi, Camilla Mills, Jay Luther, Raymond T. Chung
Gastro Hep Advances.2023; 2(4): 480. CrossRef - Association of non-alcoholic fatty liver and metabolic-associated fatty liver with COVID-19 outcomes: A systematic review and meta-analysis
Gowthami Sai Kogilathota Jagirdhar, Rakhtan K Qasba, Harsha Pattnaik, Kaanthi Rama, Akshat Banga, Shiva Teja Reddy, Anna Carolina Flumignan Bucharles, Rahul Kashyap, Praveen Reddy Elmati, Vikas Bansal, Yatinder Bains, Theodore DaCosta, Salim Surani
World Journal of Gastroenterology.2023; 29(21): 3362. CrossRef - COVID-19 and Fatty Liver Disorders
Maria Guarino, Valentina Cossiga, Francesco Cutolo, Maria Attanasio, Raffaele Lieto, Filomena Morisco
Journal of Clinical Medicine.2023; 12(13): 4316. CrossRef - Prevalence and Prognostic Significance of Liver Fibrosis in Patients With Aneurysmal Subarachnoid Hemorrhage
Tiangui Li, Peng Wang, Xiao Gong, Weelic Chong, Yang Hai, Chao You, Juan Kang, Fang Fang, Yu Zhang
Frontiers in Neurology.2022;[Epub] CrossRef
- COVID-19 and hepatic injury: Diversity and risk assessment

- Thyroid
- Thyroid Hormone Profile and Its Prognostic Impact on the Coronavirus Disease 2019 in Korean Patients
- Jiyeon Ahn, Min Kyung Lee, Jae Hyuk Lee, Seo Young Sohn
- Endocrinol Metab. 2021;36(4):769-777. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1109
- 4,353 View
- 185 Download
- 17 Web of Science
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Data on the association between coronavirus disease 2019 (COVID-19) and thyroid have been reported, including overt thyrotoxicosis and suppression of thyroid function. We aimed to evaluate the thyroid hormone profile and its association with the prognosis of COVID-19 in Korean patients.
Methods
The clinical data of 119 patients with COVID-19, admitted in the Myongji Hospital, Goyang, South Korea, were retrospectively evaluated. The thyroid hormone profiles were analyzed and compared based on disease severity (non-severe disease vs. severe to critical disease). Clinical outcomes were analyzed according to the tertiles of thyroid hormones.
Results
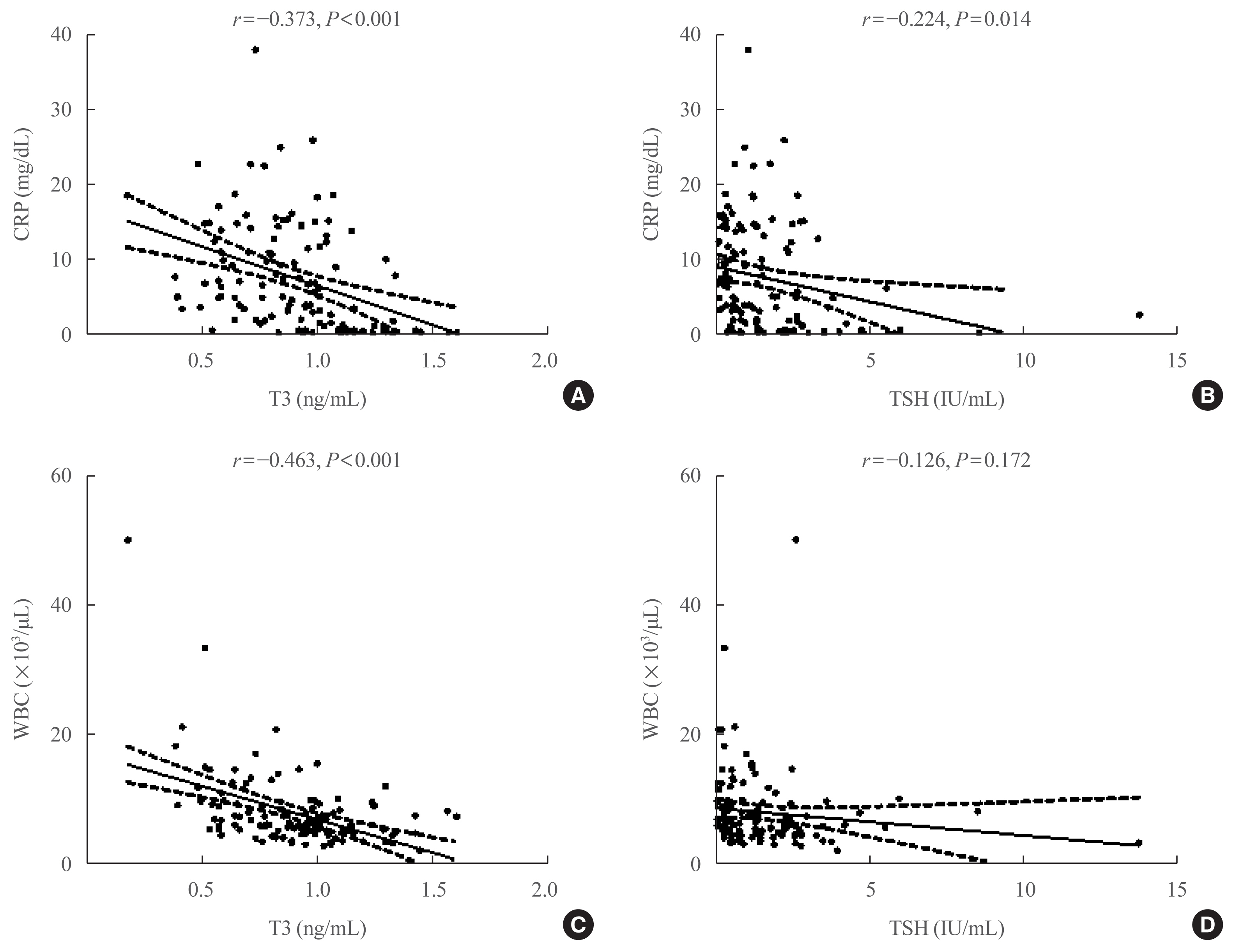
Of the 119 patients, 76 (63.9%) were euthyroid, and none presented with overt thyroid dysfunction. Non-thyroidal illness syndrome was the most common manifestation (18.5%), followed by subclinical thyrotoxicosis (14.3%) among patients with thyroid dysfunction. Thyroid stimulating hormone (TSH) and triiodothyronine (T3) levels were significantly lower in patients with severe to critical disease than in those with non-severe disease (P<0.05). Patients in the lowest T3 tertile (<0.77 ng/mL) had higher rates of mechanical ventilation, intensive care unit admission, and death than those in the middle and highest (>1.00 ng/mL) T3 tertiles (P<0.05). COVID-19 patients in the lowest T3 tertile were independently associated with mortality (hazard ratio, 5.27; 95% confidence interval, 1.09 to 25.32; P=0.038) compared with those in the highest T3 tertile.
