Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(6); 2023 > Article
-
Original ArticleMiscellaneous Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
 Keypoint
Keypoint
A study involving 44,595 patients assessed endocrine adverse events in new immune checkpoint inhibitors, or ICIs. It found a high incidence of thyroid-related issues, especially with PD-1 inhibitors. Combining durvalumab and tremelimumab significantly increased the risk of pituitary and adrenal problems. The findings emphasize the importance of predicting and managing endocrine side effects in ICI treatment. -
Won Sang Yoo1*
 , Eu Jeong Ku2*
, Eu Jeong Ku2* , Eun Kyung Lee3
, Eun Kyung Lee3 , Hwa Young Ahn4
, Hwa Young Ahn4
-
Endocrinology and Metabolism 2023;38(6):750-759.
DOI: https://doi.org/10.3803/EnM.2023.1785
Published online: November 13, 2023
1Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
2Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
3Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
4Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- Corresponding authors: Eun Kyung Lee. Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea Tel: +82-31-920-1743, Fax: +82-31-920-2789, E-mail: eklee@ncc.re.kr
- Hwa Young Ahn. Department of Internal Medicine, Chung-Ang University College of Medicine, 102 Heukseok-ro, Dongjak-gu, Seoul 06973, Korea Tel: +82-2-6299-3152, Fax: +82-2-6299-2017, E-mail: hyahnmd@cau.ac.kr
- *These authors contributed equally to this work.
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,482 Views
- 122 Download
ABSTRACT
-
Background
- This study investigated the incidence of endocrine immune-related adverse events (irAEs) for recently developed immune checkpoint inhibitor (ICI) drugs.
-
Methods
- We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through January 31, 2023. Among ICI drugs, nivolumab, pembrolizumab, and ipilimumab were excluded from the new ICI drugs because many papers on endocrine-related side effects have already been published.
-
Results
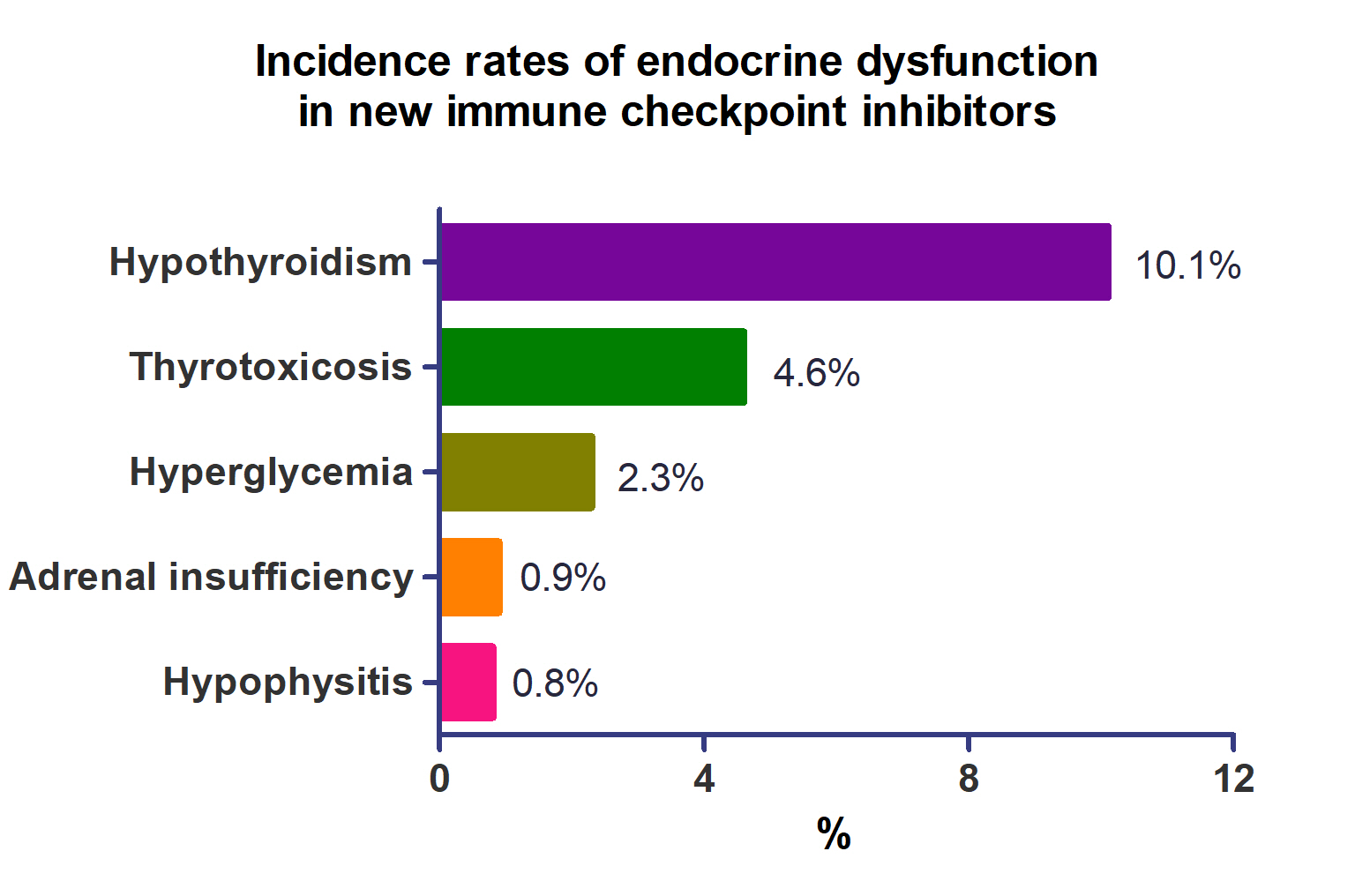
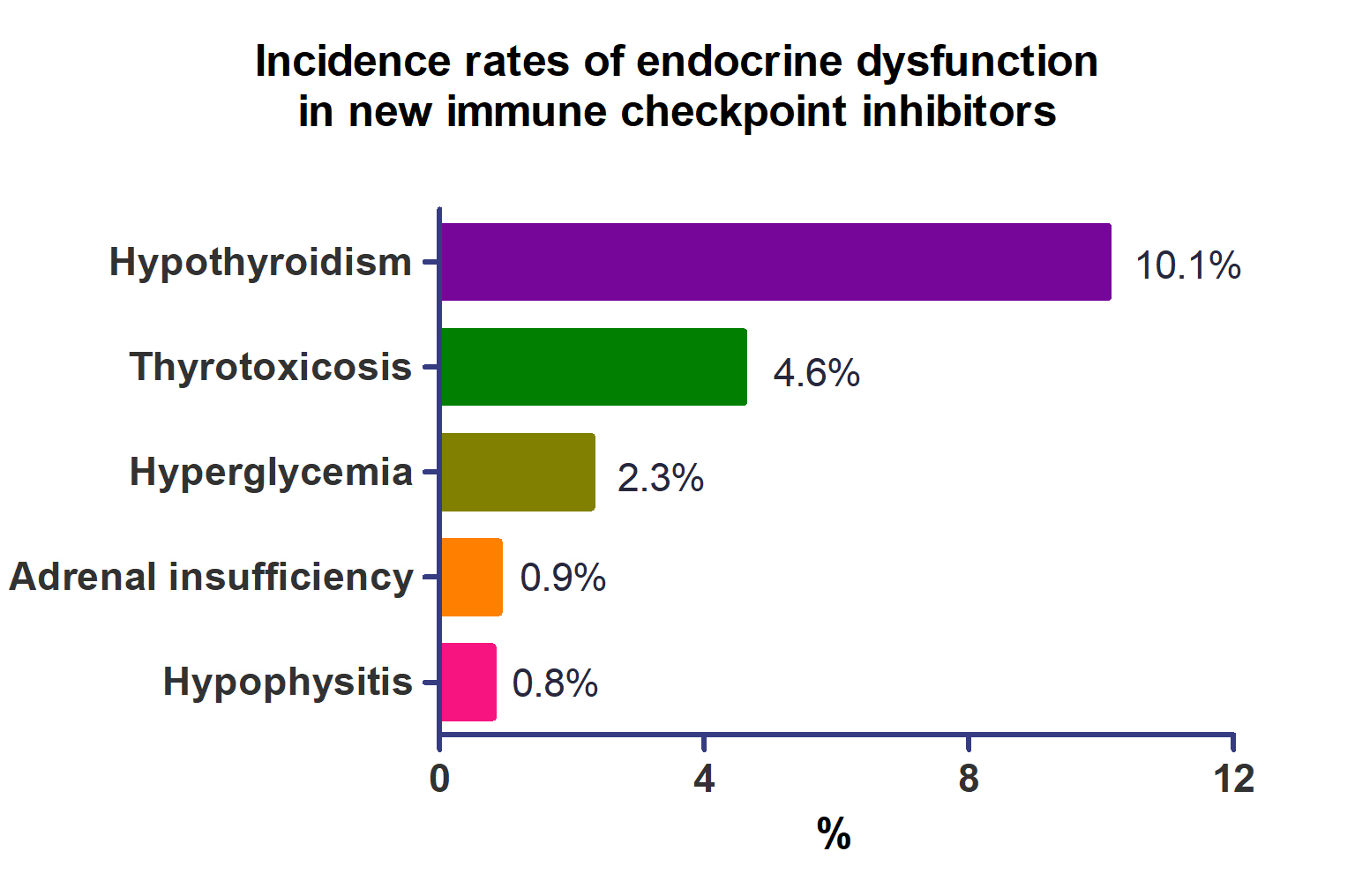
- A total of 44,595 patients from 177 studies were included in this analysis. The incidence of hypothyroidism was 10.1% (95% confidence interval [CI], 8.9% to 11.4%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%). Hypothyroidism and thyrotoxicosis occurred most frequently with programmed cell death protein-1 (PD-1) inhibitors (13.7% and 7.5%, respectively). The rate of endocrine side effects for the combination of a programmed death-ligand 1 inhibitor (durvalumab) and cytotoxic T lymphocyte-associated antigen 4 inhibitor (tremelimumab) was higher than that of monotherapy. In a meta-analysis, the combination of tremelimumab and durvalumab had a 9- to 10-fold higher risk of pituitary and adrenal-related side effects than durvalumab alone.