Conclusion
Thyroid dysfunction is common in COVID-19 patients. Changes in serum TSH and T3 levels may be important markers of disease severity in COVID-19. Decreased T3 levels may have a prognostic significance in COVID-19 related outcome. -
Citations
Citations to this article as recorded by- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis
Sadra Ashrafi, Hossein Hatami, Razieh Bidhendi-Yarandi, Mohammad Hossein Panahi
BMC Endocrine Disorders.2024;[Epub] CrossRef - Thyroid Stimulating Hormone as a Possible Additional COVID-19 Outcome Marker
Anamarija Zrilic Vrkljan, Ana Majic Tengg, Tanja Palaversa, Srecko Marusic, Lana Ruzic, Ines Bilic-Curcic, Maja Cigrovski Berkovic
Medicina.2024; 60(2): 314. CrossRef - Effect of Hypothalamic Adrenal Axis and Thyroid Function Alterations on Prognosis of Critically Ill COVID-19 Patients
Muhammet KORKUSUZ, Sulbiye KARABURGU, Tayfun ET, Rafet YARIMOĞLU, Nuh KUMRU
Namık Kemal Tıp Dergisi.2024; 12(1): 17. CrossRef - Thyroxine changes in COVID-19 pandemic: A systematic review and meta-analysis
Ziqi Li, Pengwei Hou, Shuwen Mu, Renzhi Wang, Hui Miao, Ming Feng, He Wang, Wentai Zhang, Yihao Chen, Tianshun Feng, Shousen Wang, Yi Fang
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Influence of SARS-CoV-2 Infection on the Thyroid Gland
Aleksandra Piekarska, Marta Góral, Marta Kozula, Aleksandra Jawiarczyk-Przybyłowska, Katarzyna Zawadzka, Marek Bolanowski
Biomedicines.2023; 11(2): 614. CrossRef - Thyroid Function Abnormalities and Outcomes in Hospitalized Patients
with COVID-19 Infection: A Cross-Sectional Study
Deepika Patel, Dukhabandhu Naik, Sadishkumar Kamalanathan, Kadhiravan Tamilarasu, Jayaprakash Sahoo, Ayan Roy, Chandhana Merugu, Varun Suryadevara
Hormone and Metabolic Research.2023; 55(03): 169. CrossRef - The Spectrum of Thyroid Function Tests and Autoantibodies During Hospitalization and After Six Months of Discharge in COVID-19 Patients: Does COVID-19 Trigger Autoimmunity?
Ziynet Alphan Uc, Pinar Yagcı, Zelal Adibelli, Cevdet Duran
Endocrine Research.2023; 48(2-3): 44. CrossRef - Transient low T3 syndrome in patients with COVID-19: a new window for prediction of disease severity
Mingyao Zhong, Yue Gao, Hongling Hu, Xuan Zhu, Lulu Gan, Ling Li, Cheng Xiang, Yimin Yan, Zhe Dai
Frontiers in Endocrinology.2023;[Epub] CrossRef - The Association Between COVID-19 and Thyroxine Levels: A Meta-Analysis
Yiru Chen, Xiuneng Li, Yu Dai, Jingjing Zhang
Frontiers in Endocrinology.2022;[Epub] CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Potential of Endogenous Oxytocin in Endocrine Treatment and Prevention of COVID-19
Stephani C. Wang, Fengmin Zhang, Hui Zhu, Haipeng Yang, Yang Liu, Ping Wang, Vladimir Parpura, Yu-Feng Wang
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Association Between FT3 With the Outcome and Inflammation/Coagulopathy/Fibrinolysis of COVID-19
Jiayi Deng, Siye Zhang, Fei Peng, Quan Zhang, Yi Li, Yanjun Zhong
Frontiers in Endocrinology.2022;[Epub] CrossRef - Primary hypothyroidism with an episode of ventricular tachycardia in a patient with COVID-19
Pin-Hsu Liao, Yu-Cheng Cheng, Po-Yu Liu, I-Te Lee
Medicine.2022; 101(25): e29243. CrossRef - Low triiodothyronine syndrome is associated with stroke‐associated pneumonia
Huijun Chen, Minjie Xu, Yezhi Huang, Jincai He, Wenwei Ren
European Journal of Clinical Investigation.2022;[Epub] CrossRef - Association of thyroid dysfunction and COVID-19: A systematic review and meta-analysis
Mohammad Darvishi, Mohammad Reza Nazer, Hamze Shahali, Majid Nouri
Frontiers in Endocrinology.2022;[Epub] CrossRef - The prognostic utility of serum thyrotropin in hospitalized Covid-19 patients: statistical and machine learning approaches
E. Pappa, P. Gourna, G. Galatas, M. Manti, A. Romiou, L. Panagiotou, R. Chatzikyriakou, N. Trakas, G. Feretzakis, C. Christopoulos
Endocrine.2022; 80(1): 86. CrossRef - Thyrotropin Levels in Patients with Coronavirus Disease 2019: Assessment during Hospitalization and in the Medium Term after Discharge
Abdallah Al-Salameh, Noémie Scherman, Imane Adda, Juliette André, Yoann Zerbib, Julien Maizel, Jean-Daniel Lalau, Etienne Brochot, Claire Andrejak, Rachel Desailloud
Life.2022; 12(12): 2014. CrossRef - COVID-19 and thyroid function: What do we know so far?
Camila Lüdke Rossetti, Juliana Cazarin, Fabio Hecht, Fabyan Esberard de Lima Beltrão, Andrea Cláudia Freitas Ferreira, Rodrigo Soares Fortunato, Helton Estrela Ramos, Denise Pires de Carvalho
Frontiers in Endocrinology.2022;[Epub] CrossRef
- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis

Special Article
- Miscellaneous
- COVID-19 Vaccination for Endocrine Patients: A Position Statement from the Korean Endocrine Society
- Cheol Ryong Ku, Kyong Yeun Jung, Chang Ho Ahn, Jun Sung Moon, Ju Hee Lee, Eun Heui Kim, Hyemi Kwon, Hee Kyung Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Eun Roh, Jin Hwa Kim, Mi-kyung Kim, the Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2021;36(4):757-765. Published online August 17, 2021
- DOI: https://doi.org/10.3803/EnM.2021.404
- 10,360 View
- 419 Download
- 19 Web of Science
- 21 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Since the first outbreak of coronavirus disease 2019 (COVID-19), ongoing efforts have been made to discover an efficacious vaccine against COVID-19 to combat the pandemic. In most countries, both mRNA and DNA vaccines have been administered, and their side effects have also been reported. The clinical course of COVID-19 and the effects of vaccination against COVID-19 are both influenced by patients’ health status and involve a systemic physiological response. In view of the systemic function of endocrine hormones, endocrine disorders themselves and the therapeutics used to treat them can influence the outcomes of vaccination for COVID-19. However, there are very limited data to support the development of clinical guidelines for patients with specific medical backgrounds based on large clinical trials. In the current severe circumstances of the COVID-19 pandemic, position statements made by clinical specialists are essential to provide appropriate recommendations based on both medical evidence and clinical experiences. As endocrinologists, we would like to present the medical background of COVID-19 vaccination, as well as precautions to prevent the side effects of COVID-19 vaccination in patients with specific endocrine disorders, including adrenal insufficiency, diabetes mellitus, osteoporosis, autoimmune thyroid disease, hypogonadism, and pituitary disorders.
-
Citations
Citations to this article as recorded by- COVID-19 mRNA vaccine may trigger subacute thyroiditis
Mehmet Sözen, Ömercan Topaloğlu, Berrin Çetinarslan, Alev Selek, Zeynep Cantürk, Emre Gezer, Damla Köksalan, Taner Bayraktaroğlu
Human Vaccines & Immunotherapeutics.2024; 17(12): 5120. CrossRef - The role of co-morbidities in the development of an AEFI after COVID-19 vaccination in a large prospective cohort with patient-reported outcomes in the Netherlands
C. Ouaddouh, J.W. Duijster, T. Lieber, F.P.A.M. van Hunsel
Expert Opinion on Drug Safety.2024; 23(3): 323. CrossRef - Thyroid dysfunction in COVID-19
David Tak Wai Lui, Chi Ho Lee, Yu Cho Woo, Ivan Fan Ngai Hung, Karen Siu Ling Lam
Nature Reviews Endocrinology.2024;[Epub] CrossRef - Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
Hyeyeon Moon, Sunghwan Suh, Mi Kyoung Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Prior immunization status of COVID-19 patients and disease severity: A multicenter retrospective cohort study assessing the different types of immunity
Javaria Aslam, Faisal Shahzad Khan, Muhammad Talha Haris, Hewad Hewadmal, Maryam Khalid, Mohammad Y. Alshahrani, Qurrat-ul-ain Aslam, Irrum Aneela, Urooj Zafar
Vaccine.2023; 41(2): 598. CrossRef - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(2): 253. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Inactivated SARS-CoV-2 vaccination does not disturb the clinical course of Graves’ disease: An observational cohort study
Shichen Xu, Huixin Yu, Xian Cheng, Jing Wu, Jiandong Bao, Li Zhang
Vaccine.2023; 41(38): 5648. CrossRef - Adrenal Crisis Associated With COVID-19 Vaccination in Patients With Adrenal Insufficiency
Yukako Kurematsu, Takako Mohri, Sadanori Okada, Yutaka Takahashi
JCEM Case Reports.2023;[Epub] CrossRef - Adverse Events Associated with COVID-19 Vaccination in Adolescents with Endocrinological Disorders: A Cross-Sectional Study
İbrahim Mert Erbaş, İrem Ceren Erbaş, Gözde Akın Kağızmanlı, Kübra Yüksek Acinikli, Özge Besci, Korcan Demir, Ece Böber, Nurşen Belet, Ayhan Abacı
Journal of Clinical Research in Pediatric Endocrinology.2023; 15(3): 248. CrossRef - Neue Aspekte der Glukokortikoidsubstitution bei Nebennierenrindeninsuffizienz
Tina Kienitz, Gesine Meyer
Der Internist.2022; 63(1): 12. CrossRef - Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence
Rimesh Pal, Ameya Joshi, Sanjay K. Bhadada, Mainak Banerjee, Suresh Vaikkakara, Satinath Mukhopadhyay
Endocrine Practice.2022; 28(4): 425. CrossRef - Safety of Inactivated and mRNA COVID-19 Vaccination Among Patients Treated for Hypothyroidism: A Population-Based Cohort Study
Xi Xiong, Carlos King Ho Wong, Ivan Chi Ho Au, Francisco Tsz Tsun Lai, Xue Li, Eric Yuk Fai Wan, Celine Sze Ling Chui, Esther Wai Yin Chan, Franco Wing Tak Cheng, Kristy Tsz Kwan Lau, Chi Ho Lee, Yu Cho Woo, David Tak Wai Lui, Ian Chi Kei Wong
Thyroid.2022; 32(5): 505. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism
Nikolina Markovic, Anila Faizan, Chirag Boradia, Sridhar Nambi
AACE Clinical Case Reports.2022; 8(4): 171. CrossRef - The Effect of Inactivated SARS-CoV-2 Vaccines on TRAB in Graves’ Disease
LingHong Huang, ZhengRong Jiang, JingXiong Zhou, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Osteoporosis in Patients With Respiratory Diseases
Yue Ma, Shui Qiu, Renyi Zhou
Frontiers in Physiology.2022;[Epub] CrossRef - Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology
Ach Taieb, El Euch Mounira
Vaccines.2022; 10(12): 2004. CrossRef - Forty Years Together, New Leap Forward! The 40th Anniversary of the Korean Endocrine Society
Jong Chul Won, Ki-Hyun Baek
Endocrinology and Metabolism.2022; 37(6): 851. CrossRef - No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine
Tania Pilli, Cristina Dalmiglio, Gilda Dalmazio, Alfonso Sagnella, Raffaella Forleo, Lucia Brilli, Fabio Maino, Cristina Ciuoli, Maria Grazia Castagna
European Journal of Endocrinology.2022; 187(1): K7. CrossRef - Diabetes and COVID-19 Vaccination
Hae Dong Choi, Jun Sung Moon
The Journal of Korean Diabetes.2021; 22(4): 221. CrossRef
- COVID-19 mRNA vaccine may trigger subacute thyroiditis

Original Article
- Thyroid
- Insights from a Prospective Follow-up of Thyroid Function and Autoimmunity among COVID-19 Survivors
- David Tak Wai Lui, Chi Ho Lee, Wing Sun Chow, Alan Chun Hong Lee, Anthony Raymond Tam, Carol Ho Yi Fong, Chun Yiu Law, Eunice Ka Hong Leung, Kelvin Kai Wang To, Kathryn Choon Beng Tan, Yu Cho Woo, Ching Wan Lam, Ivan Fan Ngai Hung, Karen Siu Ling Lam
- Endocrinol Metab. 2021;36(3):582-589. Published online June 8, 2021
- DOI: https://doi.org/10.3803/EnM.2021.983
- 10,878 View
- 266 Download
- 32 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The occurrence of Graves’ disease and Hashimoto thyroiditis after coronavirus disease 2019 (COVID-19) raised concerns that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may trigger thyroid autoimmunity. We aimed to address the current uncertainties regarding incident thyroid dysfunction and autoimmunity among COVID-19 survivors.