-
Conclusion
- Newly developed PD-1 inhibitors had a high incidence of thyroid-related irAEs, and combined treatment with durvalumab and tremelimumab increased the risk of pituitary- and adrenal-related irAEs. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.
- Immune checkpoints are receptors found on the T-cell membrane that have an important role in regulating the immune response and preventing autoimmunity, or the attack of healthy cells by the immune system. They act as “brakes” on the immune system, preventing it from attacking normal, healthy cells in the body [1].
- Monoclonal antibodies (mAbs) called immune checkpoint inhibitors (ICIs) block immune checkpoints, allowing T-cells to fight cancer cells. ICIs are a novel family of anti-cancer drugs that are changing the cancer treatment paradigm away from cytotoxic chemotherapy and toward immunotherapy. Currently, among several immune checkpoints, mAbs against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein-1 (PD-1) pathway have been developed and are used for cancer treatment [2].
- However, ICIs have an important role in preserving immunological self-tolerance and avoiding autoimmune disorders. As a result, ICI treatment can cause inflammatory side effects known as immune-related adverse events (irAEs) [3]. Endocrine disorders caused by ICI treatment include hypophysitis, adrenal insufficiency, thyroid dysfunction, and diabetes mellitus [4]. In a previous meta-analysis, thyroid dysfunction mainly occurred with PD-1 inhibitors, hypophysitis occurred with CTLA-4 inhibitors, and endocrine irAEs were increased by ICI combination regimens [5]. Many new ICI drugs are being developed and applied to various types of cancer treatment; however, no endocrine-related irAEs of newly developed ICI have been investigated yet.
- In this study, we investigated the incidence of various endocrine irAEs related to recently developed ICIs. In addition, programmed death-ligand 1 (PD-L1) inhibitors have been approved and used recently, and new large-scale studies have been published, so endocrine irAEs of PD-L1 inhibitors were also examined.
INTRODUCTION
- This systematic review and meta-analysis were conducted according to the principles of the Preferred Reporting Items for Systemic Review and Meta-analysis (PRISMA) [6] and registered in the International Prospective Register of Systemic Reviews (PROSPERO; CRD42022340911).