Methods
We included consecutive adult COVID-19 patients without known thyroid disorders, who were admitted to Queen Mary Hospital from July 21 to September 21, 2020 and had serum levels of thyroid-stimulating hormone, free thyroxine, free triiodothyronine (fT3), and anti-thyroid antibodies measured both on admission and at 3 months.
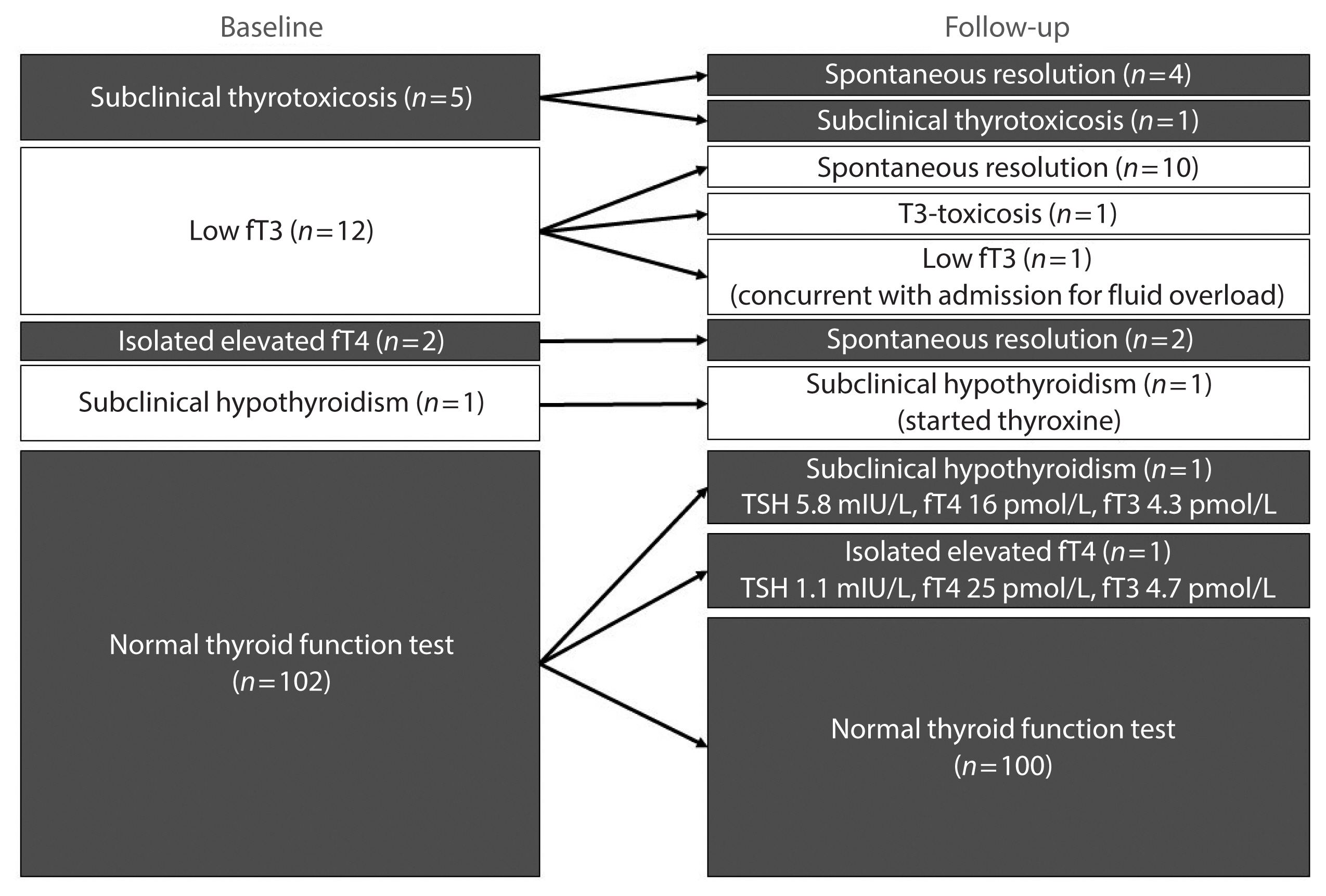
Results
In total, 122 patients were included. Among 20 patients with abnormal thyroid function tests (TFTs) on admission (mostly low fT3), 15 recovered. Among 102 patients with initial normal TFTs, two had new-onset abnormalities that could represent different phases of thyroiditis. Among 104 patients whose anti-thyroid antibody titers were reassessed, we observed increases in anti-thyroid peroxidase (TPO) (P<0.001) and anti-thyroglobulin (P<0.001), but not anti-thyroid stimulating hormone receptor titers (P=0.486). Of 82 patients with negative anti-TPO findings at baseline, 16 had a significant interval increase in anti-TPO titer by >12 U, and four became anti-TPO-positive. Worse baseline clinical severity (P=0.018), elevated C-reactive protein during hospitalization (P=0.033), and higher baseline anti-TPO titer (P=0.005) were associated with a significant increase in anti-TPO titer.
Conclusion
Most patients with thyroid dysfunction on admission recovered during convalescence. Abnormal TFTs suggestive of thyroiditis occurred during convalescence, but infrequently. Importantly, our novel observation of an increase in anti-thyroid antibody titers post-COVID-19 warrants further follow-up for incident thyroid dysfunction among COVID-19 survivors. -
Citations
Citations to this article as recorded by- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis
Sadra Ashrafi, Hossein Hatami, Razieh Bidhendi-Yarandi, Mohammad Hossein Panahi
BMC Endocrine Disorders.2024;[Epub] CrossRef - Does COVID-19 affect thyroid more than non-COVID-19 infections? A retrospective study
Yasemin Ucal, Muhittin Serdar, Hande Karpuzoğlu, Neval Yurttutan Uyar, Meltem Kilercik, Mustafa Serteser, Aysel Ozpinar
Turkish Journal of Biochemistry.2024;[Epub] CrossRef - Thyroiditis and COVID-19: focus on pediatric age. A narrative review
F. d’Aniello, M. E. Amodeo, A. Grossi, G. Ubertini
Journal of Endocrinological Investigation.2024;[Epub] CrossRef - The most common persistent symptoms in patients with COVID-19 who were evaluated in the Internal Medicine polyclinic
Zeynep KOÇ, Seydahmet AKIN
The European Research Journal.2023; 9(1): 97. CrossRef - Clinical assessment of children with long COVID syndrome
Réka Garai, Péter Krivácsy, Vivien Herczeg, Fanni Kovács, Bálint Tél, Judit Kelemen, Anna Máthé, Eszter Zsáry, Johanna Takács, Dániel Sándor Veres, Attila J. Szabó
Pediatric Research.2023; 93(6): 1616. CrossRef - T Cell Receptor Sequences Amplified during Severe COVID-19 and Multisystem Inflammatory Syndrome in Children Mimic SARS-CoV-2, Its Bacterial Co-Infections and Host Autoantigens
Robert Root-Bernstein, Elizabeth Churchill, Shelby Oliverio
International Journal of Molecular Sciences.2023; 24(2): 1335. CrossRef - A Literature Review on SARS-CoV-2 and Other Viruses in Thyroid Disorders: Environmental Triggers or No-Guilty Bystanders?