- Data sources and search terms
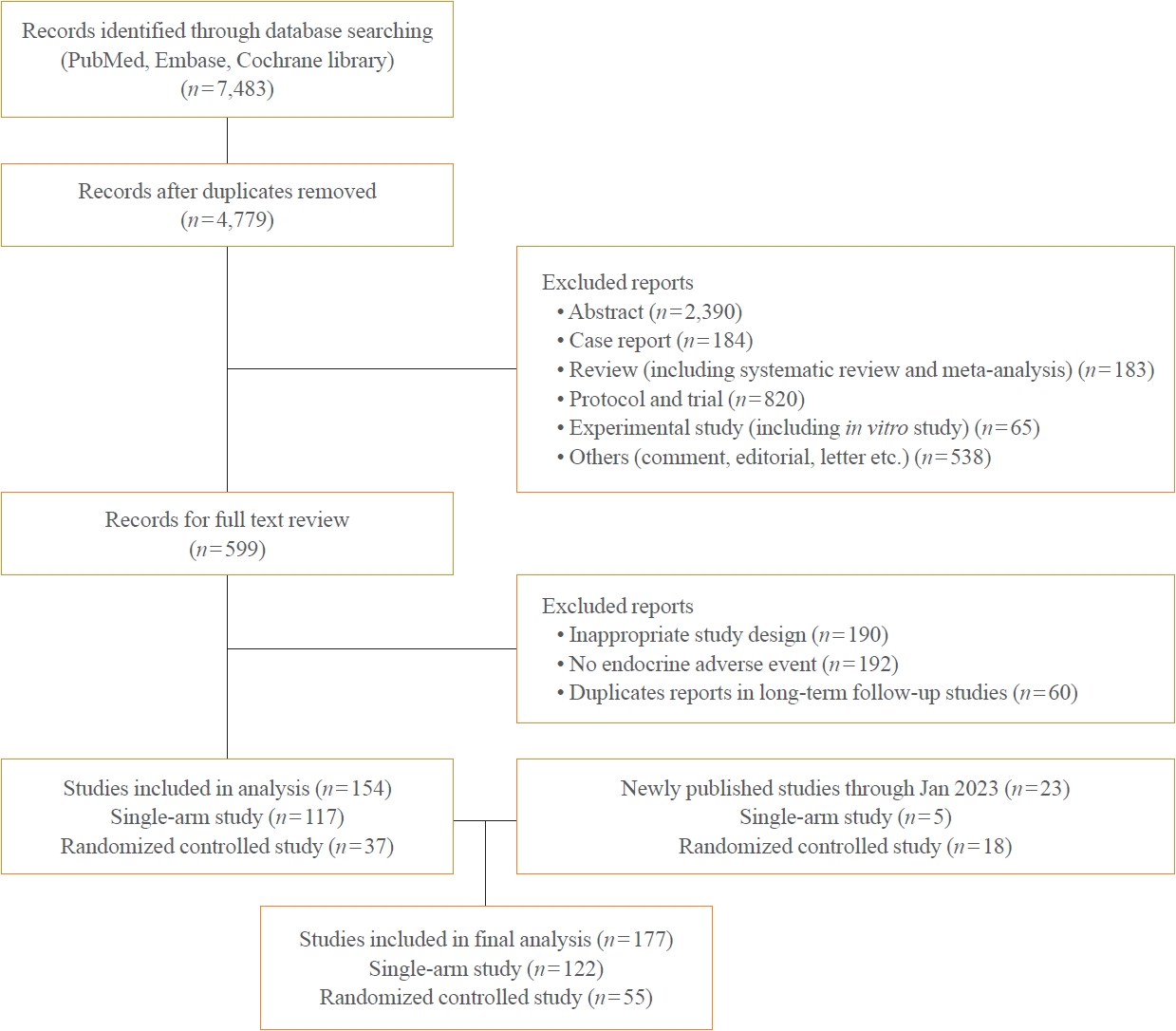
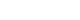
- Because the goal of this study was to analyze endocrine-related dysfunctions of newly developed ICIs, we excluded nivolumab, pembrolizumab, and ipilimumab, for which many studies have been conducted and endocrine-related adverse effects have already been analyzed [5,7,8]. Therefore, newly developed ICIs were defined as drugs approved or under development since 2015. We collected studies on newly developed ICI drugs using PubMed/Medline, Embase, and Cochrane Library from inception through to June 30, 2022. We later added 23 more studies published up to January 2023.
- We used the generic and code names of new ICIs for paper searches as follows: “cemiplimab,” “ReGN2810,” “sintilimab,” “IBI-308,” “camrelizumab,” “SHR-1210,” “toripalimab,” “JS001,” “spartalizumab,” “PDR001,” “atezolizumab,” “MPDL3280A,” “avelumab,” “MSB0010718C,” “durvalumab,” “MEDI4736,” “tremelimumab,” and “CP-67526.” All authors (E.K.L., W.S.Y., E.J.K., and H.Y.A.) independently reviewed the titles and abstracts to select literature requiring full-text review and reviewed each other’s selections.
- Study selection and data extraction
- First, phase 1 to 3 studies and retrospective observational studies were selected to identify endocrine-related dysfunctions caused by ICIs. All selected studies included only those written in English. In the same study, if the follow-up period was different, the one with the longer follow-up period was selected. Among ICI-related side effects, studies that did not report endocrine-related dysfunctions were excluded from selection. Even if endocrinerelated abnormalities were reported, the following cases were excluded from the selection process because it is difficult to evaluate the endocrine-related dysfunction of ICIs alone: (1) when the experimental group used ICIs and other chemotherapies simultaneously, and the control group uses a placebo; and (2) when ICI drugs are combined with other cancer treatments such as surgery or radiation therapy.
- Endocrine-related dysfunctions were comprehensively investigated for hypothyroidism, thyrotoxicosis, hypophysitis (including hypopituitarism), adrenal insufficiency, and hyperglycemia (including diabetes). Most clinical studies follow the Common Terminology Criteria for Adverse Events (CTCAE) classification criteria when reporting endocrine-related adverse effects. Adverse events requiring hospitalization due to side effects correspond to grade 3, and life-threatening events are grade 4. Death is classified as grade 5. Endocrine-related adverse events can rarely be found as grade 3 or higher, and most were reported as minor events of grade 1 or 2. In addition, many research papers report only endocrine-related adverse events without grade classification. Therefore, we investigated the occurrence of endocrine adverse events for all grades.