Francesca Gorini, Cristina Vassalle
International Journal of Environmental Research and Public Health.2023; 20(3): 2389. CrossRef - Thyroid dysfunction as a long-term post-COVID-19 complication in mild-to-moderate COVID-19
Vesselina Yanachkova, Teodora Stankova, Radiana Staynova
Biotechnology & Biotechnological Equipment.2023; 37(1): 194. CrossRef - The Influence of SARS-CoV-2 Infection on the Thyroid Gland
Aleksandra Piekarska, Marta Góral, Marta Kozula, Aleksandra Jawiarczyk-Przybyłowska, Katarzyna Zawadzka, Marek Bolanowski
Biomedicines.2023; 11(2): 614. CrossRef - Thyroid Function Abnormalities and Outcomes in Hospitalized Patients
with COVID-19 Infection: A Cross-Sectional Study
Deepika Patel, Dukhabandhu Naik, Sadishkumar Kamalanathan, Kadhiravan Tamilarasu, Jayaprakash Sahoo, Ayan Roy, Chandhana Merugu, Varun Suryadevara
Hormone and Metabolic Research.2023; 55(03): 169. CrossRef - The Spectrum of Thyroid Function Tests and Autoantibodies During Hospitalization and After Six Months of Discharge in COVID-19 Patients: Does COVID-19 Trigger Autoimmunity?
Ziynet Alphan Uc, Pinar Yagcı, Zelal Adibelli, Cevdet Duran
Endocrine Research.2023; 48(2-3): 44. CrossRef - Increased prevalence of autoimmune thyroid disease after COVID-19: A single-center, prospective study
Alessandro Rossini, Sara Cassibba, Francesca Perticone, Simone Vasilij Benatti, Serena Venturelli, Greta Carioli, Arianna Ghirardi, Marco Rizzi, Tiziano Barbui, Roberto Trevisan, Silvia Ippolito
Frontiers in Endocrinology.2023;[Epub] CrossRef - A prospective follow-up of thyroid volume and thyroiditis features on ultrasonography among survivors of predominantly mild to moderate COVID-19
Man Him Matrix Fung, David Tak Wai Lui, Keith Wan Hang Chiu, Sherman Haynam Lee, Chi Ho Lee, Wing Sun Chow, Alan Chun Hong Lee, Anthony Raymond Tam, Polly Pang, Tip Yin Ho, Carol Ho Yi Fong, Connie Hong Nin Loong, Chun Yiu Law, Kelvin Kai Wang To, Ching W
PeerJ.2023; 11: e15034. CrossRef - Study on Clinicopathological Features and Risk Factors of Patients with Multiple Primary Breast Cancers and Thyroid Disease
Jie Li, Yonghong Liu, Jian Jin, Qingfeng Shi, Yanting Ji, Bo Zhang, Pengfei Hu, Jinghua Pan
Mediators of Inflammation.2023; 2023: 1. CrossRef - Beyond Acute COVID-19: Investigating the Incidence of Subacute Thyroiditis in Long COVID-19 in Korea
Jeongmin Lee, Gi Hyeon Seo, Keeho Song
Endocrinology and Metabolism.2023; 38(4): 455. CrossRef - Thyroid Autoimmunity and SARS-CoV-2 Infection
Poupak Fallahi, Giusy Elia, Francesca Ragusa, Sabrina Rosaria Paparo, Armando Patrizio, Eugenia Balestri, Valeria Mazzi, Salvatore Benvenga, Gilda Varricchi, Laura Gragnani, Chiara Botrini, Enke Baldini, Marco Centanni, Clodoveo Ferri, Alessandro Antonell
Journal of Clinical Medicine.2023; 12(19): 6365. CrossRef - Autoimmune complications of COVID‐19
Niloufar Yazdanpanah, Nima Rezaei
Journal of Medical Virology.2022; 94(1): 54. CrossRef - The Independent Association of TSH and Free Triiodothyronine Levels With Lymphocyte Counts Among COVID-19 Patients
David Tak Wai Lui, Chi Ho Lee, Wing Sun Chow, Alan Chun Hong Lee, Anthony Raymond Tam, Polly Pang, Tip Yin Ho, Chloe Yu Yan Cheung, Carol Ho Yi Fong, Chun Yiu Law, Kelvin Kai Wang To, Ching Wan Lam, Kathryn Choon Beng Tan, Yu Cho Woo, Ivan Fan Ngai Hung,
Frontiers in Endocrinology.2022;[Epub] CrossRef - Comment on Khunti et al. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care 2021;44:2645–2655
Carmine Gazzaruso, Adriana Coppola, Pietro Gallotti, Ileana Terruzzi, Tiziana Montalcini, Livio Luzi
Diabetes Care.2022; 45(2): e45. CrossRef - The potential impact of COVID-19 on thyroid gland volumes among COVID-19 survivors
Emre Urhan, Zuleyha Karaca, Canan Sehit Kara, Zeynep Ture Yuce, Kursad Unluhizarci
Endocrine.2022; 76(3): 635. CrossRef - Systematic review of COVID-19 and autoimmune thyroiditis
Esra Tutal, Resat Ozaras, Hakan Leblebicioglu
Travel Medicine and Infectious Disease.2022; 47: 102314. CrossRef - The New Entity of Subacute Thyroiditis amid the COVID-19 Pandemic: From Infection to Vaccine
Mihaela Popescu, Adina Ghemigian, Corina Maria Vasile, Andrei Costache, Mara Carsote, Alice Elena Ghenea
Diagnostics.2022; 12(4): 960. CrossRef - Should we expect a wave of type 1 diabetes following SARS‐CoV‐2 pandemic?