- Through a full-text review, the following items were extracted for analysis: publication year, first author, title, trial registration number, trial name, ICI name, ICI dose (mg or mg/kg), cancer type, study type, study phase, number of participants for total and safety analysis, median follow-up time, type of control, mean or median age of participants, and number of endocrine-related dysfunctions. Supplemental Table S1 shows an overview of all the included studies.
- Statistical analyses
- Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate each risk of endocrine-related dysfunction in patients with new ICIs compared to the placebo group in randomized controlled trials (RCTs). Exact P values were used and a 2-sided P<0.05 was considered statistically significant. According to the heterogeneity test, I2≥75% or P<0.05 was considered to show significant heterogeneity and fixed or random models were used. Meta-analyses were performed by R Statistical Software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), using the packages ‘meta’ and ‘metafor.’ A random-effects model (DerSimonian and Laird method) at high heterogeneity (I2≥75%) was applied to estimate the pooled incidence across the studies. The 95% CIs for the incidence reported in individual studies were estimated from the proportion of cases of endocrine-related dysfunction and sample size using the binomial exact method (Clopper-Pearson method).
METHODS
- Flow and characteristics of included studies
- A total of 44,595 patients from 177 studies were included in this analysis (Table 1, Fig. 1, Supplemental Table S1). Of the 177 studies, 122 were single-arm studies and 55 were RCTs. Among the ICI drug types, studies of PD-L1 inhibitors were the most common with 89 studies and 31,680 patients. In the classification by cancer type, among the studies on lung cancer, non-small lung cancer-related studies were the most common (n=39). Because we collected and analyzed studies on recently developed ICI drugs, most of the studies published after 2019 were included.
- Quality assessments of included studies
- Among all included studies, we performed a meta-analysis on the risk of endocrine irAE for combination therapy and monotherapy using PD-L1 and CTLA-4 inhibitors. A quality assessment was conducted on the studies included in this meta-analysis using risk of bias tool 2.0 (ROB2.0; https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2), and the results are shown in Supplemental Fig. S1.
- Incidence and risk of endocrine-related dysfunction in patients treated with new ICIs
- The incidence of endocrine-related dysfunction was analyzed in all and each class of ICIs (Table 2). The incidence of hypothyroidism was 10.1% (95% CI, 8.9% to 11.4%; I2=87.1%), thyrotoxicosis was 4.6% (95% CI, 3.8% to 5.7%; I2=79.3%), hypophysitis was 0.8% (95% CI, 0.5% to 1.1%; I2=22.8%), adrenal insufficiency was 0.9% (95% CI, 0.7% to 1.1%; I2=0%), and hyperglycemia was 2.3% (95% CI, 1.6% to 3.4%; I2=88.3%).
- Hypothyroidism occurred most frequently with PD-1 inhibitors (13.7%) and less frequently with CTLA-4 inhibitors (4.4%). Hypothyroidism was more common among PD-1 inhibitors, especially with camrelizumab in 17% of cases and toripalimab in 18% of cases (Supplemental Fig. S2). The incidence of thyrotoxicosis was the most common at 7.5% with PD-1 inhibitors (Table 2), but the overall incidence was lower than that of hypothyroidism. As shown in Supplemental Fig. S3, among the PD-1 inhibitors, toripalimab had a particularly high incidence of thyrotoxicosis (12%). Hypophysitis and adrenal insufficiency occurred in 1.5% to 2.0% with PD-1 and CTLA-4 inhibitors, but less frequently in those with PD-L1 inhibitors (0.4% and 0.8%, respectively) (Table 2, Supplemental Figs. S4, S5). The hyperglycemia incidence was 4.3% with PD-1 inhibitors and was not reported for CTLA-4 inhibitors (Table 2). As shown in Supplemental Fig. S6, among the PD-1 inhibitors, hyperglycemia was frequently reported for toripalimab. For most endocrine irAEs, the rate of side effects in the combination of PD-L1 and CTLA-4 inhibitor groups was higher than that of the monotherapy groups receiving of PD-L1 or CTLA-4 inhibitors (Table 2).
- In the meta-analysis of ICI combination therapy (Supplemental Fig. S7), the difference in the risk of hypothyroidism between combined PD-L1 and CTLA-4 and PD-L1 monotherapy was not statistically significant (OR, 1.16; 95% CI, 0.88 to 1.52). The risk of thyrotoxicosis tended to be higher in the combination therapy group than in the monotherapy group (OR, 1.84; 95% CI, 1.00 to 3.41). The risk of hypophysitis was higher in the combination therapy group than in the monotherapy group (OR, 9.85; 95% CI, 1.84 to 52.75), and the risk of adrenal insufficiency was also higher in the combination treatment group (OR, 10.21; 95% CI, 2.38 to 43.76). However, there was no difference in the risk of hyperglycemia between the combination therapy and monotherapy groups (OR, 1.69; 95% CI, 0.66 to 4.30).
- Comparison of the incidence of endocrine-related adverse events related to conventional ICI drugs
- Previously, the incidence of endocrine-related adverse events related to ICI drugs was mainly analyzed for nivolumab and pembrolizumab among the PD-1 inhibitors, atezolizumab, avelumab, and durvalumab among the PD-L1 inhibitors, and ipilimumab among the CTLA-4 inhibitors. In this study, we did not include nivolumab and pembrolizumab in the anti-PD1 class. The incidence of hypothyroidism for the new PD-1 inhibitors was 13.7%, whereas the incidence of hypothyroidism for the previously developed PD1 inhibitors was 6.8% to 8.5% (Table 3). The occurrence of thyrotoxicosis was also high at 7.5% for the new PD-1 inhibitors compared to 2.8% to 3.9% for the previous PD-1 inhibitors. The incidence of hypophysitis among patients using PD-1 inhibitors was reported to be 0.4% to 1.1%, but in this study, it was slightly higher at 2.0%. There was no difference in the incidence of adrenal insufficiency between the previous and new PD-1 inhibitors. The occurrence of hyperglycemia after using a PD-1 inhibitor was reported as about 2.0% in one paper but was about 4.3% for new PD-1 inhibitors.