Laura Montefusco, Andrea Mario Bolla, Paolo Fiorina
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - COVID-19 and Thyroid Function: A Bi-Directional Two-Sample Mendelian Randomization Study
Gloria Hoi-Yee Li, Ching-Man Tang, Ching-Lung Cheung
Thyroid.2022; 32(9): 1037. CrossRef - Development of a prediction score (ThyroCOVID) for identifying abnormal thyroid function in COVID-19 patients
D. T. W. Lui, C. H. Lee, W. S. Chow, A. C. H. Lee, A. R. Tam, C. Y. Y. Cheung, C. H. Y. Fong, S. T. M. Kwok, C. Y. Law, K. K. W. To, C. W. Lam, K. C. B. Tan, Y. C. Woo, I. F. N. Hung, K. S. L. Lam
Journal of Endocrinological Investigation.2022; 45(11): 2149. CrossRef - Symptomatic Bradycardia Manifesting as Acute Hypothyroidism Following COVID-19 Infection: A Case Report
Jaydip Desai, Arsh N Patel, Courtney L Evans, Molly Triggs, Fulton Defour
Cureus.2022;[Epub] CrossRef - Schilddrüse und SARS-CoV-2
Georg Zettinig
Journal für Klinische Endokrinologie und Stoffwechsel.2022; 15(3): 100. CrossRef - Thyroid diseases are associated with coronavirus disease 2019 infection
Yutian Tian, Junyu Zhao, Tingting Wang, Haipeng Wang, Jinming Yao, Song Wang, Yaru Mou
Frontiers in Endocrinology.2022;[Epub] CrossRef - Thyrotropin Levels in Patients with Coronavirus Disease 2019: Assessment during Hospitalization and in the Medium Term after Discharge
Abdallah Al-Salameh, Noémie Scherman, Imane Adda, Juliette André, Yoann Zerbib, Julien Maizel, Jean-Daniel Lalau, Etienne Brochot, Claire Andrejak, Rachel Desailloud
Life.2022; 12(12): 2014. CrossRef - Long COVID in Patients With Mild to Moderate Disease: Do Thyroid Function and Autoimmunity Play a Role?
David Tak Wai Lui, Chi Ho Lee, Wing Sun Chow, Alan Chun Hong Lee, Anthony Raymond Tam, Polly Pang, Tip Yin Ho, Carol Ho Yi Fong, Chun Yiu Law, Eunice Ka Hong Leung, Kelvin Kai Wang To, Kathryn Choon Beng Tan, Yu Cho Woo, Ching Wan Lam, Ivan Fan Ngai Hung,
Endocrine Practice.2021; 27(9): 894. CrossRef - Hashimoto’s thyroiditis following SARS-CoV-2 infection
Rafael Silvestre Knack, Taliê Hanada, Renata Silvestre Knack, Kamilla Mayr
BMJ Case Reports.2021; 14(8): e244909. CrossRef - Higher SARS-CoV-2 viral loads correlated with smaller thyroid volumes on ultrasound among male COVID-19 survivors
David Tak Wai Lui, Matrix Man Him Fung, Keith Wan Hang Chiu, Chi Ho Lee, Wing Sun Chow, Alan Chun Hong Lee, Anthony Raymond Tam, Polly Pang, Tip Yin Ho, Carol Ho Yi Fong, Connie Hong Nin Loong, Wade Wei Wong, Cassandra Yuen Yan Lee, Chun Yiu Law, Kelvin K
Endocrine.2021; 74(2): 205. CrossRef - SARS-CoV-2: Emerging Role in the Pathogenesis of Various Thyroid Diseases
Avaniyapuram Kannan Murugan, Ali S Alzahrani
Journal of Inflammation Research.2021; Volume 14: 6191. CrossRef - POST-COVID ENDOCRINOPATHY :ABOUT A CASE ENDOCRINOPATHIE POST- COVID :À PROPOS D’UN CAS
S. Rafi, G. Elmghari, N, Elansari
INDIAN JOURNAL OF APPLIED RESEARCH.2021; : 13. CrossRef
- The prevalence of thyroid disorders in COVID-19 patients: a systematic review and meta-analysis

Review Article
- Diabetes
- Continuous Glucose Monitoring in the Hospital
- M. Citlalli Perez-Guzman, Trisha Shang, Jennifer Y. Zhang, Donna Jornsay, David C. Klonoff
- Endocrinol Metab. 2021;36(2):240-255. Published online March 31, 2021
- DOI: https://doi.org/10.3803/EnM.2021.201
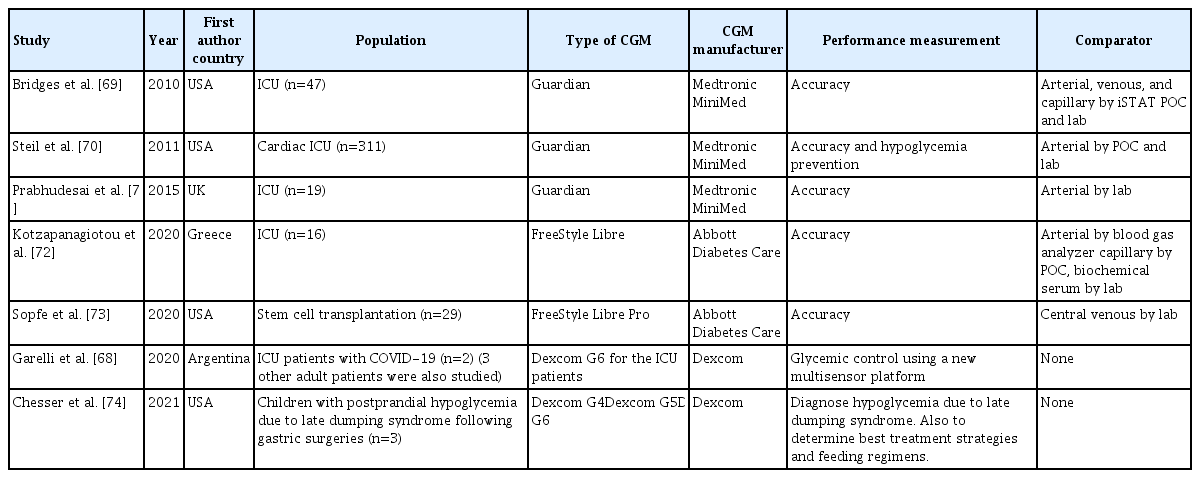
- 16,762 View
- 664 Download
- 26 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Continuous glucose monitors (CGMs) have suddenly become part of routine care in many hospitals. The coronavirus disease 2019 (COVID-19) pandemic has necessitated the use of new technologies and new processes to care for hospitalized patients, including diabetes patients. The use of CGMs to automatically and remotely supplement or replace assisted monitoring of blood glucose by bedside nurses can decrease: the amount of necessary nursing exposure to COVID-19 patients with diabetes; the amount of time required for obtaining blood glucose measurements, and the amount of personal protective equipment necessary for interacting with patients during the blood glucose testing. The United States Food and Drug Administration (FDA) is now exercising enforcement discretion and not objecting to certain factory-calibrated CGMs being used in a hospital setting, both to facilitate patient care and to obtain performance data that can be used for future regulatory submissions. CGMs can be used in the hospital to decrease the frequency of fingerstick point of care capillary blood glucose testing, decrease hyperglycemic episodes, and decrease hypoglycemic episodes. Most of the research on CGMs in the hospital has focused on their accuracy and only recently outcomes data has been reported. A hospital CGM program requires cooperation of physicians, bedside nurses, diabetes educators, and hospital administrators to appropriately select and manage patients. Processes for collecting, reviewing, storing, and responding to CGM data must be established for such a program to be successful. CGM technology is advancing and we expect that CGMs will be increasingly used in the hospital for patients with diabetes.
-
Citations
Citations to this article as recorded by- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System
Kyung-Soo Kim, Seung-Hwan Lee, Won Sang Yoo, Cheol-Young Park
Diabetes Technology & Therapeutics.2024; 26(4): 222. CrossRef - Glucose management in critically ill adults: A qualitative study from the experiences of health care providers
Miao Huang, Li Yang, Chuanlai Zhang, Xiuni Gan
Heliyon.2024; 10(3): e24545. CrossRef - The Need for Data Standards and Implementation Policies to Integrate CGM Data into the Electronic Health Record
Juan Espinoza, Nicole Y. Xu, Kevin T. Nguyen, David C. Klonoff
Journal of Diabetes Science and Technology.2023; 17(2): 495. CrossRef - Staff knowledge, attitudes and practices regarding glycaemic management in adult intensive care units: A national survey
Miao Huang, Ruiqi Yang, Chuanlai Zhang, Xiuni Gan
Nursing in Critical Care.2023; 28(6): 931. CrossRef - Continuous Glucose Monitoring for Patients with COVID-19 Pneumonia: Initial Experience at a Tertiary Care Center
Adrian G. Dumitrascu, Michelle F. Perry, Rebecca J. Boone, Maria P. Guzman, Razvan M. Chirila, Allyson W. McNally, Dorin T. Colibaseanu, Shon E. Meek, Colleen T. Ball, Launia J. White, Ana-Maria Chindris
Endocrine Practice.2023; 29(3): 155. CrossRef - Use of Continuous Glucose Monitors to Manage Type 1 Diabetes Mellitus: Progress, Challenges, and Recommendations
Jared G Friedman, Zulma Cardona Matos, Emily D Szmuilowicz, Grazia Aleppo
Pharmacogenomics and Personalized Medicine.2023; Volume 16: 263. CrossRef - Type 1 Diabetes Overview and Perioperative Management
Grace B. Nelson, Kathryn M. Sumpter
Orthopedic Clinics of North America.2023; 54(3): 287. CrossRef - Real-Time Continuous Glucose Monitoring in the Hospital: A Real-World Experience
Samantha R. Spierling Bagsic, Addie L. Fortmann, Rebekah Belasco, Alessandra Bastian, Suzanne Lohnes, Anna Ritko, Haley Sandoval, Mariya Chichmarenko, Emily C. Soriano, Laura Talavera, Athena Philis-Tsimikas
Journal of Diabetes Science and Technology.2023; 17(3): 656. CrossRef - Implementation of Continuous Glucose Monitoring in Critical Care: A Scoping Review
Eileen R. Faulds, Kathleen M. Dungan, Molly McNett
Current Diabetes Reports.2023; 23(6): 69. CrossRef - Clinical Use of Continuous Glucose Monitoring in Critically Ill Pediatric Patients with Diabetic Ketoacidosis
Esther Park, Minsun Kim
Diabetes Technology & Therapeutics.2023; 25(8): 529. CrossRef - Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality
Ji Yoon Kim, Jee Hee Yoo, Jae Hyeon Kim
Diabetes Technology & Therapeutics.2023; 25(12): 883. CrossRef - A randomized trial of continuous glucose monitoring to improve post‐transplant glycemic control
Nicholas Jandovitz, Sam J. George, Mersema Abate, Adam M. Kressel, Alexandra C. Bolognese, Lawrence Lau, Vinay Nair, Elliot Grodstein
Clinical Transplantation.2023;[Epub] CrossRef - More Green, Less Red: How Color Standardization May Facilitate Effective Use of CGM Data
Richard M. Bergenstal, Gregg D. Simonson, Lutz Heinemann
Journal of Diabetes Science and Technology.2022; 16(1): 3. CrossRef - Continuous glucose monitoring in the hospital: an update in the era of COVID-19
Chikara Gothong, Lakshmi G. Singh, Medha Satyarengga, Elias K. Spanakis
Current Opinion in Endocrinology, Diabetes & Obesity.2022; 29(1): 1. CrossRef - Breakthrough technology for in-hospital glucose monitoring
David Kerr, David Klonoff
The Lancet Diabetes & Endocrinology.2022; 10(5): 304. CrossRef - The Role of the Diabetes Care and Education Specialist in the Hospital Setting
The Science of Diabetes Self-Management and Care.2022; 48(3): 184. CrossRef - Real-Time Monitoring of Blood Parameters in the Intensive Care Unit: State-of-the-Art and Perspectives
Rebecca Bockholt, Shaleen Paschke, Lars Heubner, Bergoi Ibarlucea, Alexander Laupp, Željko Janićijević, Stephanie Klinghammer, Sascha Balakin, Manfred F. Maitz, Carsten Werner, Gianaurelio Cuniberti, Larysa Baraban, Peter Markus Spieth
Journal of Clinical Medicine.2022; 11(9): 2408. CrossRef - Novel Glycemic Index Based on Continuous Glucose Monitoring to Predict Poor Clinical Outcomes in Critically Ill Patients: A Pilot Study
Eun Yeong Ha, Seung Min Chung, Il Rae Park, Yin Young Lee, Eun Young Choi, Jun Sung Moon
Frontiers in Endocrinology.2022;[Epub] CrossRef - Stress-Induced Hyperglycemia: Consequences and Management
Deepanjali Vedantam, Devyani S Poman, Lakshya Motwani, Nailah Asif, Apurva Patel, Krishna Kishore Anne
Cureus.2022;[Epub] CrossRef - Hospital Diabetes Meeting 2022
Jingtong Huang, Andrea M. Yeung, Kevin T. Nguyen, Nicole Y. Xu, Jean-Charles Preiser, Robert J. Rushakoff, Jane Jeffrie Seley, Guillermo E. Umpierrez, Amisha Wallia, Andjela T. Drincic, Roma Gianchandani, M. Cecilia Lansang, Umesh Masharani, Nestoras Math
Journal of Diabetes Science and Technology.2022; 16(5): 1309. CrossRef - Effects of Patient-Driven Lifestyle Modification Using Intermittently Scanned Continuous Glucose Monitoring in Patients With Type 2 Diabetes: Results From the Randomized Open-label PDF Study
Hun Jee Choe, Eun-Jung Rhee, Jong Chul Won, Kyong Soo Park, Won-Young Lee, Young Min Cho
Diabetes Care.2022; 45(10): 2224. CrossRef - The Devil Is in the Details: Use, Limitations, and Implementation of Continuous Glucose Monitoring in the Inpatient Setting
Rebecca Rick Longo, Renu Joshi
Diabetes Spectrum.2022; 35(4): 405. CrossRef
- Accuracy and Safety of the 15-Day CareSens Air Continuous Glucose Monitoring System

Brief Report
- Obesity and Metabolism
- Overweight and Obesity are Risk Factors for Coronavirus Disease 2019: A Propensity Score-Matched Case-Control Study
- Wonjun Ji, Rugyeom Lee, Kyungmin Huh, Minsun Kang, In Cheol Hwang, Munkhzul Radnaabaatar, Dae Ho Lee, Jaehun Jung
- Endocrinol Metab. 2021;36(1):196-200. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.856

- 5,261 View
- 225 Download
- 7 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Although obesity is a risk factor for infection, whether it has the same effect on coronavirus disease 2019 (COVID-19) need confirming. We conducted a retrospective propensity score matched case-control study to examine the association between obesity and COVID-19. This study included data from the Nationwide COVID-19 Registry and the Biennial Health Checkup database, until May 30, 2020. We identified 2,231 patients with confirmed COVID-19 and 10-fold-matched negative test controls. Overweight (body mass index [BMI] 23 to 24.9 kg/m2; adjusted odds ratio [aOR], 1.16; 95% confidence interval [CI], 1.1.03 to 1.30) and class 1 obesity (BMI 25 to 29.9 kg/m2; aOR, 1.27; 95% CI, 1.14 to 1.42) had significantly increased COVID-19 risk, while classes 2 and 3 obesity (BMI ≥30 kg/m2) showed similar but non-significant trend. Females and those <50 years had more robust association pattern. Overweight and obesity are possible risk factors of COVID-19.