- The incidence of endocrine-related adverse events for PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) had previously been analyzed, but the number of included patients ranged from 1,000 to 4,500; therefore, we examined the incidence of endocrine adverse events in 30,000 cases from recent studies. As a result, the incidence of hypothyroidism increased from 3.5% to 6.0% in the previous report to 8.4%, and the rate of thyrotoxicosis was also high at 3.8% compared to 0.6% to 2.3% in previous studies. Hypophysitis caused by PD-L1 inhibitors was not identified in previous reports, but its incidence was 0.4% in our study.
- Among the CTLA-4 inhibitors, hypophysitis occurred in 3.8% to 5.6% of patients using ipilimumab, whereas it occurred in 1.6% to 1.8% of patients with tremelimumab. The combination therapy of durvalumab and tremelimumab had an overall lower rate of endocrine side effects than the previous combination therapy of PD-1 inhibitors (nivolumab and pembrolizumab) and ipilimumab (Table 3) [5,8-12].
RESULTS
- Through this meta-analysis and systematic literature review, we investigated the endocrine adverse event rate of newly developed PD-1 and CTLA-4 inhibitors. We also examined the endocrine adverse event rate from large-scale data for PD-L1 inhibitors. In this study, we identified four new findings. First, the newly developed PD-1 inhibitors, especially camrelizumab and toripalimab, had a higher incidence of thyroid-related irAEs compared to pembrolizumab or nivolumab. Second, a new CTLA-4 inhibitor, tremelimumab, caused less hypophysitis than ipilimumab. Third, PD-L1 inhibitors caused fewer cases of hypophysitis or adrenal insufficiency than other ICIs. Last, combination treatment with a PD-L1 inhibitor (durvalumab) and CTLA-4 inhibitor (tremelimumab) resulted in a higher incidence of pituitary and adrenal-related irAEs compared to single treatment with each drug.
- High rate of thyroid-related adverse events for newly developed PD-1 inhibitors
- Thyroid cells express PD-L1 and PD-L2 [13], which means they are susceptible to immune-mediated damage when these molecules are blocked by PD-1 or PD-L1 inhibitors. The most common thyroid-related side effect associated with ICIs is thyroiditis, which can manifest as transient thyrotoxicosis, hypothyroidism, or a combination of both [14]. ICI-induced thyroiditis is associated with T lymphocytes and shows an intra-thyroidal predominance of CD8(+) and CD4(–)CD8(–) T lymphocytes [15]. The incidence of ICI-induced hypothyroidism varies among different immunotherapy agents. In our study, CTLA-4 inhibitors had a lower rate of hypothyroidism than PD-1 or PD-L1 inhibitors, and this result is similar to a previous meta-analysis [5]. Even within the anti-PD-1 class, the incidence rates of hypothyroidism were higher for camrelizumab and toripalimab, 17% and 18%, respectively, compared with cemipalimab or spartalizumab. In a retrospective analysis of lung cancer patients treated with PD-L1 inhibitors, it was also reported that camrelizumab had a higher incidence of treatment-related adverse effects than other PD-L1 inhibitors [16]. However, it is not clear why there is a difference in adverse effects related to drugs in the same anti-PD-1 class. There are several possible explanations for the observed differences in thyroid-related side effects between the newly developed and established PD-1 inhibitors. First, differences in the pharmacokinetics and pharmacodynamics of these agents could be responsible for the variable incidence of thyroid-related irAEs. For example, it is possible that the newer agents have a longer half-life or higher affinity for the PD-1 receptor, leading to more sustained immune activation and a higher incidence of irAEs. Toripalimab mainly interacts with regions of the heavy chain (VH) of the former and the FG loop of the latter, whereas nivolumab mainly binds to the N-terminal loop of PD-1, and pembroizumab interacts with the C’ D loop [17]. The binding of camrelizumab to PD-1 involves the BC and FG loops [18]. These differences of binding to PD-1 may be the cause of the differences in immune responses. In addition, in the camrelizumab study, the median progression-free survival period was extended in the group with a high number of thyroid-related irAEs, suggesting that the higher the affinity of the drug, the more thyroid dysfunction occurs [19].
- Second, differences in patient populations or study design could have influenced the results. For example, the patients included in the studies of the newer PD-1 inhibitors may have had more risk factors associated with thyroid-related irAEs. Known risk factors for the occurrence of thyroid-related adverse events after using ICIs include positive thyroid autoantibodies, existing thyroiditis, and a family history of thyroid disease [20,21].
- Because thyroid-related side effects are so common, several expert guidelines and position statements recommend thyroid function assessment prior to the initiation of ICI treatment and prior to each cycle for up to 6 months [22-24].
- Low rate of hypophysitis related to newly developed CTLA-4 inhibitors
- Our meta-analysis revealed that hypophysitis was less common with tremelimumab compared to ipilimumab, although the reason for this is unknown. Both ipilimumab and tremelimumab are fully human monoclonal antibodies of immunoglobulin G 1 (IgG1) and IgG2 [25-27]. Ipilimumab and tremelimumab bind to the same region of CTLA-4 using similar VH framework regions, whereas the light chain (VL) framework regions differ from each other [28]. Ipilimumab has a half-life of 12 to 14 days, whereas tremelimumab has a longer half-life of 22 days [29]. The difference in irAEs might be related to the structural differences and half-lives of these two drugs. Although further studies are needed to confirm these findings, our results suggest that tremelimumab may be a safer option for patients who are at higher risk of developing hypophysitis. Human leukocyte antigen (HLA) markers DQ8 and DR53 were associated with the occurrence of lymphocytic hypophysitis [30]. Kobayashi et al. [31] reported that anti-pituitary antibodies and susceptible HLA alleles were predictable markers for pituitary dysfunction induced by ICIs. However, it is not yet common in clinical settings to evaluate anti-pituitary antibodies or HLA analysis prior to ICI treatment.
- Low rates of hypophysitis or adrenal insufficiency related to PD-L1 inhibitors
- PD-L1 inhibitors had lower rates of adrenal or pituitary irAEs compared to PD-1 or CTLA-4 inhibitors. One reason for this difference may be related to the different mechanisms of action of ICIs. PD-L1 inhibitors block the interaction between PD-L1 and its receptor, PD-1, whereas PD-1 inhibitors block the interaction between PD-1 and its ligands, PD-L1 and PD-L2 [32]. CTLA-4 inhibitors block the interaction between CTLA-4 and its ligands, CD80 and CD86 [33]. Because PD-L1 inhibitors only target one of the interactions between the immune system and cancer cells, it is possible that they may be associated with more targeted immune responses and therefore, a lower risk of irAEs compared to PD-1 or CTLA-4 inhibitors, which block multiple interactions between the immune system and cancer cells. Another possible explanation for the lower rates of endocrine irAEs with PD-L1 inhibitors is that they may have a lower affinity for immune cells in the endocrine system, which could reduce the risk of damage to these tissues. In a meta-analysis comparing PD-1 inhibitors and PD-L1 inhibitors, PD-L1 inhibitors had a lower incidence of other organ immune-related side effects such as rash, pneumonitis, and colitis compared with PD-1 inhibitors [34].
- High rates of hypophysitis or adrenal insufficiency related to ICI combination therapy
- Our analysis revealed that combined treatment with durvalumab and tremelimumab resulted in a higher incidence of pituitary and adrenal-related irAEs compared to single treatment with durvalumab. Specifically, the incidence of pituitary-related irAEs was 1.5% for the combined treatment compared to 0.4% for durvalumab alone. Similarly, the incidence of adrenal-related irAEs was 2.7% for the combined treatment compared to 0.8% for durvalumab alone.
- There are several possible explanations for the observed differences in pituitary and adrenal-related irAEs between the combined treatment and single treatment with each drug. The combination of durvalumab and tremelimumab may have a synergistic effect on the immune system, leading to a greater risk of irAEs. Previously, more endocrine-related side effects were reported for combination therapy with a PD-1 inhibitor and a CTLA-4 inhibitor [35]. Because each drug has a different mechanism of action and acts on different lymphocyte subtypes at different sites, more widespread and severe immune-related side effects may occur. Therefore, our findings have important implications for the management of patients receiving ICI combination treatment. First, the increased incidence of pituitary and adrenal-related irAEs associated with the combined treatment of durvalumab and tremelimumab suggests that these drugs may have unique effects on the immune system when used in combination, leading to a greater risk of endocrine-related irAEs. Second, our findings highlight the importance of monitoring pituitary and adrenal-related irAEs in patients receiving combination treatments, especially those at higher risk of developing irAEs.
- There were several limitations in our study. First, the studies included in our meta-analysis varied in terms of patient populations, dosing regimens, and treatment duration, which could have influenced the results. However, it is worth noting that the average onset time of iAEs after ICI use has been reported as 8 to 16 weeks in previous studies [36]. Therefore, careful observation in the early stages of use is necessary. Second, the definition of endocrine-related irAEs in the included studies was variable, which may have affected the accuracy of our estimates. In particular, distinguishing between transient and Graves’ disease-related thyrotoxicosis proved to be challenging. However, based on references to previous reports, it is inferred that a majority of cases are more likely to be transient thyrotoxicosis rather than Graves’ disease-related, despite this difficulty in differentiation. Finally, our analysis was limited to the available published data and did not include unpublished data or data from ongoing studies.
- In conclusion, among the newly developed ICIs, PD-1 inhibitors had a high rate of thyroid-related side effects, whereas PD-L1 inhibitors had a relatively low rate of hypophysitis and adrenal insufficiency. Tremelimumab also had relatively low rate of hypophysitis. However, combination therapy with durvalumab and tremelimumab was associated with a high risk of pituitary and adrenal adverse events. Based on these facts, it is necessary to predict the endocrine side effects corresponding to each ICI drug, diagnose and treat them appropriately, and try to reduce the morbidity and mortality of patients.
DISCUSSION
Supplementary Material
Supplemental Fig. S1.
Supplemental Fig. S2.
Supplemental Fig. S3.
Supplemental Fig. S4.
Supplemental Fig. S5.
Supplemental Fig. S6.
Supplemental Fig. S7.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: W.S.Y., E.J.K., E.K.L., H.Y.A. Acquisition, analysis, or interpretation of data: W.S.Y., E.J.K., E.K.L., H.Y.A. Drafting the work or revising: W.S.Y., E.J.K., E.K.L., H.Y.A. Final approval of the manuscript: W.S.Y., E.J.K., E.K.L., H.Y.A.
Article information
-
Acknowledgements
- This work was supported by the Korean Endocrine Society of EnM Research Award 2021, a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC19C0103) and a National Cancer Center Research Fund (grant number: 2210522-2).
| Study characteristic | No. of studies | No. of patients | |
|---|---|---|---|
| Total | 177 | 44,595 | |
| Study type | |||
| Single-arm study | 122 | 11,986 | |
| Randomized controlled study | 55 | 32,609 | |
| ICI drug types | |||
| PD-1 inhibitorsa | 69 | 9,827 | |
| PD-L1 inhibitorsb | 89 | 31,680 | |
| CTLA-4 inhibitorsc | 9 | 1,717 | |
| PD-L1 and CTLA-4 inhibitors | 10 | 1,371 | |
| Cancer type | |||
| Head and neck cancer | 9 | 2,149 | |
| Breast cancer | 10 | 3,141 | |
| Gastrointestinal cancer (esophageal, gastroduodenal, colorectal cancer) | 22 | 3,538 | |
| Hepato-biliary cancer | 11 | 897 | |
| Non-small cell lung cancer | 39 | 16,363 | |
| Small cell lung cancer | 5 | 1,072 | |
| Melanoma | 7 | 1,236 | |
| Genitourinary cancer | 19 | 8,139 | |
| Reporting year | |||
| Before 2017 | 6 | 1,059 | |
| 2017 | 7 | 2,101 | |
| 2018 | 8 | 3,650 | |
| 2019 | 19 | 1,825 | |
| 2020 | 42 | 8,806 | |
| 2021 | 64 | 16,755 | |
| 2022 | 31 | 10,399 | |
ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T lymphocyte-associated antigen 4.
a PD-1 inhibitor: camrelizumab, cemiplimab, sintilimab, spartalizumab, toripalimab;
b PD-L1 inhibitor: atezolizumab, avelumab, durvalumab;
c CTLA-4 inhibitor: tremelimumab.