-
Citations
Citations to this article as recorded by- Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study
Jong Han Choi, Kyoung Min Kim, Keeho Song, Gi Hyeon Seo
Endocrinology and Metabolism.2023; 38(2): 245. CrossRef - Systematic review with meta‐analysis: COVID‐19 outcomes in patients receiving anti‐TNF treatments
Georgios Kokkotis, Konstantina Kitsou, Ioannis Xynogalas, Vana Spoulou, Gkikas Magiorkinis, Ioannis Trontzas, Panagiotis Trontzas, Garyphallia Poulakou, Konstantinos Syrigos, Giorgos Bamias
Alimentary Pharmacology & Therapeutics.2022; 55(2): 154. CrossRef - Association between COVID-19 morbidity, mortality, and gross domestic product, overweight/ obesity, non-communicable diseases, vaccination rate: A cross-sectional study
Kuat Oshakbayev, Zulfiya Zhankalova, Meruyert Gazaliyeva, Khalit Mustafin, Gulnara Bedelbayeva, Bibazhar Dukenbayeva, Nurzhan Otarbayev, Attila Tordai
Journal of Infection and Public Health.2022; 15(2): 255. CrossRef - Association between being underweight and excess body weight before SARS coronavirus type 2 infection and clinical outcomes of coronavirus disease 2019: Multicenter study
João Araújo Barros-Neto, Carolina Santos Mello, Sandra Mary Lima Vasconcelos, Gabriel Soares Bádue, Raphaela Costa Ferreira, Maria Izabel Siqueira de Andrade, Carlos Queiroz do Nascimento, Mateus de Lima Macena, José Adailton da Silva, Heleni Aires Clemen
Nutrition.2022; 101: 111677. CrossRef - Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study
Ángel Arturo López-González, Bárbara Altisench Jané, Luis Masmiquel Comas, Sebastiana Arroyo Bote, Hilda María González San Miguel, José Ignacio Ramírez Manent
Nutrients.2022; 14(14): 2795. CrossRef - Trends of overweight and obesity prevalence in school-aged children among Henan Province from 2000 to 2019
Yuhao Zhang, Hao Lou, Ye Huang, Ruijuan Wang, Xiao Wen, Cuiping Wu, Changfu Hao, Ran Li, Genli Gao, Xiaomin Lou, Xian Wang
Frontiers in Public Health.2022;[Epub] CrossRef - The effects of 105 biological, socioeconomic, behavioral, and environmental factors on the risk of SARS-CoV-2 infection and a severe course of COVID-19: a prospective, explorative cohort study
Jaroslav Flegr, Pavel Flegr, Lenka Příplatová
Biology Methods and Protocols.2022;[Epub] CrossRef - Current status of health promotion in Korea
Soo Young Kim
Journal of the Korean Medical Association.2022; 65(12): 776. CrossRef - Obesity and Coronavirus Disease 2019
Min-Ji Kim, Jae-Han Jeon
Journal of Metabolic and Bariatric Surgery.2021; 10(1): 1. CrossRef
- Risk for Newly Diagnosed Type 2 Diabetes Mellitus after COVID-19 among Korean Adults: A Nationwide Matched Cohort Study

Review Article
- Thyroid
- Best Achievements in Clinical Thyroidology in 2020
- Eun Kyung Lee, Young Joo Park
- Endocrinol Metab. 2021;36(1):30-35. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2021.103
- 4,443 View
- 246 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - This review highlights the most interesting research in thyroidology conducted in 2020. The publications of interest discussed below dealt with the following topics: thyroid dysfunction, risk of thyroid cancer, molecular diagnostics and new therapeutics for thyroid cancer, and thyroid disease in the coronavirus disease 2019 pandemic era.
-
Citations
Citations to this article as recorded by- Compensation for iodine deficiency conditions with drugs based on duckweed substrate
M. Kh. Sadulaev, M. I. Usmanova, T. T. Tataev, A. M. Inderbiev, A. S.-A. Zhamalullayla, A. Salamova
BIO Web of Conferences.2023; 76: 03002. CrossRef - Use of long non-coding RNAs for the molecular diagnosis of papillary thyroid cancer
Daham Kim, Juyeon Yu, Jiwon Kim, Yoon-a Hwang, Jin Kyong Kim, Cheol Ryong Ku, Jung Hyun Yoon, Jin Young Kwak, Kee-Hyun Nam, Eun Jig Lee
Frontiers in Oncology.2022;[Epub] CrossRef - Ultrasound-Guided Fine-Needle Aspiration with or without Negative Pressure for Different Types of Thyroid Nodules
Qi Zhou, Wenjun Wu, Fang Wang, Xiaohua Gong, Xiaojun Chen
International Journal of General Medicine.2021; Volume 14: 5475. CrossRef
- Compensation for iodine deficiency conditions with drugs based on duckweed substrate


 KES
KES

 First
First Prev
Prev