| Study | Name of ICI drugs | ICI class | No. of subject | Hypothyroidism | Thyrotoxicosis | Hypophysitis | Adrenal insufficiency | Hyperglycemia |
|---|---|---|---|---|---|---|---|---|
| Barroso-Sousa et al. (2018) [5] | Nivolumab, pembrolizumab | PD-1 | 4,953 | 7.0 (3.0–12.3) | 3.2 (1.7–5.7) | 1.1 (0.8–1.6) | NR | NR |
| Atezolizumab | PD-L1 | 1,010 | 3.9 (1.7–8.4) | 0.6 (0.2–1.8) | NR | NR | NR | |
| Ipilimumab | CTLA-4 | 1,013 | 3.8 (1.9–7.8) | 1.7 (0.8–3.8) | 3.8 (2.7–5.2) | NR | NR | |
| Ipilimumab+nivolumab | PD-1+CTLA-4 | 575 | 13.2 (6.9–23.8) | 8.0 (4.1–15.3) | 8.0 (5.9–10.8) | NR | NR | |
| Lu et al. (2019) [9] | Nivolumab, pembrolizumab | PD-1 | 13,519 | NR | NR | 0.41 (0.22–0.66) | 0.49 (0.16–0.93) | NR |
| Atezolizumab, averlumab, durvalumab | PD-L1 | 4,532 | NR | NR | NR | 0.43 (0.02–1.20) | NR | |
| Tremelimumab, ipilimumab | CTLA-4 | 9,000 | NR | NR | 4.53 (2.62–6.82) | 5.32 (3.30–7.68) | NR | |
| Ipilimumab+nivolumab, ipilimumab+pembrolizumab, duvalumab+tremelimumab | PD-1+CTLA-4 | 2,952 | NR | NR | 7.68 (5.99–9.54) | 4.05 (2.81–5.45) | NR | |
| PD-L1+CTLA-4 | ||||||||
| de Filette et al. (2019) [8] | Nivolumab | PD-1 | 3,317 | 8.0 (6.4–9.8) | 2.8 (2.1–3.8) | 0.5 (0.2–1.2) | 2.0 (0.9–4.3) | 2.0 (0.7–5.8) |
| Pembrolizumab | PD-1 | 4,485 | 8.5 (7.5–9.7) | 3.7 (2.8–4.7) | 1.1 (0.5–2.6) | 0.8 (0.3–2.0) | 0.4 (0.2–1.3) | |
| Atezolizumab | PD-L1 | 998 | 6.0 (4.2–8.4) | NR | NR | NR | 1.4 (0.2–9.4) | |
| Avelumab | PD-L1 | 316 | 5.5 (3.5–8.7) | 2.3 (0.6–8.6) | NR | 1.1 (0.3–4.2) | 1.1 (0.2–7.6) | |
| Durvalumab | PD-L1 | 191 | 4.7 (2.5–8.8) | NR | NR | NR | NR | |
| Ipilimumab | CTLA-4 | 4,430 | 3.8 (2.6–5.5) | 1.4 (0.8–2.4) | 5.6 (3.9–8.1) | 1.4 (0.9–2.2) | NR | |
| Tremelimumab | CTLA-4 | 1,171 | Up to 5.2 | Up to 5.2 | 1.8 (1.1–2.9) | 1.3 (0.7–2.4) | NR | |
| Ipilimumab+nivolumab | PD-1+CTLA-4 | 816 | 16.4 (11.7–22.5) | 9.4 (7.1–12.3) | 8.8 (6.2–12.4) | 5.2 (2.9–9.2) | NR | |
| Ipilimumab+pembrolizumab | PD-1+CTLA-4 | 163 | 15.1 (10.6–21.8) | 10.4 (6.6–16.1) | 10.5 (6.5–16.4) | 7.6 (1.2–36.8) | 2.0 (0.6–5.9) | |
| Durvalumab+tremelimumab | PD-1+CTLA-4 | 99 | 10.2 (5.6–17.9) | NR | NR | NR | NR | |
| Wang et al. (2017) [10] | Nivolumab | PD-1 | 2,445 | 6.8 (5.34–8.62) | 2.84 (2.07–3.89) | 0.54 (0.2–1.43) | 1.74 (0.44–6.63) | NR |
| Pembrolizumab | PD-1 | 3,806 | 8.0 (6.77–9.43) | 3.88 (2.93–5.12) | 0.89 (0.58–1.38) | 0.98 (0.52–2.31) | NR | |
| Atezolizumab | PD-L1 | 189 | 3.49 (0.3–30.62) | NR | NR | NR | NR | |
| Xu et al. (2019) [11] | Ipilimumab | CTLA-4 | 3,280 | 2.5 (2.0–3.1) | 0.3 (0.1–0.5) | 3.9 (3.3–4.6) | 0.6 (0.3–0.9) | NR |
| Tremelimumab | CTLA-4 | 705 | 2.7 (1.6–4.2) | 0.0 (0.0–0.5) | 0.4 (1.1–1.2) | 0.9 (0.3–1.8) | NR | |
| Almutari et al. (2020) [12] | Nivolumab | PD-1 | 1,019 | 7.02 (4.37–10.19) | 3.01 (1.96–4.24) | 0.31 (0.0–1.14) | 1.68 (0.16–4.34) | NR |
| Pembrolizumab | PD-1 | 1,632 | 8.34 (7.01–9.77) | 3.34 (1.6–5.61) | 0.66 (0.05–1.7) | 0.33 (0.03–0.82) | NR | |
| Ipilimumab | CTLA-4 | 2,729 | 2.84 (1.46–4.57) | 0.9 (0.14–2.16) | 4.13 (2.35–6.31) | 0.67 (0.28–1.17) | NR | |
| Pembrolizumab+ipilimumab | PD-1+CTLA-4 | 153 | 16.34 (11.32–23.01) | 11.11 (7.05–17.07) | 10.46 (6.54–16.31) | 3.27 (1.4–7.42) | NR | |
| Nivolumab+ipilimumab | PD-1+CTLA-4 | 630 | 16.39 (13.5–19.49) | 10.16 (5.94–15.28) | 10.4 (6.6–14.88) | 4.21 (2.67–6.04) | NR | |
| Present study | Camrelizumab, cemiplimab, sintilimab, spatalizumab, toripalimab | PD-1 | 9,827 | 13.7 (11.4–16.4) | 7.5 (5.2–10.7) | 2.0 (0.9–4.6) | 1.7 (0.6–4.6) | 4.3 (1.9–9.3) |
| Atezolizumab, averlumab, durvalumab | PD-L1 | 31,680 | 8.4 (7.3–9.7) | 3.8 (3.1–4.6) | 0.4 (0.3–0.6) | 0.8 (0.7–1.1) | 1.8 (1.2–2.7) | |
| Tremelimumab | CTLA-4 | 1,717 | 4.4 (2.0–9.3) | 4.1 (0.9–16.5) | 1.6 (0.8–3.1) | 1.5 (0.6–3.5) | NR | |
| Durvalumab+tremelimumab | PD-L1+CTLA-4 | 1,371 | 9.7 (7.9–11.9) | 5.9 (3.8–9.0) | 1.6 (0.5–5.4) | 2.7 (1.6–4.6) | 3.1 (1.2–7.8) |
- 1. Paluch C, Santos AM, Anzilotti C, Cornall RJ, Davis SJ. Immune checkpoints as therapeutic targets in autoimmunity. Front Immunol 2018;9:2306.ArticlePubMedPMC
- 2. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–82.ArticlePubMedPMC
- 3. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68.ArticlePubMed
- 4. Sznol M, Postow MA, Davies MJ, Pavlick AC, Plimack ER, Shaheen M, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–6.ArticlePubMed
- 5. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–82.ArticlePubMedPMC
- 6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.ArticlePubMedPMC
- 7. Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol 2016;12:413–25.ArticlePubMed
- 8. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res 2019;51:145–156.ArticlePubMed
- 9. Lu J, Li L, Lan Y, Liang Y, Meng H. Immune checkpoint inhibitor-associated pituitary-adrenal dysfunction: a systematic review and meta-analysis. Cancer Med 2019;8:7503–15.ArticlePubMedPMCPDF
- 10. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-pd-1/ pd-l1 treatment for malignancies: a meta-analysis. Front Pharmacol 2017;8:730.ArticlePubMedPMC
- 11. Xu H, Tan P, Zheng X, Huang Y, Lin T, Wei Q, et al. Immune-related adverse events following administration of anti-cytotoxic t-lymphocyte-associated protein-4 drugs: a comprehensive systematic review and meta-analysis. Drug Des Devel Ther 2019;13:2215–34.PubMedPMC
- 12. Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol 2020;10:91.ArticlePubMedPMC
- 13. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, et al. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 2017;27:894–901.ArticlePubMed
- 14. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 2016;101:4431–9.ArticlePubMedPMC
- 15. Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid 2020;30:1440–50.ArticlePubMedPMC
- 16. Zheng X, Tao G, Sun S, Jin X, Chen Y, Zhang Y, et al. Adverse events of different PD-1 inhibitors in lung cancer patients: a real-world study. Ann Transl Med 2022;10:183.ArticlePubMedPMC
- 17. Zhang L, Hao B, Geng Z, Geng Q. Toripalimab: the first domestic anti-tumor PD-1 antibody in China. Front Immunol 2022;12:730666.ArticlePubMedPMC
- 18. Liu K, Tan S, Jin W, Guan J, Wang Q, Sun H, et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep 2020;21:e51444.ArticlePubMedPMCPDF
- 19. Chen Y, Zhuang L, Zhang D, Du X, Sheng L. Thyroid dysfunction as a predictive indicator in camrelizumab of advanced esophageal squamous cell carcinoma. J Immunol Res 2022;2022:4015897.ArticlePubMedPMCPDF
- 20. Ruggeri RM, Spagnolo CC, Alibrandi A, Silvestris N, Cannavo S, Santarpia M. Predictors of thyroid adverse events during cancer immunotherapy: a real-life experience at a single center. J Endocrinol Invest 2023;46:2399–409.ArticlePubMedPDF
- 21. Yoon JH, Hong AR, Kim HK, Kang HC. Characteristics of immune-related thyroid adverse events in patients treated with PD-1/PD-L1 inhibitors. Endocrinol Metab (Seoul) 2021;36:413–23.ArticlePubMedPMCPDF
- 22. Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer 2018;124:1111–21.ArticlePubMedPDF
- 23. Ruggeri RM, Campenni A, Giuffrida G, Trimboli P, Giovanella L, Trimarchi F, et al. Endocrine and metabolic adverse effects of immune checkpoint inhibitors: an overview (what endocrinologists should know). J Endocrinol Invest 2019;42:745–56.ArticlePubMedPDF
- 24. Kwon H, Roh E, Ahn CH, Kim HK, Ku CR, Jung KY, et al. Immune checkpoint inhibitors and endocrine disorders: a position statement from the Korean Endocrine Society. Endocrinol Metab (Seoul) 2022;37:839–50.ArticlePubMedPMCPDF
- 25. Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol 2010;37:533–46.ArticlePubMed
- 26. Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Onco Targets Ther 2016;9:1767–76.PubMedPMC
- 27. Ribas A, Hanson DC, Noe DA, Millham R, Guyot DJ, Bernstein SH, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist 2007;12:873–83.ArticlePubMedPDF
- 28. He M, Chai Y, Qi J, Zhang CW, Tong Z, Shi Y, et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget 2017;8:67129–39.ArticlePubMedPMC
- 29. Callahan MK, Postow MA, Wolchok JD. Immunomodulatory therapy for melanoma: ipilimumab and beyond. Clin Dermatol 2013;31:191–9.ArticlePubMedPMC
- 30. Heaney AP, Sumerel B, Rajalingam R, Bergsneider M, Yong WH, Liau LM. HLA markers DQ8 and DR53 are associated with lymphocytic hypophysitis and may aid in differential diagnosis. J Clin Endocrinol Metab 2015;100:4092–7.ArticlePubMedPDF
- 31. Kobayashi T, Iwama S, Sugiyama D, Yasuda Y, Okuji T, Ito M, et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J Immunother Cancer 2021;9:e002493.ArticlePubMedPMC
- 32. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–8.ArticlePubMedPDF
- 33. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011;332:600–3.PubMedPMC
- 34. Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol 2021;17:2545–58.ArticlePubMed
- 35. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473–86.ArticlePubMedPDF
- 36. Kotwal A, Perlman JE, Goldner WS, Marr A, Mammen JS. Endocrine dysfunction from immune checkpoint inhibitors: pearls and pitfalls in evaluation and management. JCO Oncol Pract 2023;19:395–402.ArticlePubMed
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite